Peripheral blood B cells include lymphocytes at various stages of differentiation, each with a specific function in the immune response. All these stages show variations in percentage and absolute number throughout human life. The numbers and proportions of B subpopulation are influenced by factors such as gender, age, ethnicity, and lifestyle. This study establishes reference values according to age of peripheral blood B cell subtypes in healthy Mexican population.
MethodsPeripheral blood from healthy new-borns and adults were analysed for total B cell subpopulations, using surface markers such as CD19, IgM, IgD, CD21, CD24, CD27, and CD38, to identify naïve, memory with and without isotype switch, double-negative, transitional, and plasmablast cells.
ResultsWe observed a significant variation in terms of frequency and absolute counts between all groups analysed. Values from each B cell subpopulation show variations according to age.
ConclusionsIn order to attempt to elucidate reference values for B cell subpopulation, the present study evaluated a population sample of healthy blood donors from this region. Values reported here can also be used as a tool for diagnosis of diseases in which B cell maturation is affected.
Human peripheral blood cell counts may vary among healthy individuals according to age. These variations may also be due to pathologies that involve the immune system, i.e., primary immunodeficiency (PIDs).1 B cell differentiation is a highly regulated process that varies according to age; in embryonic development, human B cells are generated in fetal liver; from new-borns to adults, early B cell development occurs in bone marrow, whereas differentiation to generate mature B cells, memory, or plasma cells occurs in peripheral lymphoid organs such as the spleen or lymph nodes. Peripheral B cells include immature B cells, also called transitional B cells, a population that recently emigrated from bone marrow; these cells are in transit to peripheral lymphoid organs such as the spleen or lymph nodes where they fulfill their maturation.2,3 Antigen recognition by follicular B cells together with co-stimulation received from follicular helper T cells induces class-switch recombination and B cell differentiation to generate memory B cells. These cells are characterised by the loss of IgD expression, the presence of either IgM, IgG, or IgA and are usually identified by the surrogate marker CD27, are long-lived and, upon reencounter with antigen, differentiate into antibody-producing cells (plasma cells).3
PIDs have a calculated incidence of 1–5 patients per 100,000 habitants. Annually, the European and Latin-American societies for immunodeficiencies (ESID and LASID, respectively) report at least 1000 new cases for PIDs.4 The statistics in LASID show that in a single national health institute at least 30 new PIDs cases were identified per month,5,6 indicating that the calculated incidence might be underestimated. Common Variable Immune Deficiency (CVID) is, after IgA deficiency, the most frequently diagnosed PID. CVID is characterised by low immunoglobulin levels (at least two of the main isotypes are decreased) due to alterations in peripheral B cell development.7 As CVID is diagnosed at two peaks of age (children and adults; it is important to have reference ranges of all B cell subpopulations in peripheral blood.8 Three major classifications of CVID have been proposed, all of them based on the impairment of the B cell phenotypes.9–11 To predict associated clinical complications, we used Freiburg Classification; this classification is based on the decrease of memory B cells and reported that the expansion of B cells CD21low subpopulation allows the detection of patients with the highest risk to develop splenomegaly and/or granuloma.10 Additionally, most PIDs are diagnosed during childhood.5 Several primary immunodeficiencies have shown impairment of central or peripheral B cell development, such as X-linked agammaglobulinaemia (XLA), CVID, or X-linked lymphoproliferative disease (XLP); these patients have a defective differentiation of precursors (XLA) or mature B cells (XLP).7
Immunophenotyping by flow cytometry has been used to delineate the stages of peripheral B cell maturation in healthy population with the purpose of establishing reference values according to age, and to create the basis for correlating the clinical data and the laboratory findings from patients with immunological diseases.12,13 It has been demonstrated that lymphocytes subsets vary significantly depending on ethnicity, exposure to infectious disease, environmental factors and age.14 For that reason, we analysed peripheral B cell populations in a cohort of healthy Mexican individuals ranging from neonates to adults with the aim of establishing age-dependent reference values for distinct B cell populations in peripheral blood. We found that total memory and switched memory B cells increase throughout life and are maintained in adults, while transitional B cells decrease throughout life. These data may help to identify defects in peripheral B cell development in individuals suspected of suffering from PIDs. The results shown here demonstrate that important variations occur in B cell numbers and this should be taken into account when considering patients with potential immunological diseases.
