High sensitive C-reactive protein (hs-CRP) has been shown to be associated with asthma in recent studies. However, the relationship between hs-CRP and the control of asthma has not been clearly identified yet.
ObjectiveTo investigate the association of hs-CRP with asthma control test (ACT), which reveals the degree of asthma control, and to compare hs-CRP in adults with mild and moderate asthma in chronic, stable asthmatic patients.
MethodsThirty patients with physician-diagnosed asthma (11 mild, 19 moderate), and 30 healthy patients were enrolled in the study. In addition to medical history and physical examination, asthma was assessed according to GINA guideline. Respiratory function tests (RFT) and ACT were performed. The serum hs-CRP levels of all cases patients were measured.
ResultsThe levels of hs-CRP in asthmatic patients were significantly higher than those in the control cases (p=0.002). The serum hs-CRP levels in the moderate asthmatics were significantly higher than those in the mild asthmatic ones (p=0.04). When asthmatic cases were divided into two groups according to ACTs; the levels of hs-CRP in the groups of ACT≤20 (uncontrolled groups) were significantly higher than the groups of ACT≥20 (controlled groups) (p=0.02). The hs-CRP levels showed significant correlations with ACT (p=0.00, r=−0.91) and asthma severity (p=0.04, r=038) in asthmatic patients.
ConclusionIn conclusion it was shown that hs-CRP is related with asthma severity and ACT, and hs-CRP is a potential sensitive marker which reveals the severity and the control of asthma.
The C-reactive protein (CRP), so named for its capacity to precipitate the somatic C-polysaccharide of Streptococcus pneumoniae is an exquisitely sensitive non-specific marker of acute inflammation and tissue damage.1,2 The CRP is predominantly synthesised in the liver and is regulated by pro-inflammatory cytokines, primarily the tumour necrosis factor-alpha and interleukin-6 (IL-6). During an acute-phase response, there is a rapid increase in the production of CRP (10,000-fold), resulting in the release of elevated quantities into the circulation. The CRP may serve as a general scavenger protein and play an important role in opsonisation, phagocytosis, and cell-mediated cytotoxicity. The CRP can also act as a potent proinflammatory agent and activates the classical complement cascade by binding directly to the complement fragment C1q.3,4 Standard assays for CRP lack the sensitivity needed to determine the levels of inflammation, and thus, clinical utility of standard CRP evaluation is extremely limited. Recent improvements have resulted in a new generation of highly sensitive assays that can detect the CRP at levels 100-fold lower than the earlier assays.5 The CRP determined using a highly sensitive assay is referred to as high sensitivity-CRP (hs-CRP). Using hs-CRP, assessment of conditions indicative of chronic, low-grade inflammation is now possible. Large-scale prospective studies have demonstrated that hs-CRP is a strong independent predictor of future myocardial infarction, stroke, peripheral arterial disease and sudden cardiac death among healthy men and women, and recurrent events and death in patients with acute or stable coronary syndromes.1,6
Also, low-level inflammation, as indicated by increased hs-CRP serum concentrations, has been described in both chronic obstructive pulmonary lung diseases (COPD) and asthma.7 As asthma is characterised by variable degrees of airway inflammation, cytokines such as IL-1, IL-6 and nuclear factor κ–β, which regulate hs-CRP6 had a role in airway inflammation.8 So it is reasonable to consider that there may be a direct association between severity of inflammation and level of hs-CRP level.
In asthma, not only local but also systemic inflammation occurs and hs-CRP may play a role in the pathogenesis of asthma.9 In one study, hs-CRP was significantly higher in all asthmatics compared to healthy controls and in uncontrolled asthmatics hs-CRP was significantly higher than other asthmatic children. This result was in agreement with a study conducted by Navratil et al. who found higher serum hs-CRP levels in children with uncontrolled asthma compared to the levels in children with controlled asthma and healthy ones.10
Positive association was found between hs-CRP levels and both the presence and the severity of asthma in adults.11–15 But there was no comparison between uncontrolled asthmatic patients and controlled ones in terms of hs-CRP levels in adult chronic, stable asthmatic patients in the literature.
In the present study, our aim was to assess the relationship between hs-CRP levels and asthma control test (ACT) in chronic, stable asthmatic patients. We wonder about the answer to this question: Is it useful to measure hs-CRP levels in order to evaluate the degree of inflammation and the control of asthma in policlinic follow up?
MethodsStudy designThirty asthmatic patients (11 mild, 19 moderate) and healthy controls were recruited randomly from the pulmonary clinic between April 2008 and June 2008. The diagnosis of asthma and the assessment of severity were performed according to the Global Initiative for Asthma (GINA).16
Inclusion criteria:
- 1.
