Introduction.Acoustic Radiation Force Impulse (ARFI) elastography evaluates hepatic fibrosis non-invasively and has been mainly validated in viral hepatitis. Data on rare liver diseases such as autoimmune hepatitis (AIH), overlap syndrome, primary biliary cholangitis (PBC) or primary sclerosing cholangitis (PSC) are sparse.
Material and methods. 85 patients (including 31 AIH, 26 PBC, 16 PSC and 3 PSC-and 9 PBC-AIH-overlap syndromes) were retrospectively analysed pointing at ARFI elastography of the liver and the correlation with histologic Ishak fibrosis score (F0-6). Results of shear wave velocities (m/s) were expressed as mean ± standard deviation.
Results. The mean shear wave velocity of all 85 patients showed 1.80 ± 0.84 m/s (0.74-3.98). The ARFI elastography values correlated with the degree of fibrosis in all patients overall and in patients with AIH, overlap syndrome and PSC, respectively. The subgroup of 26 patients with PBC (only with Ishak F > 3) revealed no correlation between ARFI and these early fibrosis stages (r = 0.019, p = 0.927). ARFI elastography correlated with bilirubin, AST, but not with patient age, body mass index or measurement depth. The cut-off of 2.04 m/s for detecting cirrhosis (Ishak F > 5) leads to a sensitivity of 90.0% and specificity of 74.7% (AUROC 87.2%).
Conclusion. ARFI elastography can evaluate fibrosis in AIH, PSC and PSC-/PBC-AIH-overlap syndrome with good accuracy for the detection of hepatic cirrhosis. Shear wave velocities in PBC should be interpreted with caution in early stages of fibrosis.
Chronic liver diseases may lead to hepatic fibrosis and finally to cirrhosis. An ongoing progress of fibrosis impairs the prognosis. Hepatic cirrhosis may cause complications such as ascites, esophageal varices, renal failure or hepatocellular carcinoma, yielding a relevant morbidity and mortality. The most frequent causes of liver injury are alcoholism, viral hepatitis B and C, fatty liver disease and more rarely hemochromatosis, primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC) or autoimmune hepatitis (AIH).
The gold standard for evaluating the degree of fibrosis is liver biopsy yielding additional information about degree of inflammation and etiology of liver disease. Due to its invasiveness and sampling error more simple, non-invasive and repeatable methods are needed to evaluate liver fibrosis. Transabdominal ultrasound can detect hepatic cirrhosis, but often fails in cases of early stages of fibrosis.1 Transient elastography and point shear wave elastographic methods such as acoustic radiation force impulse elastography have been evolving for some years. They evaluate tissue elastic properties non-invasively and can be used for evaluating fibrosis and cirrhosis of the liver. Meta-analyses of different elastographic techniques report that the values of transient elastography or shear wave velocities of the hepatic parenchyma correlate well with the degree of fibrosis.2,3 Some studies contain cohorts consisting of mixed diseases and for the single etiologies mainly chronic viral hepatitis B or C have been evaluated. The rather rare hepatic diseases such as PBC, PSC or AIH are underrepresented with only up to 20% of patients.4 Few studies using transient elastography for detection of fibrosis and cirrhosis show good accuracy in PBC and PSC, thus demonstrating its prognostic relevance.5,6 ARFI elastography data in PBC, PSC or AIH are sparse. In one study, ARFI was evaluated in PBC, PSC, AIH and overlap syndrome, but only 9 patients were included in that analysis indicating differentiation of patients with hepatic fibrosis from healthy controls.7 In a further study, a differentiation of significant from non-significant fibrosis by ARFI was shown in 15 patients with AIH.8
Our retrospective analysis aimed at evaluating the performance of ARFI point shear wave elastography in patients with PBC, PSC, AIH and overlap syndrome.