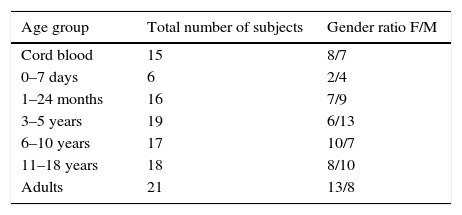
Materials and methodsStudy populationBlood samples from a total of 112 healthy subjects, taken between 2009 and 2014, were included in this study. Blood samples included 15 taken from umbilical cord, and 97 individuals ranging from birth to 40 years old. All blood samples were used for routine laboratory analysis of common haematological parameters. Immunological, infectious, and haemato-oncological diseases were ruled out in all individuals. Neonatal cord blood was obtained by venipuncture immediately after clamping. Samples were divided in the following groups: cord blood, new-borns, 1 month to 2 years, 3–5, 6–10, 11–18, and 19–40 years old. Demographic details of the tested population are presented in Table 1. Additionally, CD21low B cells were analysed in 28 samples; 18 adult healthy donors from 20 to 40 years old and 10 young healthy donors from 1 to 17 years old (supplementary Table 1). The study is part of a protocol approved by the local ethics committee of the Instituto Nacional de Pediatría (26/2009) and the protocol of Hospital Juárez de México (HJM2264/13-A), both of which were developed according to the rules declared under the Declaration of Helsinki.
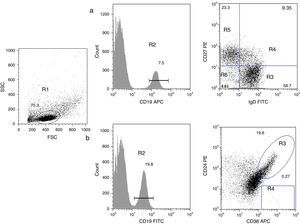
B cell subpopulation immunophenotypingVenous blood was collected using ethylenediaminetetraacetic acid (EDTA)-coated tubes (Vacutainer; Becton Dickinson, San Jose, California, USA) and processed within 24h. Peripheral blood mononuclear cells (PBMCs) were obtained by density-gradient centrifugation using standard procedures. PBMCs were harvested and washed with phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA). To identify all B subpopulations, 0.5×106 cells were stained with combinations of the following monoclonal antibodies: CD19 APC, CD19 FITC, IgD FITC, CD27 PE, CD21 PE, CD24 PE, and CD38 APC (all from Becton Dickinson). B cell subsets were defined by the following markers on their surface, according to Morbach et al.15: naïve B cells (CD19+CD27−IgD+), class-switched memory B cells (CD19+CD27+IgD−), non-switched memory/marginal-zone-like B cells (CD19+IgD+CD27+), double-negative