Cases who are older than 18 years of age.
- 2.
Cases diagnosed according to the Global Initiative for Asthma (GINA).16 Patients were included if they had a clinical diagnosis of asthma and spirometric values showing reversibility of more than and/or equal to 12% in forced expiratory volume in 1s (FEV1), or at least 200mL from baseline after inhalation of salbutamol (4×100mcg) given by metered dose inhaler using a spacer device.
- 3.
Cases who are non-smokers.
Exclusion criteria:
- 1.
Cases whose Body Mass Index (BMI) was ≥25kg/m2.
- 2.
Cases who have had an exacerbation of asthma or respiratory tract infection during the last three months prior to enrolment.
- 3.
Patients with hepatic, renal, cardiovascular diseases, diabetes mellitus, cancer and systemic inflammatory disorders.
All cases were examined and asthmatic patients were assessed according to severity as mild and moderate asthma.16
This study was approved by the Ethics Committee of the Ataturk Training and Research Hospital (Ankara, Turkey) and written informed consents were obtained from all subjects. All the patients underwent a detailed symptom enquiry, physical examination and investigations including a complete blood count, chest radiograph (postero-anterior view), ACT and respiratory function tests (RFT), serum total immunoglobulin-E (IgE) and skin prick test (SPT). The hs-CRP levels were measured in the peripheral venous blood samples in all cases patients by a semi-quantitative assay using Latex enhanced immunonephelometry assay. Elevated total IgE level and positive SPT were used to define the atopic status.
Measurement of serum hs-CRPSerum hs-CRP levels were measured using an available high sensitive-CRP commercial kit (Siemens, CardioPhase, Marburg, Germany). The measurement method used is based on a particle enhanced immunonephelometry assay.
Lung function testSpirometric values were measured using a spirometer (Spirolab, Italy). The best value of three manoeuvres was expressed as a percentage of the predicted value and as absolute value.
Body Mass IndexBody Mass Index (BMI) was calculated as weight in kilograms divided by the square of height in metres.
Skin prick testSkin prick test was conducted with a panel of common aeroallergen extracts in the presence of a positive histamine control and a negative vehicle control on the forearm. Sixteen aeroallergens including Dermatophagoides pteronyssinus, Dermatophagoides farinae, Grasses mix, Cereals mix, Feathers mixture, Dog hair, Moulds, Salicaceae, Trees mixtures, Compositea, Canis familiaris, cockroach and pollen (ALK-Abelló A/S, Hørsholm, Denmark) were utilised. The test was considered positive if the wheal was greater than 3mm in mean diameter.
Asthma control testACT was used to evaluate asthma control. The ACT questionnaire is a validated self-administered questionnaire including five questions related to the last four weeks: episodes of breathlessness, nocturnal awakenings, limitations of daily activities, need for rescue medication and patient's self-rating of asthma control. Each question includes five response modalities with a score ranging from one to five by increasing level of asthma control, so the global arithmetic score ranges from five to twenty-five. Well-controlled asthma by ACT was defined by a score ≥20.16,17
According to GINA 2007, controlled asthma was defined as: the need for rescue medications twice or less a week (short-acting β2-agonists), no limitation of day activity, no nocturnal symptoms, twice or less a week day-time symptoms and FEV1%>80% predicted. There were no exacerbations and no use of systemic steroids in the previous 12 months. The patients with uncontrolled asthma were defined as having three or more features of the following: the need for rescue medications more than twice a week, any limitation of day activity, any nocturnal symptoms, day-time symptoms more than twice a week and FEV1%<80% predicted or had required one or more hospitalisations for asthma in the last year. They had been taking high-doses of ICS (fluticasone propionate >500–800μg/day) and long acting β2-agonists for at least six months.
Forty healthy volunteers were used as a control group. They were free of respiratory tract infection within three months prior to the study, and also from other significant illnesses known to affect hs-CRP levels (which have been described previously).
Statistical analysisData were presented as median (minimum–maximum). Statistics were performed with SPSS 16 (SPSS, Chicago, IL, USA). As the normality distribution assumption did not hold, Mann–Whitney U-test was used to analyse differences between two independent groups. Correlations between data were analysed using Spearman's rank correlation test. A p value of <0.05 was considered as statistically significant.
