Anaphylaxis is a sudden, severe, and potentially life-threatening allergic reaction, affecting a portion of allergic patients. Adrenaline is the first-line medication for anaphylaxis and available in many parts of the world as adrenaline autoinjectors (AAIs).
ObjectiveAim of this study was to determine attitudes and knowledge levels of patients/parents regarding the use of AAIs, frequency, and rate of appropriate AAI use and to give a standardized and better education by improving on mistakes during administration.
Method190 patients aged 1–18 years who were prescribed AAIs for any reason between 2012 and 2017 in Hacettepe University Pediatric Allergy Unit. Demographic data were collected during face-to-face interview or by telephone. Parents completed a mini-survey regarding use, carriage, and storage of AAI.
ResultsSome 190 patients (64.7% male) aged 7.83 (4.99–12.08) years, median (inter-quartile), were included in the study. The indications for AAI prescription were food allergy (78.9%); venom allergy (14.2%); idiopathic anaphylaxis (3.7%); mastocytosis (2.1%); and drug allergy (1.0%). One-fourth of AAI-prescribed patients experienced anaphylaxis requiring the use of AAI within the past five years. However, only 30% of the patients dared to use AAI; only three-quarters of whom had managed to use it correctly.
ConclusionAfter prescription of AAI and initial training, patients and parents’ concerns and fears should be taken into consideration and necessary support should be provided. At every opportunity and each clinical visit, not only should training sessions be repeated but also the patients and parents should be psychologically supported.
Anaphylaxis is a sudden, severe, and potentially life-threatening allergic reaction which affects a portion of allergic patients. Anaphylaxis cannot be prevented entirely, so at-risk patients should be ready for this severe reaction.1
According to the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group, the lifetime prevalence of anaphylaxis is between 0.05–2.00%.2 In Europe, the lifetime prevalence was found to be 0.3%.3 The frequency is increasing worldwide and in all age groups.4 The annual incidence of anaphylaxis in Turkey was estimated to be 1.95 per 100,000.5 The frequency of anaphylaxis was found to have increased by 5–7 times in the last 10–15 years according to a multicenter study from Turkey.6
Adrenaline is the standard care and first-line medication for anaphylaxis and is available in many parts of the world as adrenaline autoinjectors (AAIs). Despite its vital and life-saving role, adrenaline is still underused or usually administered with a delay in the management of anaphylaxis.7 In previous studies, the rate of AAI usage was found to be quite low in children with recurrent episodes of anaphylaxis.8–10
Predictors of appropriate and inappropriate use of AAIs in clinical practice have not been well described. The aim of this study was to determine the attitudes and knowledge levels of patients/parents regarding the use of AAIs, frequency, and the rate of appropriate AAI use and to provide standardized and better education by improving the errors made during administration.
MethodThe present study included patients aged 1–18 years who were prescribed AAIs for any reason (food allergy, drug allergy, venom allergy, idiopathic anaphylaxis, mastocytosis etc.) between 2012–2017 years in Hacettepe University Pediatric Allergy Unit.
Patients were invited to enrol in the study during a face-to-face interview or by telephone (95%). 190 of 200 patients who were interviewed were included in the analysis.
Demographic data were collected during the interview. The parents completed a mini-survey regarding the use, carriage, and storage of AAI. They were asked whether the patients experienced a condition that they should use AAI, in this case, they were asked whether they used AAI or not, the resulting complaints, the location in which anaphylaxis occurred, whether they had taken any other medication before AAI, the misuse of AAI, and the adverse effects. They were also questioned about training on AAI usage and how competent they felt after training.
Informed consent was obtained from both the parents and the patients if they were over six years of age. The study was approved by the Medical Ethics Committee of Hacettepe University (Hacettepe University, GO 17/330).
Statistical analyses were performed using the SPSS 22 software program (IBM SPSS Statistics for Windows, Armonk, NY, USA). Variables were investigated with visual and analytic methods to decide whether they were normally distributed or not. Quantitative variables were non-normally distributed and expressed as median [Interquartile Range (IQR)]. Frequencies and proportions for categorical variables were used to describe patient characteristics.
