Asthma control represents the main goal of asthma management and different strategies aim to avoid the long term downsides of inhaled corticosteroids. We investigated in real-life conditions the contribution of sublingual immunotherapy in achieving the control of birch-related mild persistent asthma compared to two usual step-up therapeutic options.
MethodsA three-year open randomised study included 84 asthmatics, uncontrolled during the previous birch pollen season, despite a treatment with budesonide 400μg/day. Patients randomly received budesonide 800μg/day, budesonide 1600μg/day, budesonide 400μg/day plus montelukast 10μg/day and budesonide 400μg/day plus carbamylated allergoid of betulaceae pre-coseasonally. Asthma Control test, combined allergy symptoms and medications score, albuterol consumption, lung function, nasal eosinophils and nasal steroids usage were assessed as changes from the first to last pollen season.
ResultSeventy-six patients concluded the study. All options, except budesonide 800μg/day, produced an improvement of mean monthly Asthma Control test (p<0.05). Patients undergoing low-dose budesonide plus immunotherapy achieved, after three years, an appreciable control (ACT mean score 24). A significant improvement was seen in all groups for allergy symptoms plus medications and bronchial reactivity. Albuterol consumption and lung function improved in all but the first group. Only budesonide plus immunotherapy reduced nasal eosinophils and nasal steroids usage. Two mild self-resolving adverse events were reported.
ConclusionsFor patients with respiratory allergy due to birch pollen and mild persistent asthma, sublingual immunotherapy added to low-dose inhaled corticosteroids appears effective in maintaining long-term seasonal asthma control, representing a safe opportunity to reduce the cumulative amount of delivered corticosteroids.
Asthma is a multifaceted disease; consequently numerous markers of its severity and activity are available. Among them, functional parameters, clinical assessment and biomarkers of inflammation are widely used. In recent years the primary goal of asthma treatment has been identified in disease ‘control’, a composite outcome including all the primary clinical and functional aspects.1
The Asthma Control Test (ACT) allows a quick patient's self-assessment of the degree of disease control.2 The periodical assessment of this degree permits the treatment adjustment with a step-wise approach.
In persistent asthma of any level of severity, treatment guidelines recommend regular inhaled corticosteroids (ICSs), being the most effective drugs for achieving disease control, reducing mortality for asthma, preventing exacerbations, reducing symptoms and reliever medications use, improving lung function and suppressing airway inflammation, albeit the evidences of preventing disease progression are not consistent.1,3,4
In order to face the risk of downsides in the long-term period, usual strategies aim to reduce the amount of delivered ICSs with the addition of pharmacological agents, such as long-acting bronchodilators (LABAs) or anti-leukotrienes. Moreover, since allergic asthma is often associated with allergic rhinitis (AR), particular attention should be paid to the total intake of corticosteroids, simultaneously delivered through nasal and bronchial routes.5,6
Allergen specific immunotherapy (SIT), as a biological response modifier, is the only treatment handling causes and not only symptoms.7,8 Administered either subcutaneously or sublingually (SLIT), it reduces the symptoms of AR and asthma, as shown by numerous controlled trials and metanalyses, representing a complementary option to traditional pharmacotherapy.9
A number of studies have compared different asthma medication regimen, nevertheless studies directly comparing therapeutic approaches including the use of SIT in allergic asthma are lacking or reached controversial conclusions. In some circumstances injective SIT enhanced the effect of ICSs and showed a steroid-sparing effect in both adults and children with mild to moderate asthma.10–12 Conversely in others it failed to reach, or marginally affected the clinical endpoint.13,14 Add-on SLIT reduced the delivery of ICSs and improved lung function,15 but did not provide any additional benefit to children optimally controlled by standard treatment.16 Recently long-term SLIT was more beneficial than ICSs in grass allergic patients, reducing the nasal local inflammation and the non-specific bronchial responsiveness,17 and it was able to step-down seasonal birch-induced asthma.18
This study investigated whether SLIT provides any advantage in real-life conditions, in achieving the control of mild persistent asthma related to birch pollen, compared to increasing doses of ICSs or combination of low-dose ICSs with anti-leukotrienes.