ResultsThe comparison of asthmatic and control subjectsThe characteristics of the participants are shown in Table 1. Age, BMI and sex distribution in the asthmatic group were the same as the control subjects. The levels of hs-CRP in asthmatic patients were significantly higher than in the control cases [median (min–max) hs-CRP levels of asthmatics and the controls were 1.97 (0.16–10.2) and 0.45 (0.21–4.2), respectively, p: 0.002].
Demographic and clinic characteristics of the patients of study.
| Asthmatic cases (n=30) | Control cases (n=30) | p | |
| Females–males (n) | 24–6 | 19–11 | 0.15 |
| Median (min–max) age | 51.1 (18.0–71.0) | 44.5 (20–73) | 0.88 |
| Median (min–max) BMIa (kg/m2) | 23.05 (19.2–24.8) | 23.0 (19.6–24) | 0.74 |
| Median (min–max) hs-CRP (ng/L) | 1.97 (0.16–10.5) | 0.45 (0.21–4.2) | 0.00a |
| Median (min–max) ACTb | 16.5 (8–25) | ||
| Median (min–max) FEV1 (ml) | 2550.0 (1480–4840) | 2350 (2030–2880) | 0.243 |
| Median (min–max) FEV1% | 92.5 (60–114) | 105 (100–124) | <0.00a |
| Median (min–max) PEF (lt/h) | 5.67 (2.14–10.9) | 6.24 (5.48–8.14) | 0.01a |
| Median (min–max) PEF (%) | 77 (38–112) | 94 (82–103) | 0.00a |
| Mild asthmatic patients (%) | 36.7 | ||
| Moderate asthmatic patients (%) | 63.3 |
When asthmatics were evaluated according to the atopy status, 19 of the cases were found atopic and 11 were non-atopic. No statistical relation was found between atopic asthmatic cases and non-atopic ones according to hs-CRP (p>0.05).
The association between levels of serum hs-CRP and severity of asthmaThere were 11 (36.7%) mild and 19 (63.3%) moderate asthmatic patients in our study. A significant difference was determined between the mild and moderate groups. The serum hs-CRP levels in the moderate asthmatic groups were significantly higher than those in the mild asthmatic ones [median (min–max) hs-CRP levels of the mild and the moderate asthmatics were 0.72 (0.16–5.7) and 2.21 (0.36–10.2), respectively, p=0.04] (Fig. 1).
A significant positive correlation was detected between the levels of hs-CRP and the severity of asthma (p=0.04, r=0.38). However, no significant correlation among hs-CRP and forced expiratory volume (FEV1), FEV1%, Peak Expiratory Flow (PEF) and PEF% values (FEV1, p=0.54, r=−0.11; FEV1%, p=0.91, r=0.02; PEF, p=0.44, r=0.14 and PEF%, p=0.95, r=−0.11, respectively) was found.
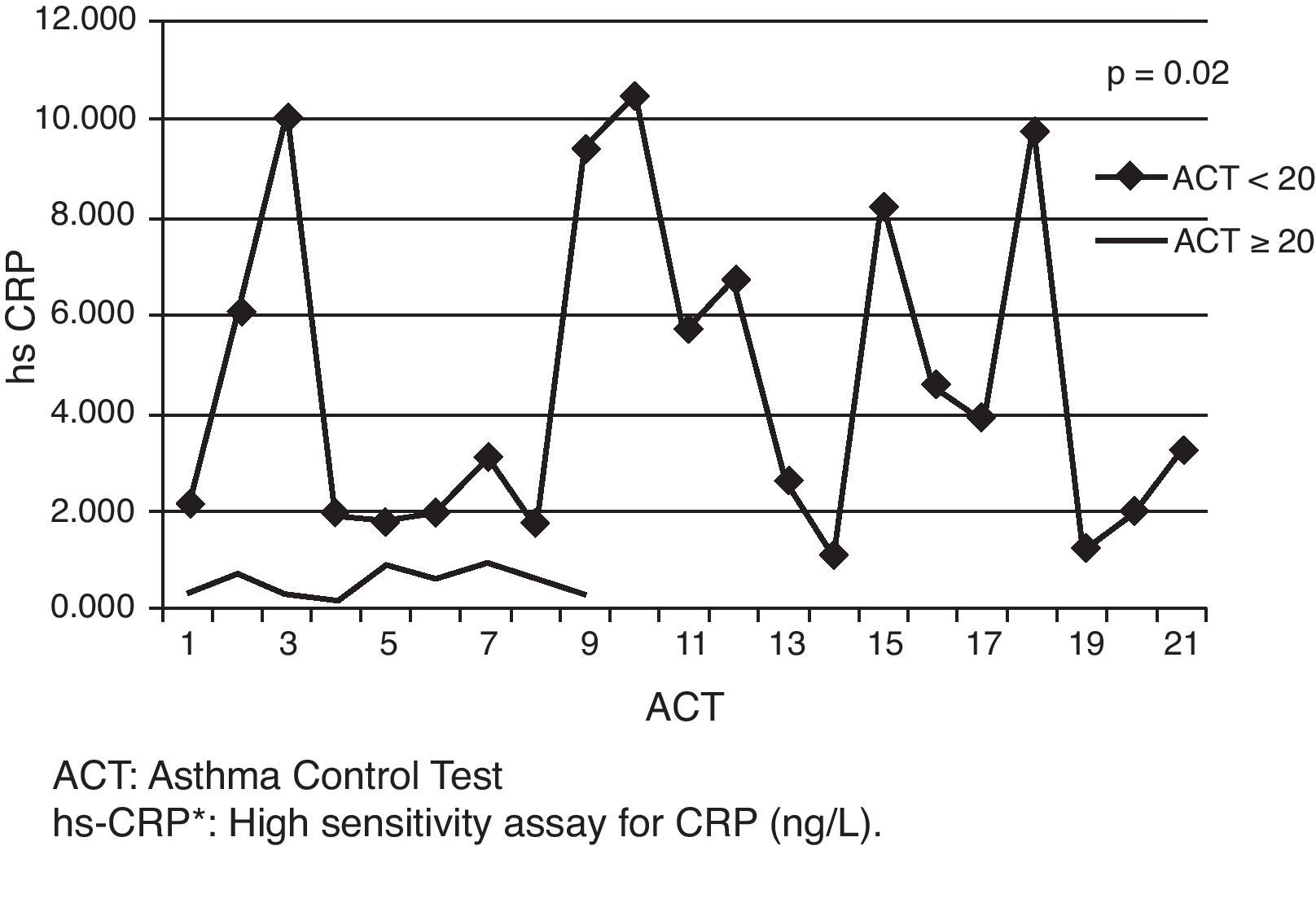