B cells (CD19+CD27−IgD−), transitional B cells (CD19+CD38++CD24++), and plasmablasts (CD19+CD38+++CD24−) (Fig. 1); and for CD21low B cells (CD19+CD38lowCD21low) (supplementary Fig. 1). Isotype controls used were anti-CD45 PerCP/γ1 FITC/γ2 PE (Becton Dickinson). After staining, cells were washed, fixed with 1% paraformaldehyde in PBS and stored until analysis. A FACS Aria 1 flow cytometer (Becton Dickinson) was used to acquire 30,000 events and FlowJo (Treestar) was used for analysis of each B cell subpopulation. Leucocyte counts and total lymphocytes per mm3 were determined using a haematology analyser (Coulter LH 500).
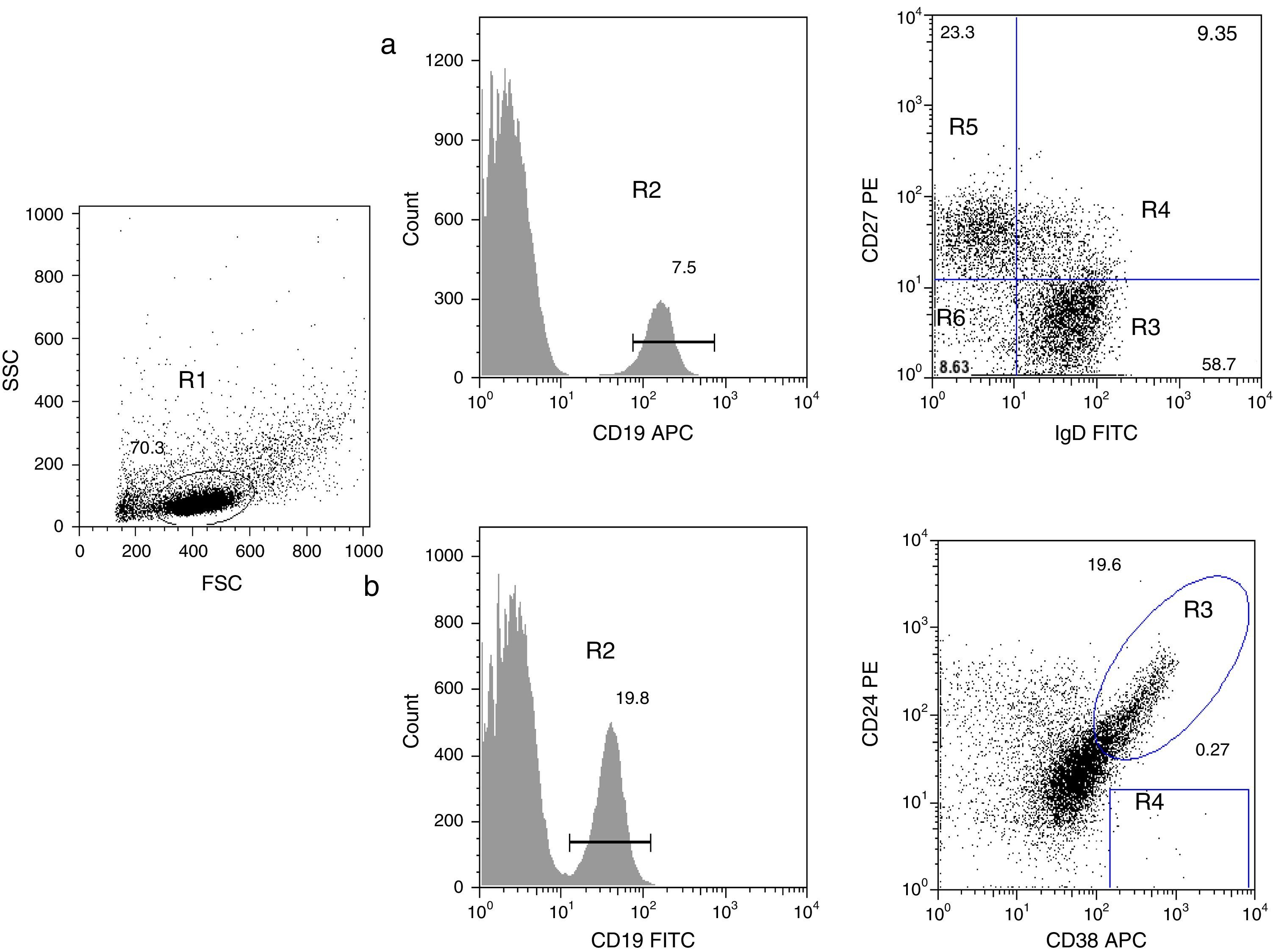
Analysis of B cell subpopulations by flow cytometry. (a) CD19+ B cells (R2) were gated within the lymphocyte side- and forward-scatter region (R1). Using panel 1, the cells can be subdivided into several subpopulations: naïve B cells (R3, CD19+CD27−IgD+), non-switched memory/marginal-zone-like (R4, CD19+IgD+CD27+), class-switched memory B cells (R5, CD19+CD27+IgD−), double-negative B cells (R6, CD19+CD27−IgD−). (b) By using panel 2, it is possible to distinguish transitional B-cells (CD19+CD38++CD24++) and plasmablasts (CD19+CD38+++CD24−).
All results are presented as median values with ranges between the 5th and 95th percentile, to avoid distributional assumptions. Data were analysed using Kruskal–Wallis test for non-parametric data using Sigma Plot v12.0, to verify statistical difference of results between individual age groups. Two-group comparisons were performed using a Mann–Whitney test.
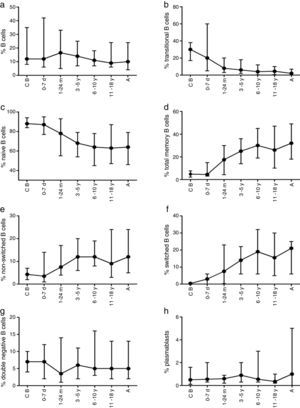
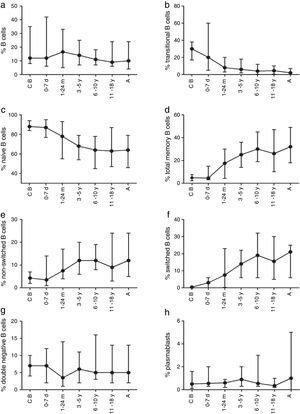
ResultsVariations in total B cellsThe tested population was composed of 112 healthy children and adults from seven groups of age, ranging from new-borns to young adults, as noted in Materials and Methods. Absolute B cell numbers during the first months of life were higher than values observed in adults; additionally, total numbers gradually decreased throughout life. Furthermore, an expansion of the total naïve B cells pool (transitional cells) was observed in the youngest age group (Table 2).