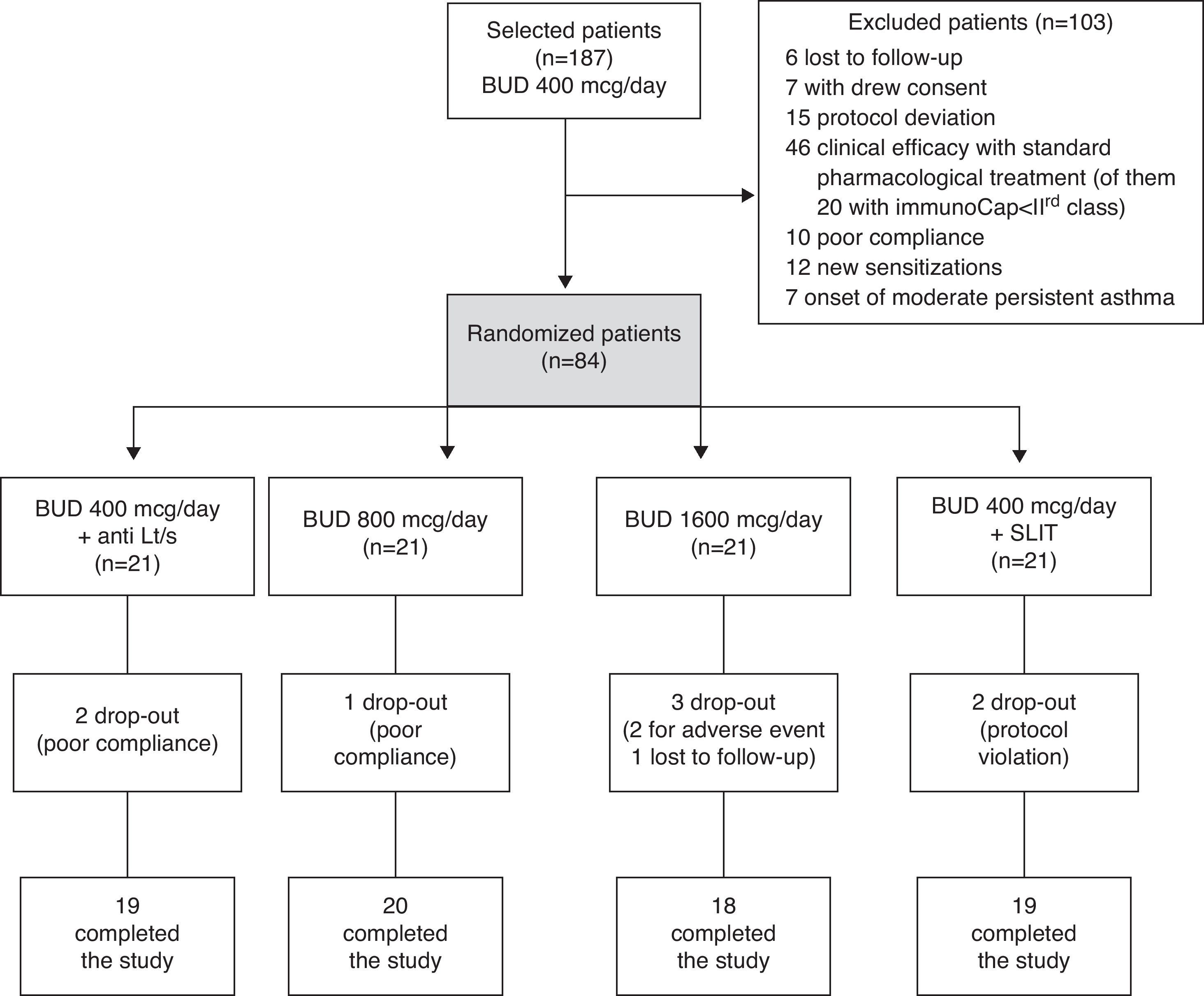
Materials and methodsStudy designThis study was carried out in the allergy department of the Macchi Hospital Foundation, Varese. One hundred and eighty-seven outpatients, sensitised to birch pollen and suffering from AR and mild persistent asthma (according to GINA guidelines classification) during the pollen season, were recruited in 2004.1 During the 2005 birch pollen season (run-in period), all patients were treated with standard pharmacological treatment for step 2 of GINA guidelines, including a fixed low-dose of inhaled budesonide (400μg/day), on demand albuterol spray (100μg) and oral antihistamines (cetirizine 10mg/day), from February to April.1 For patients non-adequately responding, identified by a lack of control assessed by asthma control test (mean monthly ACT score less than 20), four alternative step-up options were randomly adopted: (1) medium (800μg/day) or (2) high (1600μg/day) dose of inhaled budesonide, (3) low-dose (400μg/day) of budesonide plus leukotrienes inhibitor (montelukast 10mg/day), and (4) low-dose (400μg/day) of budesonide plus SLIT (Fig. 1). The study was approved by the local ethical committee and all patients signed an informed consent at enrolment.