The relationship between the levels of hs-CRP and asthma controlAsthmatic cases were divided into two groups according to their ACTs. The two groups, ACT≥20 (n: 7) and ACT<20 (n: 23), were significantly different from each other via hs-CRP levels [median (min–max) hs-CRP levels of the groups according to ACTs were given respectively as follows: [ACT<20; 2.21 (0.32–10.5), ACT≥20; 0.7 (0.16–3.29), p=0.02] (Fig. 2). A significant negative correlation between hs-CRP and ACT was shown (p=0.00; r=−0.91) (Fig. 3).
Our study has revealed that there is a positive association between hs-CRP and the severity of asthma and a negative association between hs-CRP and ACT. But no association was found between the parameters of RFT and the levels of hs-CRP throughout asthmatic patients in our study.
In asthma, the importance of airway inflammation has been well established. Besides the airway inflammation, systemic inflammation may also exist in asthma. The relevance of high sensitivity assays for hs-CRP, which is known to be a sensitive marker of low-grade systemic inflammation, has not been fully studied in asthma. Studies have attempted to validate the use of hs-CRP as a surrogate marker of airway inflammation in bronchial asthma. The study by Takemura et al.18 cross-sectionally examined the serum hs-CRP levels in steroid-naive and steroid-inhaling adult non-smoker patients with asthma and healthy controls. Serum hs-CRP levels were significantly increased in steroid-naive patients as compared to controls, but not in patients on inhaled corticosteroids. Recent population-based studies have shown increased levels of hs-CRP both in asthma and exacerbations of COPD and it was even detected in the stable statement of COPD.19–21
The relationship between hs-CRP levels and the severity of asthma has been shown in two previous studies.22,23 There was a positive correlation only within severe asthmatic patients in the study by Qian et al.22 Regarding the previous studies, the relationship between levels of hs-CRP and the severity of asthma indicates that CRP is a proinflammatory agent. Different proinflammatory cytokines such as interleukin-1β (IL-1β), IL-6, IL-8 and IL-18 have been synthesised by the activated proteins hs-CRP. These cytokines have increased due to asthmatic inflammation.24,25 Severity of asthma correlates positively with the asthmatic inflammation.26 Significant differences in hs-CRP levels between subjects with severe asthma and controls without any respiratory symptoms have recently been demonstrated in one study.27 In contrast, in a study by Takemura et al.,18 hs-CRP levels were only increased in steroid-naive patients compared with controls.18 Also, serum hs-CRP levels in moderate asthmatic cases were significantly higher than in mild ones in our study.