Total numbers of lymphocytes and B cells according to age.
| Age group | % lymphocytes | Absolute counts total lymphocytes (mm3) | % B cells | Absolute counts B cells (mm3) |
|---|---|---|---|---|
| Cord blood | 46 (29–73) | 4420 (903–8288) | 12 (8–35) | 486 (108–1786) |
| 0–30 days | 46 (13–77) | 4297 (949–6160) | 12 (6–42) | 468 (57–2341) |
| 1–24 months | 51 (34–75) | 4198 (2062–7643) | 17 (5–31) | 577 (227–1305) |
| 3–5 years | 47 (30–67) | 3248 (2298–4410) | 14 (7–25) | 423 (235–998) |
| 6–10 years | 44 (27–62) | 2672 (1525–4353) | 11 (7–18) | 288 (163–592) |
| 11–18 years | 37 (30–55) | 2503 (1650–3411) | 9.1 (6–23) | 231 (135–772) |
| Adults | 35 (24–55) | 2263 (1279–3648) | 10 (6–22) | 240 (126–534) |
Values within parenthesis represent median (5th–95th percentile range).
Total, switched memory, non-switched memory, naïve, transitional, double-negative B cells, and plasmablasts were identified in all age groups. The frequency and total number of distinct B cells are shown as median values as well as the interquartile ranges (5th and 95th percentiles) in Tables 3 and 4.
Percentages of B cell subpopulations in healthy donors according to age.
| B cell subpopulations | Transitional B cells CD24+++CD38+++ (%B) | Naïve CD27−IgD+ (%B) | Total memory CD27+ (% B) | Non-switched memory/marginal zone like CD27+IgD+ (%B) | Switched memory CD27+IgD− (%B) | Double-negative CD27−IgD− (%B) | Plasmablast CD24−CD38+++ (%B) |
|---|---|---|---|---|---|---|---|
| Age group | |||||||
| Cord blood | 30 (17–38) | 88 (84–94) | 4.8 (2.2–7.7) | 4.3 (2–7) | 0.4 (0.04–0.8) | 7 (4–10) | 0.5 (0.1–1.6) |
| 0–7 days | 20 (5–60) | 87 (77–95) | 4.5 (3–15) | 3.5 (1–14) | 3 (0.0–6) | 7 (2–12) | 0.5 (0.3–2) |
| 1–24 months | 8 (3–19.7) | 78 (57.4–92.1) | 17.5 (5.1–28.2) | 7.5 (4–16.7) | 7.5 (1.1–20.3) | 3.5 (1.3–13.4) | 0.6 (0.2–0.9) |
| 3–5 years | 6 (2–18) | 68 (55.3–79) | 25 (15.3–35.5) | 12 (6.5–18.5) | 14.1 (6.4–21.6) | 5.8 (2–10.3) | 0.9 (0.3–2) |
| 6–10 years | 4 (0.3–12) | 64 (45.7–76.6) | 30 (19.7–44.3) | 12 (8–18.3) | 19 (7.7–31.6) | 5 (3–15) | 0.55 (0.3–3) |
| 11–18 years | 4.5 (0.5–10) | 62.8 (49–86.2) | 26 (10.4–44.2) | 9.2 (3.16–22.4) | 15.5 (5.4–28) | 5 (2–13) | 0.35 (0.2–1) |
| Adults | 2 (0.05–7) | 64 (46–78.4) | 32 (19.1–44) | 12 (6.1–23.4) | 21 (7.6–24.4) | 5 (2–11.3) | 1 (0.05–4.4) |
Values within parenthesis represent median (5th–95th percentile range).
Absolute numbers of B cell subpopulations in healthy donors according to age.