Inclusion criteria: age 18–65 years; clinical history of AR with mild persistent asthma (according to GINA classification) for at least two years, with symptoms limited to the birch exposition period; skin prick test (>5mm) positivity to birch; minimum class II positivity to birch assessed with ImmunoCAP (Unicap; Pharmacia, Uppsala, Sweden); pre-bronchodilator forced expiratory volume in one second (FEV1) of 80% or more of the predicted value; positive methacholine challenge (PD20 FEV1 <800μg).
Exclusion criteria: sensitisation to perennial allergens (mites, cat, dog) and seasonal confounding allergens (grass, parietaria, cypress), systemic immunological disorders, moderate to severe asthma, malignancies, diabetes, chronic heart failure, chronic obstructive pulmonary disease, pregnancy or lactation, any SIT course in the last five years, major psychiatric disorders, or major anatomical abnormalities of the nose.
Skin prick test and verification of inclusion/exclusion criteria were carried out during the screening phase and confirmed before randomisation. A standard panel (Lofarma S.p.A., Milan, Italy), including mites, grass, parietaria, cypress, birch, olive, mugwort, ragweed, cat, dog, alternaria, and cladosporium, positive (1% histamine) and negative (diluent) controls was applied.
Evaluation of efficacy and safetyThe primary end-point was the change in mean monthly ACT for each pollen season, from baseline to 2008. The questionnaire consists of five questions enquiring about the frequency of symptoms, the limitation of daily activities and the use of rescue medications in the previous four weeks. Each item has five possible answers scored from 1 (worst) to 5 (best). The higher the ACT score (maximum 25 points), the better controlled is asthma.
Secondary end-points were the assessment of symptoms-medications scores (sum of scores for symptoms and total rescue medications use) and delivery of bronchodilators and intranasal corticosteroids, registered in a 3-month diary card during 2005 and 2008 seasons only. Each symptom (nasal itching, sneezing, rhinorrhoea, nasal obstruction, eye itching, redness cough, wheezing and dyspnoea) was scored as follows: 0, absent; 1, mild; 2, moderate and 3, severe. Intranasal budesonide, two puffs per nostril daily (100μg/day), oral cetirizine one tablet daily (10mg/day), inhaled alburerol spray (100μg), prednisone tablets (50mg) were prescribed as rescue medications on demand and scored as: 1 point for bronchodilator, 2 points for antihistamine and topical steroid; 3 points for oral corticosteroid.
These scores were used to calculate for each patient a mean monthly symptoms-medications score (SMS) and a mean monthly score for the bronchodilators and intranasal corticosteroids usage.
Pulmonary function (FEV1 and MEF25), bronchial reactivity to methacholine (MCH) and nasal eosinophilia (EOS) were evaluated during the run-in (2005) and in the last pollen season (2008). The MCH challenge was performed with an inspiration-activated dosimeter (Masterlab Yaeger), delivering methacholine from 30 to 1200μg in refracted doses. A computerised dose-response curve identified the provocation dose causing a 20% decrease in FEV1 (PD20).
Nasal smear, obtained from the inferior turbinate with a cotton tip, was put on a glass slide, air-dried, and stained with May-Grunwald-Giemsa. Eosinophils count by optical microscopy was expressed as a percentage of 100 cells in 10 fields.
The occurrence of adverse events (AEs) was registered by patients and was inquired by investigators at each monthly visit. The judgements on compliance to SLIT and pharmacological treatment were based on checking returned blisters and patient's feedback respectively.
Investigational treatmentTreatment was administered at the beginning of February (eight weeks before the expected pollen peak) up to the end of April, for at least 12 weeks. Two groups of patients assumed respectively inhaled budesonide 400μg twice/day (group 1) and budesonide 800μg twice/day (group 2). A third and fourth group received budesonide 200μg twice/day plus morning oral montelukast 10mg (group 3) and plus SLIT (group 4) respectively. The randomisation procedure was based on a computer-generated list.
Carbamylated monomeric allergoid in tablet (Lais®, Lofarma S.p.A., Milan, Italy), standardised with an in-house-reference, was administered in accordance with the recommendations of the manufacturer, with a four-day build-up phase followed by a maintenance phase of three years (1000 Allergic Unit once a day for five days/week) in a pre-coseasonal regimen, from early February to late April for at least 12 weeks. Cumulative annual average dose was approximately 60,000AU (equivalent to 214,200μg of modified major allergen).