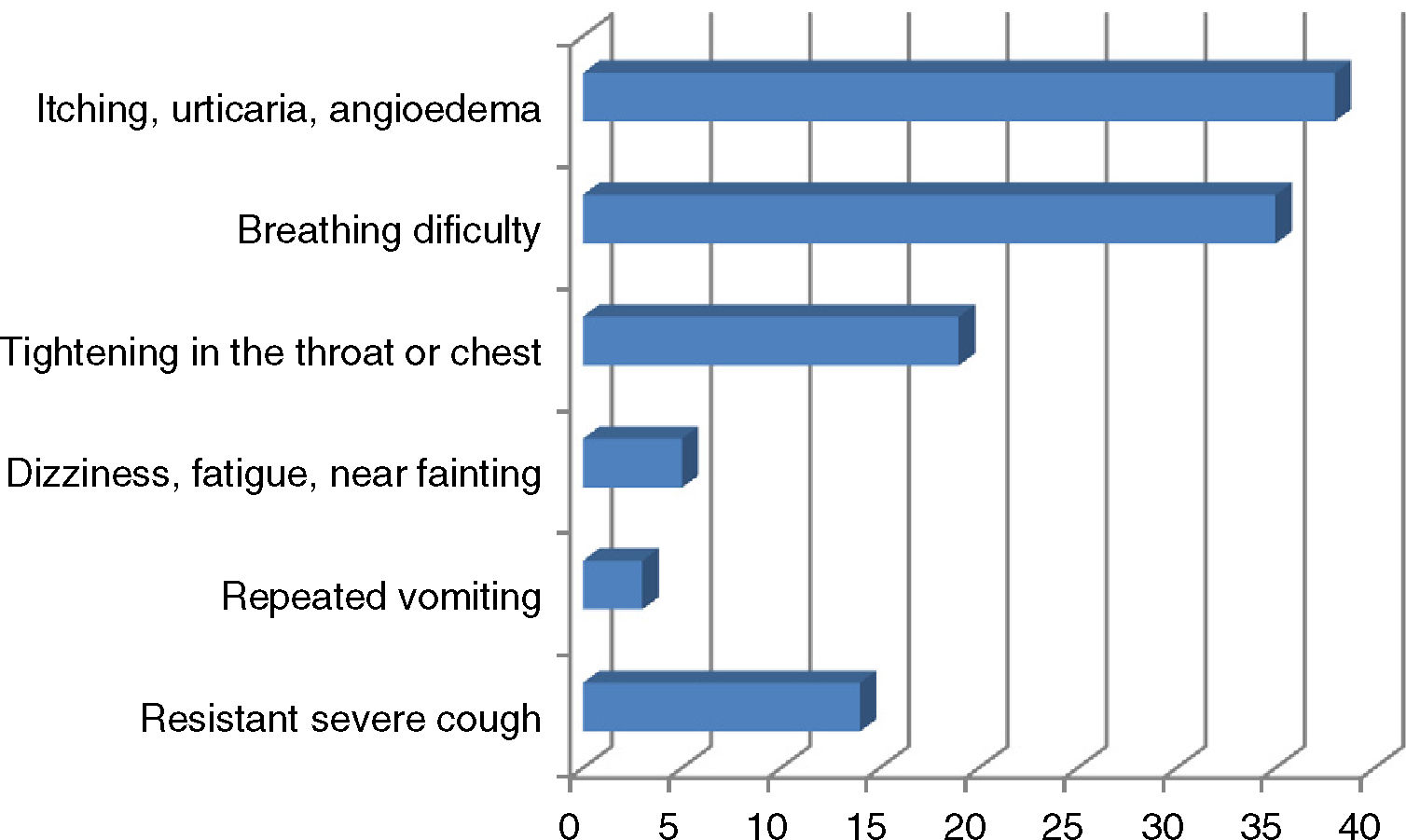
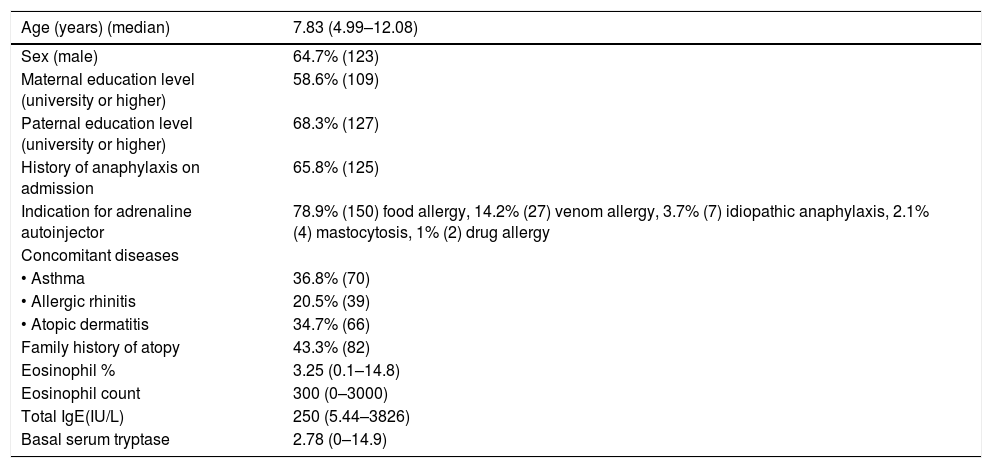
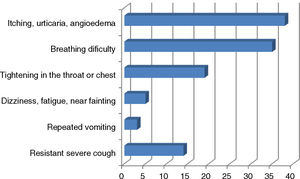
ResultsA total of 190 patients aged 7.83 (4.99–12.08) years, median (inter-quartile), were included in the study. The percentage of boys/girls was 64.7/35.3. Some 65.8% experienced anaphylaxis prior to admission. The most common indication for AAI prescription was food allergy (n=150, 78.9%); other indications were venom allergy (n=27; 14.2%), idiopathic anaphylaxis (n=7; 3.7%), mastocytosis (n=4; 2.1%) and drug allergy (n=2; 1.0%). The median time elapsed since AAI was first prescribed was 4.00 (0.83–5.50) years. The percentage of parents with a university or higher level of education was 58.6% for mothers and 68.3% for fathers (Table 1). The personal and family histories of atopy are shown in Table 1. Of the 44 patients with a single episode of anaphylaxis that required AAI; 42 episodes were due to food allergen intake (95.5%) and the other reasons were hymenoptera sting in one patient and idiopathic anaphylaxis in another patient. The symptoms of the patients during anaphylaxis are shown in Fig. 1.