| B cell subpopulations | Transitional B cells CD24+++CD38+++ (mm3) | Naïve CD27−IgD+ (mm3) | Total memory CD27+ (mm3) | Non-switched memory/marginal zone like CD27+IgD+ (mm3) | Switched memory CD27+IgD− (mm3) | Double-negative CD27−IgD− (mm3) | Plasmablast CD24−CD38+++ (mm3) |
|---|---|---|---|---|---|---|---|
| Age group | |||||||
| Cord blood | 117 (37–500) | 438 (96–1607) | 24 (4.5–74) | 22 (4.3–7) | 1.6 (0.3–5) | 34 (7–107) | 2.2 (0.7–7) |
| 0–7 days | 131 (3–491) | 394.5 (44–2060) | 16 (9–117) | 16.5 (3–73) | 12.5 (0.0–70) | 50 (4–164) | 9.5 (0.0–17) |
| 1–24 months | 77 (10–168.2) | 454 (139.2–1127.1) | 80.5 (29–157.6) | 39 (16.6–115.2) | 41.5 (6.2–81.2) | 21 (8.9–63.7) | 4 (1–9) |
| 3–5 years | 29 (10–166) | 252 (175.5–653.7) | 107 (45.5–303.4) | 45.5 (21.2–154.6) | 65.5 (20–176.4) | 26 (8.2–81) | 5 (2–7) |
| 6–10 years | 12 (0.7–50) | 170 (100–329) | 115 (33–248.4) | 43 (15–75.7) | 54 (12.8–174.7) | 23 (5–61) | 2.5 (0.5–8) |