Statistical analysesHomogeneity of demographical and clinical parameters was assessed using Kruskal–Wallis test or Monte Carlo simulation.19,20 Variations in clinical parameters were computed as percentage change from baseline to the third year and tested. Within treatments changes were compared using a Generalised Linear Model approach for repeated measures. Post hoc test for homogeneous (Least Significant Difference) or not homogeneous variances were used for pair-wise comparison of the efficacy. Sample size calculation estimated that a total of 108 patients should enter the efficacy analysis. The probability is 80% that the study will detect a treatment difference at one-sided 0.05 significance level, if the true difference between treatments is 8%, with the assumption that the SD of response variable is 16.53.21 All the statistical analyses have been computed using the Statistical Package for Social Sciences ver. 17.01 (SPSS® Inc., Chicago, USA).
ResultsAfter the run-in period, 84 patients non-adequately responding to standard drug therapy, identified by a lack of asthma control assessed by ACT, were randomised to the four treatment options (21 pts each group). Eight subjects dropped-out during the study; details are provided in Fig. 2, together with the reasons for exclusion of 103 screened patients. The efficacy analysis included 76 subjects. Age and clinical parameters did not differ between the four treatment groups (p>0.05). The mean age was 24.797 years (SD 0.8) with range 18–43 years.
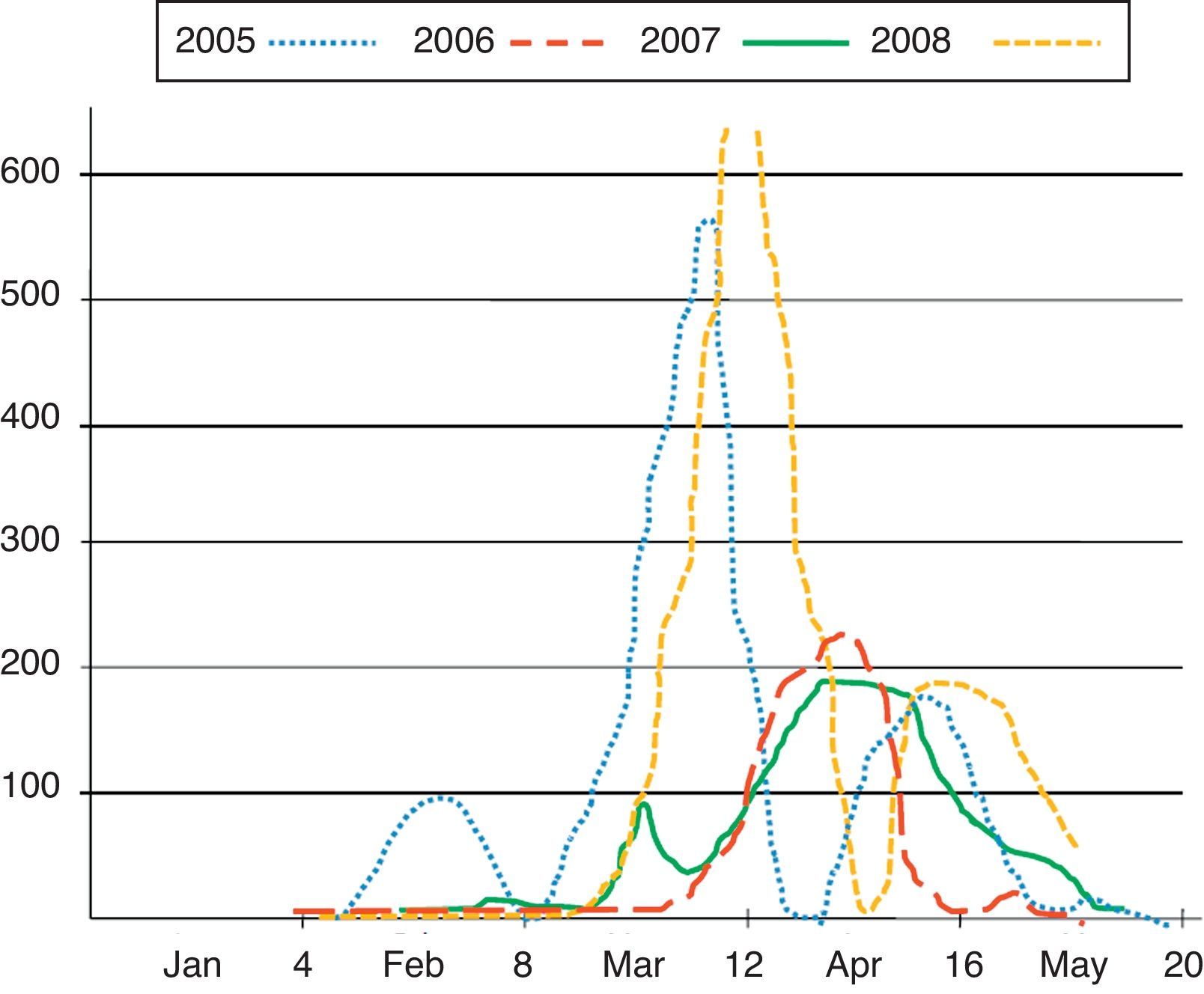
The pollen count showed a similar pattern in the four seasons, with median beginning at week 9.5, peak at week 13 and conclusion at week 18 (Fig. 3). However, the pollen peak reached higher levels during 2005 and 2008 (over 550grains/mm3) with respect to 2006 and 2007 (around 200grains/mm3).