Characteristics of the patients (n=190).
| Age (years) (median) | 7.83 (4.99–12.08) |
|---|---|
| Sex (male) | 64.7% (123) |
| Maternal education level (university or higher) | 58.6% (109) |
| Paternal education level (university or higher) | 68.3% (127) |
| History of anaphylaxis on admission | 65.8% (125) |
| Indication for adrenaline autoinjector | 78.9% (150) food allergy, 14.2% (27) venom allergy, 3.7% (7) idiopathic anaphylaxis, 2.1% (4) mastocytosis, 1% (2) drug allergy |
| Concomitant diseases | |
| • Asthma | 36.8% (70) |
| • Allergic rhinitis | 20.5% (39) |
| • Atopic dermatitis | 34.7% (66) |
| Family history of atopy | 43.3% (82) |
| Eosinophil % | 3.25 (0.1–14.8) |
| Eosinophil count | 300 (0–3000) |
| Total IgE(IU/L) | 250 (5.44–3826) |
| Basal serum tryptase | 2.78 (0–14.9) |
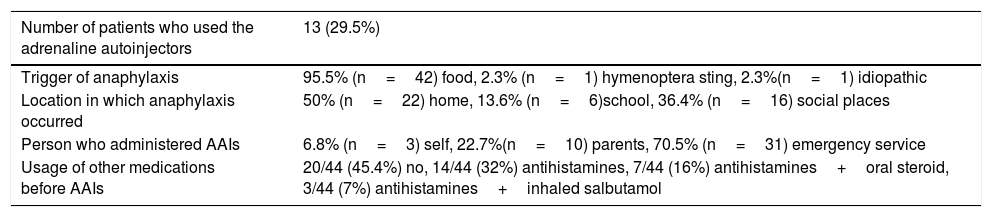
Among the 44 patients who experienced anaphylaxis, AAIs was administered in three patients by themselves, ten by parents and 31 by physicians at the emergency department. Two patients described restlessness upon administration of AAIs as an adverse effect.