| 11–18 years | 13.5 (1–50) | 150 (88–646.4) | 62.5 (21.8–138.4) | 23.5 (6.4–73) | 37 (11.4–77.8) | 13 (4.7–30.2) | 2 (0.5–4) |
| Adults | 3 (0.1–17) | 153 (74.2–363.4) | 68 (43.1–142.6) | 31 (14.2–63.4) | 46 (16–95.4) | 13 (3.5–30.3) | 2 (0.05–18) |
Values within parenthesis represent median (5th–95th percentile range).
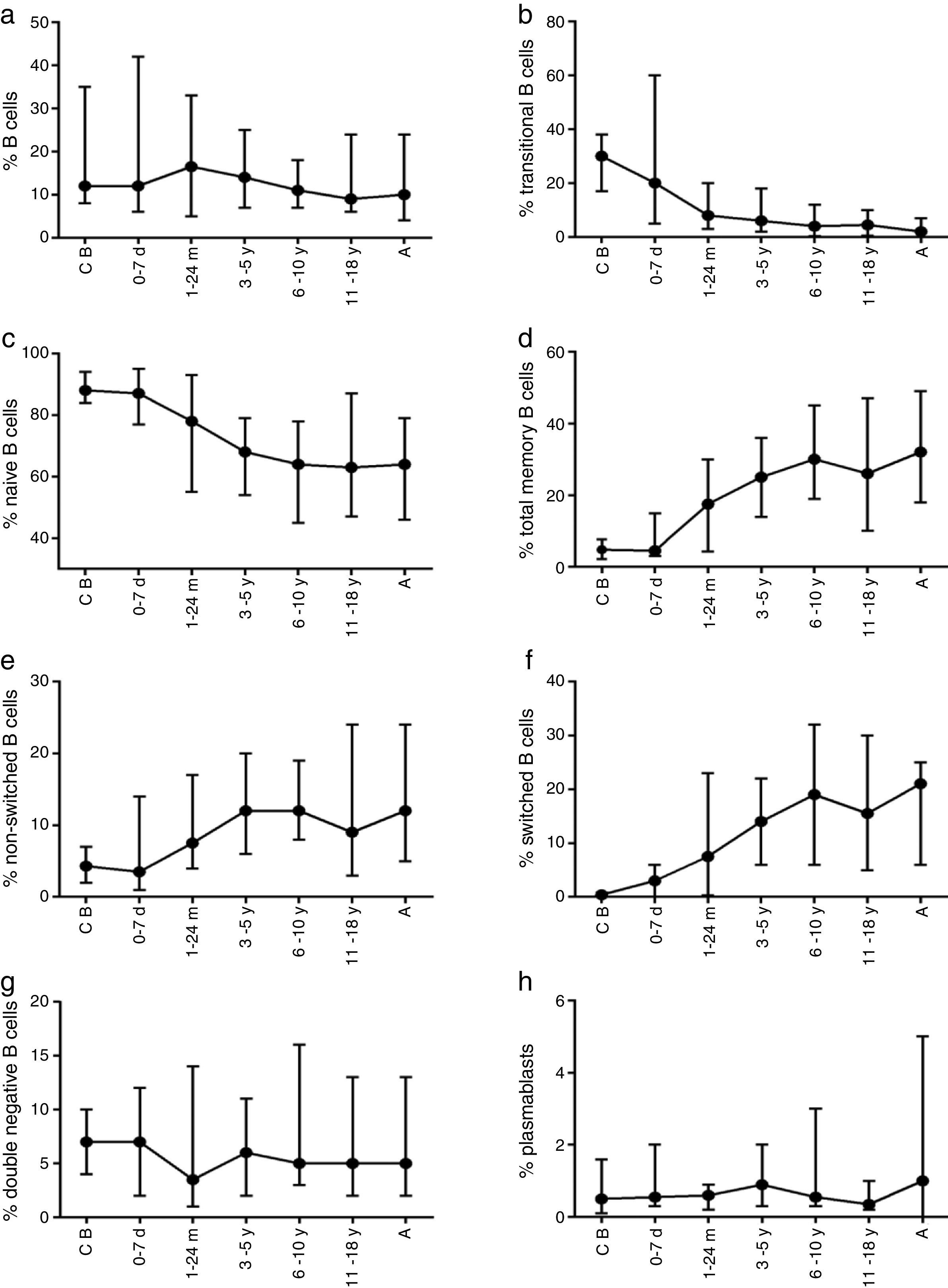
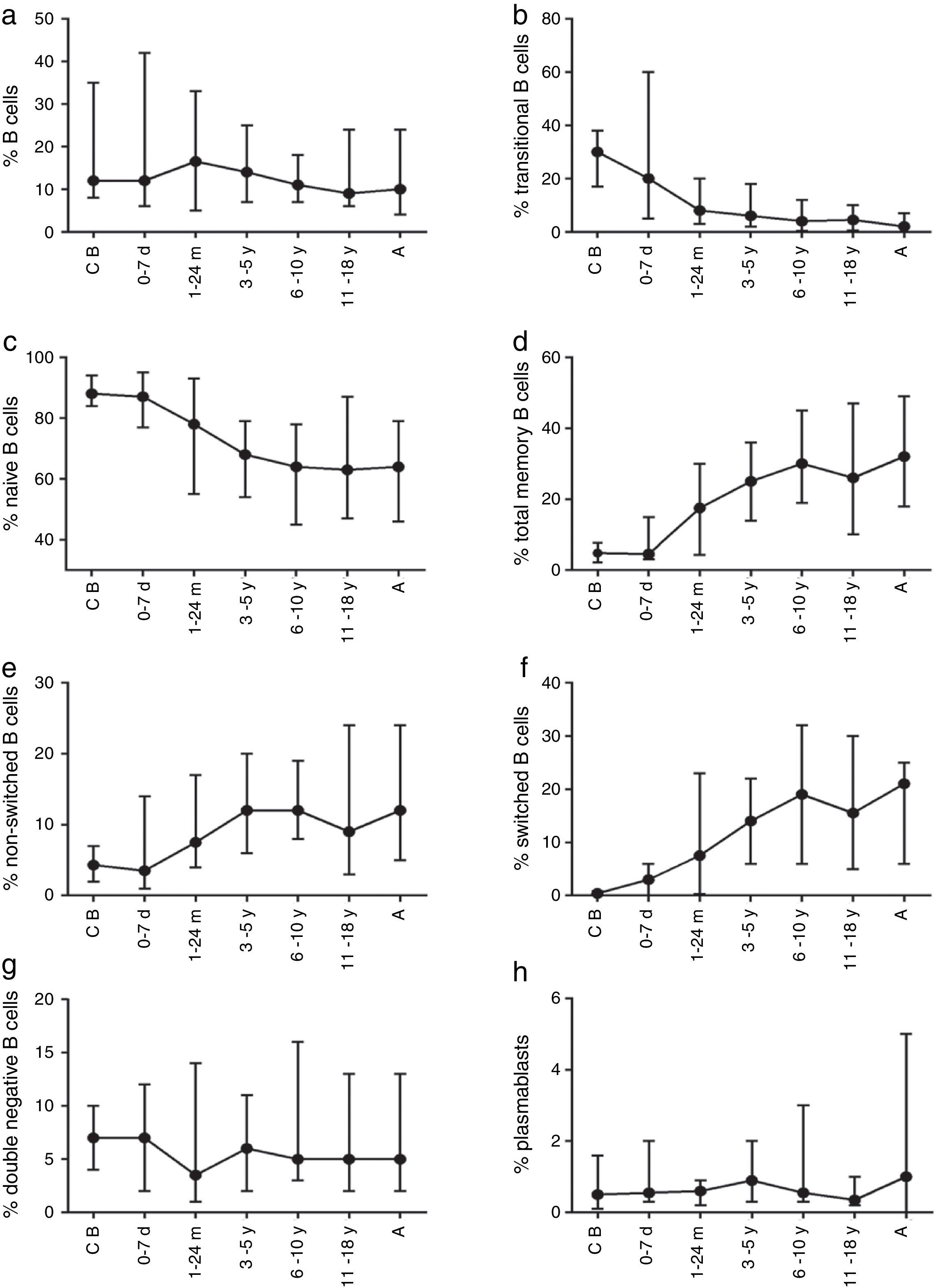
The composition of the B cell subsets showed age-dependent variations; whereas the frequency of transitional B cells, as well as naïve B cells, decreased rapidly during the first years of life (Fig. 2b and c), the frequencies of switched and non-switched memory B cells increased slowly throughout life (Fig. 2d–f). The frequency of double-negative B cells (CD27−IgD−) increased during the first days of age and did not show further age-related variations. Plasmablasts were rarely detected in the peripheral blood (Fig. 2g and h).