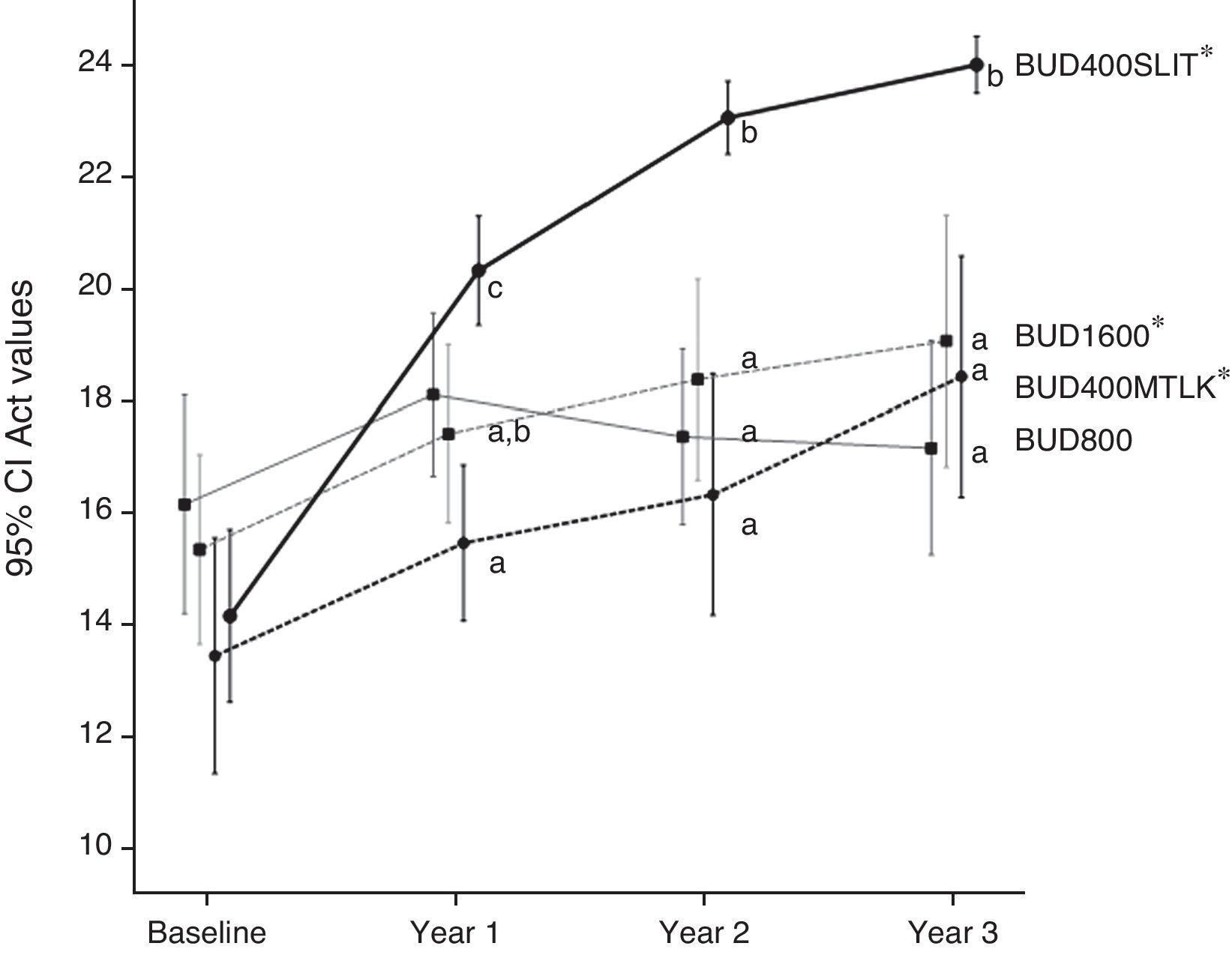
As overall, the changes in clinical outcomes showed an increased efficacy from group 1 to group 4 (Table 1). In particular, only the treatment with budesonide 800μg/day did not exert a significant change after three years in the level of mean monthly ACT. A significantly better performance was associated with the use of SLIT; only patients of group 4, in fact, finally achieved an appreciable control (mean 24; SEM 0.242). Groups 1, 2 and 3 had a mean monthly ACT ranging from 17.2 to 19.1 (Fig. 4).