The locations where anaphylaxis occurred were home (n=20, 50%) followed by social places (n=16, 36.4%) and school (n=6, 13.6%). Nearly half of the patients used other medications including antihistamines, oral steroid or inhaled salbutamol before AAI (Table 2). Some of the patients (23%) used AAI incorrectly; two made the drug out, one unintentionally injected into the finger. Incorrect applications were performed by parents.
Characteristics of the patients who experienced anaphylaxis (n=44, 23.15%).
| Number of patients who used the adrenaline autoinjectors | 13 (29.5%) |
|---|---|
| Trigger of anaphylaxis | 95.5% (n=42) food, 2.3% (n=1) hymenoptera sting, 2.3%(n=1) idiopathic |
| Location in which anaphylaxis occurred | 50% (n=22) home, 13.6% (n=6)school, 36.4% (n=16) social places |
| Person who administered AAIs | 6.8% (n=3) self, 22.7%(n=10) parents, 70.5% (n=31) emergency service |
| Usage of other medications before AAIs | 20/44 (45.4%) no, 14/44 (32%) antihistamines, 7/44 (16%) antihistamines+oral steroid, 3/44 (7%) antihistamines+inhaled salbutamol |
The mini survey for the appropriate use and storage of AAI resulted in correct answers in most, as shown in Table 3.
Knowledge level of participants regarding usage and storage of AAIs.
| Questions | % of patients/parents with the correct answer |
|---|---|
| What should you do after using AAIs? (I should immediately go to emergency service after using AAI.) | 98.4 |
| How often do you change your AAI? (I change it when used or according to the expiration date.) | 89.5 |
| Where do you carry and store your AAIs? (I carry it in my bag all the time and keep it at room temperature.) | 88.9 |
| Where should you apply AAI? (I should apply it to the lateral side of the thigh) | 95.8 |
The initial training was provided by an allergy nurse in 42.2% of the patients, by an allergy specialist in 35.1%. Nearly 7% of the patients/parents have watched education videos from the internet, others learned either from family doctor, nurse, pharmacist or read from the prospectus. 4.7% of the patients said that they did not have training for usage. Only 36.7% of patients or parents felt confident about using the AAI after training, whereas 42.6% felt anxious, 15.4% felt fear and 5.3% stated that they did not understand the use of AAI. The type of trainer was not associated with the outcome in terms of administration or mini survey results. Three-fourths of patients had one auto-injector, and 21.6% had two AAIs.
There was no significant difference between the patients who used AAI themselves and applied to the emergency department, with regard to sex, trigger of anaphylaxis, education level of the parents, positive history of anaphylaxis on admission, family history of atopy, type of trainer or feeling confident or anxious after training.
DiscussionIn this study, we observed that one-fourth of the AAI-prescribed patients experienced anaphylaxis and had required the use of AAI within the past five years. However, only 30% of the patients had the courage to use AAI and furthermore, only three-quarters of those users had managed to use it correctly.