Variation in absolute numbers of naïve, memory, transitional B cells, and plasmablastsThe absolute counts of distinct B cell subsets depend on the relative frequency of each B cell subset as well as the developmental changes in the total B cell count during ageing. The number of total B cells decreased throughout life (Fig. 3a). Naïve B cells upon encounter with antigen gradually differentiate into memory B cells (CD27+) (Fig. 3b). We observed that the memory B cell pool is complete in children after 10 years of age, reaching the levels seen in adults (Fig. 3c). The absolute numbers of non-switched memory B cells is very low and almost invariable in the first months of life; this population increases rapidly in childhood and gradually decreases in adults (Fig. 3e). Class-switched memory B cells are very important for the secondary immune responses and include B cells that are able to produce immunoglobulins other than IgM. Total numbers of memory B cells increase in adults and are almost absent in new-borns. Double-negative B cells (CD27−IgD−) are particularly numerous in new-borns, in contrast to children from other age groups (Fig. 3f). Transitional B cells are a relatively high percentage of total B cells in cord blood but decrease significantly in peripheral blood during the first years of life (Fig. 3b). The plasmablasts represent the last step in the differentiation of B cells. They include a minor B cell subpopulation that is usually not more than 20cells per mm3 (Fig. 3h).
B cells with low expression of CD21The expression of CD21 was further analysed on B cells (supplementary Fig. 1) only in two groups: 18 healthy donors from 20 to 40 years old and 10 healthy donors from 1 to 17 years old. The CD21low population was similar between both groups, the adult healthy donors showed a media of 4% (range 0.3–11, 5th and 95th percentile), while the 1–17 year old healthy donors showed a media of 4% (range 0.5–17) (supplementary Table 1); no significant differences were found between both groups (p=0.7145) (supplementary Fig. 2). As the Freiburg classification suggests a cut-off of more than 20% for CD21low B cells, our results indicate that this cut-off parameter is suitable for Mexican individuals to differentiate them from patients with CVID.
DiscussionSeveral markers have recently been used to characterise B cell subpopulations. The maturation of these cells is a tightly regulated process that depends, among many other factors, on age and the immunological status of individuals. Their development is a complex process that begins in the bone marrow and continues in peripheral lymphoid tissues.3 After emerging from bone marrow and migrating to the periphery, B cells pass through several consecutive developmental stages. The naïve B cells differentiate into memory B cells as a result of antigen-induced activation. Memory B cells rapidly differentiate into plasma cells when they are exposed to a second boost with the same antigen.2 The maturation process of B cells is accompanied by changes in expression of specific markers on the cell surface. Several B cell subpopulations, reflecting individual maturation stages, and can be detected in peripheral blood by multicolour flow cytometry.2,16
An impaired generation of B cell subpopulations is characteristic of several PIDs and other immunological, haematological, and infectious diseases. B cells that have recently emigrated from bone marrow can be detected in peripheral circulation; they have been denominated as transitional B cells. These cells can be distinguished by expression of CD10, together with high expression of CD38, CD24, and IgM.17 Significantly high levels of these cells in cord blood and peripheral blood have been reported in several pathological conditions such as human immunodeficiency virus infection,18 B cell-activating factor receptor deficiency and other diseases.12,19
Plasmablasts can also be found, albeit in low numbers, in peripheral blood, and they are recognised by their high expression of CD38. They are found in similar proportions in all age groups and never exceed, under normal circumstances, 5% of the total B cell population.15 Increased number of plasmablasts correlates with disease activity, and have been observed in systemic lupus erythematous.20
Human naïve B cells comprise about 60–70% of all circulating B cells and have un-mutated IgV regions. In contrast, memory B cells (20–30%) display mutations within their IgV regions.2 Different approaches for the characterisation of B cell subpopulations have been proposed, although the differential expression of CD27 and IgD has become accepted as standard for their characterisation.21 Both CD27 and surface IgD were used in this work to identify and characterise these subpopulations, and the numbers are similar to those which have been described in similar reports.12