Mean (and standard error of mean, SEM) of the clinical outcomes measured at baseline (2005) and after three years (2008). Statistical significance is calculated in changes from baselines.
| Outcome | ||||||||||||||||
| ACT | SMS | FEV1 | MEF25 | MCH | B2 | EOS | NCS | |||||||||
| Year | 2005 | 2008 | 2005 | 2008 | 2005 | 2008 | 2005 | 2008 | 2005 | 2008 | 2005 | 2008 | 2005 | 2008 | 2005 | 2008 |
| Treatment | ||||||||||||||||
| BUD800 | 16.1 (0.9) | 17.2 (0.9) | 424.2 (16.3) | 322.3 (21.6)* | 88.3 (0.8) | 90.3 (2.1) | 47.0 (1.9) | 59.1 (3.8)* | 226.9 (22.6) | 520.0 (64.7)* | 11.1 (0.6) | 10.4 (1.2) | 25.7 (1.6) | 25.6 (2.5) | 18.0 (1.1) | 17.2 (1.4) |
| BUD1600 | 15.3 (0.8) | 19.1 (1.0)* | 401.4 (14.1) | 258.3 (28.5)* | 87.0 (0.8) | 92.4 (2.0)* | 49.1 (2.4) | 64.2 (4.7)* | 199.8 (24.7) | 644.9 (89.3)* | 11.2 (0.6) | 8.3 (1.3)* | 27.4 (1.8) | 24.3 (2.1) | 20.1 (1.3) | 23.0 (5.9) |
| BUD400MTLK | 13.4 (1.0) | 18.4 (1.0)* | 396.1 (22.8) | 158.1 (21.2)* | 86.2 (0.6) | 96.5 (2.9)* | 43.5 (1.9) | 75.3 (4.4)* | 165.7 (17.0) | 728.7 (76.0)* | 11.9 (0.9) | 7.4 (1.1)* | 24.5 (1.9) | 23.5 (2.7) | 21.3 (1.2) | 19.9 (1.4) |
| BUD400SLIT | 14.1 (0.7) | 24.0 (0.2)* | 450.8 (18.4) | 58.8 (11.64)* | 85.2 (0.6) | 103.3 (1.5)* | 43.4 (1.9) | 91.2 (1.3)* | 166.8 (18.3) | 997.1 (39.7)* | 11.1 (0.6) | 1.0 (0.2)* | 27.7 (1.8) | 6.3 (1.1)* | 22.2 (1.0) | 3.3 (0.6)* |
*p<0.05
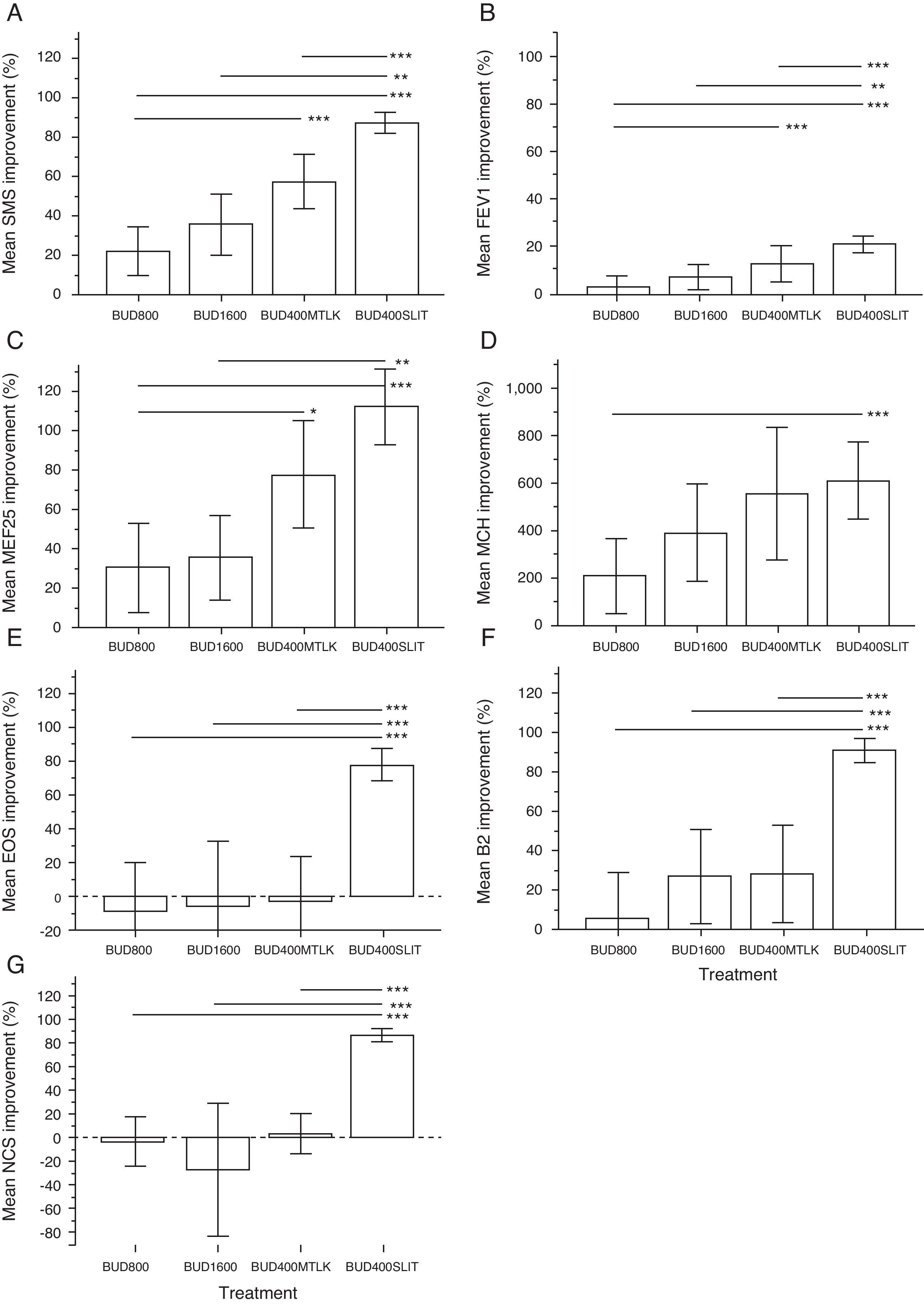