The increasing prevalence of allergic diseases, especially food allergies, leads to the prescription of AAIs by physicians more often. In our patient population, the most common cause for the prescription of AAI was a food allergy (78.9%). Also, the most common trigger factor for anaphylaxis among the 44 patients was food allergen intake in 95.5%. According to the European Anaphylaxis Registry, the most common cause of anaphylaxis in children is food allergies (88% in children aged <6 years, 57% in children aged 6–12 years).11 A study from Japan reported the clinical background that led to the prescription and AAI usage as being immediate food allergy in 240 cases among 266 cases (90%).12 In a study from the USA, the most common cause of AAI use was found to be food allergy (79.3%).13
In the present study, 44 of 190 (23.1%) patients included during a five-year period had experienced anaphylaxis. The frequency of AAI use is relatively higher in our study compared with the literature: among the 190 patients who were prescribed AAIs, we found the frequency of AAI use as being 6.84% (13 of 190 patients), 29.5% among the patients needing an AAI, the use of AAI at 6.84% is still very low compared with current recommendations and standards. In a study from Western Japan, Korematsu et al. found that 1330 AAIs were prescribed in one year and 6% of patients used them.14 Consistently another study from Japan: between the years 2011–2014, they reported that none of the 217 patients with anaphylaxis used an AAI before going to the emergency department.15 Fleisher et al. reported the annualized reaction rate as 0.81(367/512 subjects reporting 1171 reactions) over a median follow-up of 36 months (range: 0–48.4). Of the 11.4% of reactions (n=134) that were severe, 29.9% were treated with epinephrine.16 According to the European Anaphylaxis Registry, 12% of patients used AAI before presenting to the emergency department.11 Fatal anaphylaxis was found to be associated with the absence of adrenaline or inappropriate administration.17 Therefore patients and parents should be encouraged to administer adrenaline if necessary.
The most commonly reported misuse of adrenaline autoinjectors is in the form of unintentional injections involving the finger or thumb.18,19 In a study of 266 anaphylaxis cases from Japan, no erroneous application was detected.12 In the present study three of the 13 patients who applied AAI misused it (23%) which shows the necessity to train with placebo trainers and repeat the demonstration.
The majority of the patients received training for AAI use from the allergy nurse and/or physician. During the evaluation of the educational tools of the patients, we noticed that internet usage is low despite the technological developments in today’s world. During the initial education after the prescription of AAI, the appropriate training videos may contribute to the quality and utility of learning and the internet links to be accessed by the patients will provide the patient and his/her family with the opportunity to remember and update their information and to cooperate with the doctor.
Although our country is a developing country, the education and socio-cultural level of parents in our study population were high. The percentage of correct answers to questions about AAI use, carriage and storage were >85% for all questions. However, it was remarkable that the use of AAI was still low. When patients were asked how they felt about using AAI after training, only 36.7% of the patients stated that they felt competent in that regard, 42.6% were anxious, and 15.4% felt fear. In previous studies, it was found that food allergy had adverse effects on the quality of life of the patients and their parents.20,21 The psychological burden and the fear and concern of the patient’s family in this emergent and life-threatening situation may be an important factor on the underuse of AAIs. Like the present study, Kim et al. also found that the level of education or knowledge does not have a direct correlation with parental empowerment but psychological factors and parental comfort have.22
In our study, the majority of patients had only one AAI. This may be due to the absence of packages with double injectors in our country as well as the fact that more than one AAI was not covered by the social security institution at that time. Additionally, a recent adult study reported that although patients have two AAIs, 82% of them did not carry two AAIs and 84% preferred to keep one AAI in another location.23 Given that the need for using second dose AAI in anaphylaxis is common, the importance of carrying two AAIs should be counselled for parents/patients on each training and clinical visit.
Our study has several limitations. First of all, the retrospective design of the study may lead to a lower frequency of anaphylaxis due to recall bias. Secondly, the results would be more objective if the interviews were performed face-to-face and the mini-survey were applied on anaphylaxis models. The strength of our study lies in the fact that it is the first study of our country with a high number of patients from one center which also includes psychological factors. Many new studies may inspire this work: future studies planned with appropriate psychological surveys will provide more professional suggestions for those patients and parents experiencing anaphylaxis.
In summary, after the prescription of AAI and initial training, the patients and parents’ concerns and fears should be taken into consideration and necessary support should be provided. The rate of use of AAIs, which are vital in the treatment of anaphylaxis, may be increased in this way. At every opportunity and on each clinical visit, not only should training sessions be repeated but also the patients and parents should be psychologically supported.
Conflict of interestAuthors declare that there is no conflict of interest and no funding. All authors approve the final version of the manuscript.