Naïve B cells, defined by the expression of CD19 and IgD and the lack of CD27 on their surface, gradually decrease with age. The increase in the numbers of these cells seen in some pathologies is likely due to a decrease in memory B cells and not to a higher B cell lymphopoiesis by the bone marrow. Some primary antibody deficiencies are characterised by an increase in naïve B cell levels, such as X-linked hyper-IgM syndrome,22 and in some patients with CVID.23
It has been suggested that CD27 distinguishes two types of memory B cells. CD27+IgD+ memory B cells may be generated independently of the germinal centre reaction, and may correspond to or may include circulating marginal zone B cells, which respond to T-independent antigens.24 In contrast, CD27+IgD− memory B cells represent post-germinal centre B cells. These populations are almost undetectable in some diseases with abnormal splenic function.25,26 Reduced numbers of these B cell subpopulations have also been described in monogenic deficiencies such as CD19, CD81, CR2, MS4A1, and LRBA, defects that have been associated with CVID27–31 and X-linked lymphoproliferative disease.32 The absence of switched memory B cells (CD27+IgD−) is highly suggestive of a disrupted germinal centre reaction. Humans with defective germinal centre reactions due to CD40L and ICOS deficiencies lack this subpopulation of B cells.16 Abnormal proportions of switched memory B cells have been described in several conditions, such as Wiskott–Aldrich syndrome,33 X-linked lymphoproliferative syndrome,32 AID, UNG, PMS, CD4034 and TACI deficiency,35 as well as some patients with CVID.36
The double-negative CD27−IgD− B cells, although lacking CD27 surface expression, have properties of memory B cells since they are hyper-mutated. A previous study has demonstrated that this subset is also particularly numerous in cord blood but may represent two different populations with similar phenotypes that rapidly decrease in number during the first year of life.12 These memory B cells have been observed to be expanded in lupus,37 chronic infectious disease (HIV, malaria)38,39 and in some patients with CVID (unpublished observations). The role of the CD27−IgD− B cell subpopulation is currently not well understood.
Finally, the CD21low B cells and CD27+IgD− memory B cells were described for the Freiburg Classification.10 Peripheral B cell homeostasis is severely disturbed in CVID patients and the expansion of CD21low B cells are detected in a group of CVID patients; the expansion of these cells correlate with the presence of splenomegaly and autoimmune diseases in CVID.40 Additional groups of CVID patients show severely reduced class-switched memory B cells (CD27+, IgD+), (<0.4% of lymphocytes), or nearly normal number of class-switched memory B cells (>0.4% of lymphocytes).10 Those patients with low memory B cells have been subdivided into a group with expanded CD21low B cells (> 20% CD19+ B cells) and a group with less than 20% of CD21 low B cells. In other reports show that CD21low B cells levels were low throughout life.12 We analysed 18 healthy adults and 10 young healthy individuals (1–17 years old) (supplementary Table 1), and we found no significant differences in the percentages of CD21low B cells (0.3–11% in 1–17 years old and 0.5–17% in adults) (Supplementary Fig. 2). These data agree with previous reports, suggesting that the increase of CD21 low B cells is associated with immune dysregulation.41
Importantly, this study has established references ranges for B cell subpopulations of healthy Mexican population, while it may also help to understand different diseases associated with deregulation of humoral immune responses. Our results show significant changes in both percentages and absolute numbers of all subpopulations in all age groups studied; such data would be beneficial to clinicians in identifying possible pathologies that affect B cells.
ConclusionB cell subpopulation levels show significant variation throughout life, information that could be valuable in immunology or rheumatology clinics. The reference values established in this study may be useful in the diagnosis of pathologies in which B cell maturation is affected.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
AuthorshipWe assure that all authors included on a paper fulfil the criteria of authorship. All authors have participated in conception and design, or analysis and interpretation of data. All authors gave approval of the final version to be published.
Conflict of interestThe authors have no conflict of interest to declare.
This work was partially supported by the Instituto de Ciencia y Tecnología del Distrito Federal (grant # ICYTDF/226/2012), the Consejo Nacional de Ciencia y Tecnología (CONACYT, México) (grants # FOSISS-2013-01-202111, CB-2010-01-154472, and FOSISS-161089), and the Fundación Mexicana para los Niños con Inmunodeficiencias Primarias (FUNEMI), A.C.
Laura Berrón-Ruiz, Gabriela López-Herrera, Francisco Espinosa-Rosales, and Leopoldo Santos-Argumedo are SNI fellows. Laura Berrón-Ruiz received a postdoctoral scholarship (CVU 44813) from CONACYT, México.
The authors thank Héctor Romero-Ramírez, Víctor Hugo Rosales-García, and Mariela Trujillo-de la Cruz for their technical assistance.