All groups achieved a significant improvement in allergy symptoms-medications scores (SMS), MEF25 and bronchial reactivity (MCH) after three years. Comparing the efficacy among treatments, the SMS improved more in patients of group 4 (decrease of 87%) than in all other groups (p<0.01). Similarly, group 3 had a better outcome (57% decrease) than group 1 (24%), but not significantly higher than group 2 (36%). No significant difference was detected between group 1 and group 2 (Fig. 5A).
A between group difference was detected only with groups 1 and 4 on MCH (Fig. 5D). MEF25 improved largely in group 4 with respect to groups 1 and 2 (Fig. 5C). The FEV1 increase (Fig. 5B) followed a pattern similar to SMS, whereas the albuterol intake in group 4 was significantly lower compared to all the other groups after three years (p<0.001), with a trend to incremental performance from group 1 to group 4 (Fig. 5F).
Only patients treated with budesonide and SLIT showed a significant reduction of nasal eosinophils (EOS, Fig. 5E) and nasal corticosteroids usage (NCS, Fig. 5G).
During the three years of SLIT course, two patients reported one episode of generalised itching within 30min from the uptake. These two AEs occurred during the maintenance phase and self-resolved without any therapy in less than two hours.
DiscussionIn this real-life study, patients with mild persistent asthma and AR due to birch pollen exposition, treated with low-doses of ICSs according to the step 2 of GINA guidelines, and unable to achieve an acceptable control, were randomised to three common step-up options (medium-dose and high-dose of ICSs, low-dose of ICSs plus anti-leukotrienes) or low-dose of ICSs plus SLIT. Since patients had normal lung function and no daily symptoms, associations with LABA were not introduced.