A significant difference was determined between asthmatic subjects and controls in terms of RFT. But no significant relation was found between RFT and the levels of hs-CRP of the asthmatic patients in our study; similar to the previous reports by Qian et al.,22 Büyüköztürk et al.,28 and Ramirez et al.29 On the contrary, there was positive correlation between the levels of RFT and hs-CRP according to the reports by Takemura et al., Fujita et al. and Kim et al.18,30,31
Asthma symptoms represent the main reason for visits to chest specialists. Symptoms of asthma constitute an essential element for asthma control and currently form the basis of the recommended strategy. It therefore seemed important to evaluate the true relation between inflammation and asthma symptoms. For this reason, the authors aimed to investigate that relationship between inflammatory markers and asthma symptoms, and also asthma control.26 Significant relationships were found between increased CRP levels and respiratory symptoms, such as wheeze, attacks of breathlessness after effort and nocturnal cough in two studies.32,33 The latter study also concluded that non-allergic asthma in particular is strongly associated with higher CRP levels, whereas allergic asthma is not.33 Also, another study showed that non-atopic asthma is strongly associated with higher hs-CRP levels, whereas allergic asthma is not.1 We did not determinate any correlation between hs-CRP and respiratory symptoms (p>0.05).
A single study with 33 participants34 evaluated the changes in serum markers of inflammation in asthmatic children. After discontinuation of corticosteroids, 71% of the children presented symptoms. Blood eosinophil counts and serum eosinophil cationic protein levels were significantly higher in children who became symptomatic.34 Rytila et al.35 compared systemic markers of eosinophil inflammation (blood eosinophil counts, serum eosinophil peroxidase concentration—EPO) in symptomatic asthmatic and non-asthmatic subjects suffering from respiratory symptoms. Both asthmatic patients and non-asthmatic symptomatic subjects had a rise in these two systemic markers in comparison with healthy subjects, even if the level was significantly higher in asthmatic subjects.35 To conclude, analysis of these studies shows that there is an unequivocal relation between asthma symptoms and inflammation. Most of the studies reported that uncontrolled asthmatics had more severe inflammation than controlled asthmatic children.26 However, no studies have yet been specifically designed to answer these questions both in children and adults.
The main objectives in the treatment of asthma are: control of asthma and the maintenance of wellness according to GINA 2008 guideline. There are many kinds of methods, such as the asthma control test (ACT)17 and Asthma Control Questionnaire Form (ACQF)36 for evaluating the control and severity of asthma. After determining the control of asthma, the treatment can be planned. The control of asthma has been evaluated by ACT in our study. We investigated the relationship between hs-CRP levels and ACT in our study and found that ACT negatively correlated with the levels of hs-CRP. When ACT decreases, the level of hs-CRP increases in our study. There was a significant difference between the cases with uncontrolled asthma (ACT<20), and the cases with controlled asthma (ACT≥20). This is the first study that shows the association between hs-CRP levels and ACT in asthmatic adults, according to our knowledge.
There were some limitations in this study. The sample size was small and it was not a longitudinal study.
All of the asthmatic cases should be evaluated for the control of asthma by using ACT or ACQF according to GINA guidelines. In our study, we have shown the correlation between ACT and the levels of hs-CRP. The role of hs-CRP in evaluating asthma control advances the field in that way; the measurement of hs-CRP is simple, it is an easy applicable method, and is useful in the routine polyclinic follow-up. The hs-CRP can be used for assessing the control of the patient's symptoms, thus we suggested that hs-CRP may be a potential indirect marker of systemic inflammation that reflects both asthma severity and asthma control.
The clinical implications of this study can be summarised as follows: in the future, hs-CRP may be a good indicator of the dosage of inhaled corticosteroids in asthmatic patients due to its easy clinical use. The role of hs-CRP may also be important in evaluating the mortality of asthma similar to acute coronary syndrome. Further expanded longitudinal investigations are needed to examine the role of hs-CRP in both the severity and control of asthma.
Conflict of interestAll authors disclose that they have no conflict of interest.
In this original research, patients were examined, lung function tests were performed and data were collected by Dr. Hatice Kılıç and Dr. Ayşegül Karalezli. Data were analysed with SPSS statistical program by Can Ateş. The results were analysed, evaluated and discussed by all authors (Dr. Hatice Kılıç, Dr. Ayşegül Karalezli, Dr. H. Canan Hasanoğlu, Dr. Özcan Erel). The manuscript was written by the contribution of all authors.