All groups at baseline had comparable and unsatisfactory level of asthma control. Throughout the study all but medium-dose ICSs improved this outcome, with a progressive trend year by year from 2005 to 2008, despite the pollen counts being rather dissimilar in 2006 and 2007 and this occurrence could have affected the real difference between treatments during that period. Analogous change was observed for lung function and use of short-acting bronchodilators. Notably, only patients receiving budesonide plus SLIT were close to full control (mean 24.0 SEM 0.242) at the end of the study, whereas all the others resulted only partially controlled. The four therapeutic options determined a global reduction of allergy symptoms-medications scores. Low-dose ICSs plus SLIT provided a marked advantage over the other options on symptoms plus medications decrease, FEV1 increase, rescue medications usage, and was comparable to low-dose ICSs plus montelukast on MEF25 and bronchial reactivity.
These findings are rather surprising because in previous studies budesonide proved to be effective in controlling symptoms of mild to moderate persistent asthma, even at doses lower than 800μg/day.17,22–25 Moreover, Cochrane reviews concluded that no significant dose-dependent improvements in FEV1, peak expiratory flow rate (PEFR) or symptoms were evident in asthmatics with mild to moderate disease treated with budesonide.26,27 On the other hand, these investigations did not adopt asthma control, expressed by the absence of symptoms, nocturnal awakening, limitation in work or daily activities, and by a reduced use of beta-2-agonists and normal lung function, as main study outcome. In addition they focused mainly on bronchial symptoms, whereas our combined symptoms plus medication assessment reflected both upper and lower airways impairment. For this reason we hypothesise that the persistence of nasal inflammation could indeed explain the unsatisfactory asthma control associated to all therapeutic options, except for the combination SLIT plus ICS. Both upper and lower compartments, in fact, could benefit from treating the united airways disease with SLIT, which is expected to down-regulate both organ-specific inflammations.28–31 Actually only the group receiving SLIT showed a significant decrease of nasal eosinophilic infiltration, along with a lower usage of nasal corticosteroids. No corresponding changes were detected in the other groups, as expected since nasal inflammation is not unequivocally affected by ICSs and is only partially faced by the inhibition of leukotrienes.
On the other hand, the lack of compliance to inhaled treatment is a well known critical issue, thus also unequal levels of adherence among groups could explain the observed outcome differences.32 In this study, conducted in a real-life setting, the compliance to anti-asthmatic treatment could not be precisely assessed. Finally, since patients not responding to the respective step of GINA guidelines entered the study, we cannot exclude that the unmet control is explained by a selection of subjects, less responsive to pharmacological treatment. These considerations could also justify the very large clinical improvement observed in respect to baseline in patients receiving SLIT.
Although we did not use a specific design to verify the influence of SLIT on ICSs intake, the goal of the treatment could be reached with a thrifty use of nasal steroids and with a low-dose of inhaled budesonide, thus sustaining the previous experiences of a potential steroid-sparing effect of SIT in persistent asthma. This observation acquires particular relevance when considering the risk of treatment downsides in patients exposed to both nasal and bronchial corticosteroids.
Guidelines appear cautious concerning the use of SIT in severe persistent or difficult to control asthma, probably because of the marginal role of the allergens in these circumstances and owing to the risk of side effects.1 SLIT with allergens specifically modified, with the purpose of improving the safety profile, represents an interesting hypothesis for these conditions, as observed in previous experience.33 Only two patients receiving monomeric carbamylated allergoid, during the three years of follow-up, reported one episode of self-resolving generalised itching without any occurrence of asthma deterioration.
The main limitations of this investigation concern the real-life conditions in which it was conducted; however, these experimental models aim to explore the applicability of therapeutic strategies in real scenarios.
A relatively small number of patients with regard to the open-fashion design were recruited, because of the restriction to those patients requiring a treatment step-up and without confounding sensitisations.
Asthma control was evaluated with a validated questionnaire, without assessing day-by-day the individual aspects concurring to its determination; eosinophils and methacholine test were assessed only twice, preventing a correct evaluation of their behaviour over time. Similar considerations can be moved to the diary card assessment, although the pollen count was similar only during the first and the last pollen season. We chose to give a snapshot of these outcomes only at the beginning and at the end of the study without overcharging the study procedures of a real-life investigation. For the same reason we avoided the burden of an ideal blinding-dummy design with placebo.
This real-life study, comparing different therapeutic strategies in a relatively long-term period, pointed out that a favourable option to achieve the goals of the treatment, in patients with seasonal mild asthma and normal lung function associated with AR, is the combination of ICSs at low-dose with a SLIT course. This approach is safe and permits an appreciable asthma control, with benefits on the nasal allergic condition and a steroid-sparing effect.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentRight to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors have no conflict of interest to declare. Dr Marco Emanuele Bruno is employee at Lofarma S.p.A., industry producing the vaccine used in this study, and Dr Enrico Compalati has a scientific consultancy with Lofarma S.p.A.