Material and MethodsBetween January 2011 and February 2017, 85 patients with histologically proven cholestatic liver disease (PBC, PSC) or AIH or PSC-/PBC-AIH-overlap-syndrome and a hepatic ARFI elastography within at least 2 weeks after biopsy were retrospectively analysed. Only patients with at least 5 valid measurements available were included into the retrospective analysis. For all patients, demographic data (age, gender, BMI), and laboratory values (aspartate transaminase [AST], alanine transaminase [ALT], bilirubin, APRI score [AST/platelet ratio]) were evaluated. The study complies with the ethics guidelines of the Helsinki Declaration.
ARFI techniqueThe transducer sends high-frequency, short-duration ultrasound pulses to generate localized displacements in tissue. These displacements result in shear-wave propagation transversely to the impulse delivered. This shear wave is tracked using ultrasonic correlation-based methods and can be monitored both spatially and temporally. Displacement magnitude is inversely proportional to local tissue stiffness, shear-wave velocity is directly proportional. A single transducer is used to apply localized radiation forces within tissue for short time periods and to track the resulting tissue displacements in the form of shear wave velocity propagation. This shear wave velocity is proportional to the square root of tissue elasticity.9
ARFI shear wave elastography was performed using the ultrasound system Acuson S2000 (Siemens Medical Solutions, Erlangen, Germany) in the Virtual touch tissue quantification mode. The curved-array transducer (4C1 or 6C1HD) was placed intercostally to the right flank and a region of interest (10 x 5 mm) was set in the right hepatic lobe (segment 7/8) during real-time B-mode imaging in a breath hold position. All patients were fasting. The ARFI technique provides quantitative values of hepatic elasticity that are expressed as meters per second (m/s). Results are presented as mean, median with standard deviation (SD) and the interquartile range (IQR).
Confirmation of DiagnosisAll patients were diagnosed at our university hospital. Chronic viral hepatitis B and C had been excluded by serological laboratory testing. A relevant alcohol-induced hepatitis was excluded by medical history and histology. AIH, PBC, PSC and overlap syndrome were diagnosed on clinical, biochemical, serological and histopathological findings and in accordance with the dedicated EASL guidelines.10,11
HistopathologyAll biopsy specimens were analyzed by an experienced pathologist blinded to the patients elastography results. The histological features of the liver biopsy specimens were interpreted in accordance with the Ishak score classification.12 Ishak fibrosis score for fibrosis (silver staining) was staged on a scale ranging from F0 to F6, in which 0 indicates no fibrosis and 5 indicates cirrhosis: F0 - no fibrosis, F1 - fibrous expansion of some portal areas, F2 - fibrous expansion of most portal areas, F3 - fibrous expansion of most portal areas with occasional portal to portal bridging, F4 - marked fibrous bridging as well as portal to central, F5 - marked bridging with occasional nodules (incomplete cirrhosis), F6 - cirrhosis.
Biopsy was obtained either by ultrasound-or laparo-scopic-guided biopsy. For ultrasound-guided biopsy, specimens were obtained using a core tissue biopsy needle with an external diameter of 1.3 mm and a sample notch length of 2.2 cm. For laparoscopic guided biopsy, a core biopsy instrument with an external diameter of 1.2 mm and a sample notch length of 1.7 cm was used via a Silverman needle. The specimens (median n = 2) were fixed in formalin (10%) and then submitted to the Department of Pathology for a histopathological diagnosis.
Statistical AnalysisClinical and laboratory characteristics of patients as well as the ARFI values were expressed as the mean ±SD together with the range in brackets. The Spearman’s correlation coefficient (r) was used for analysis of relationships between variables. The assessment of the diagnostic performance of ARFI was calculated by the area under the receiver operating characteristic (AUROC) curves.
The results were illustrated as boxplots with the median as a thick line through each box which represents the interquartile range within which 50% of values are located. Error bars mark minimum and maximum values (i.e. range). Small circle or star mark outliners. A p < 0.05 indicated a significant correlation or difference. All reported p values are two-sided. Statistical analysis was performed using the Statistical Package for the Social Sciences (version 19.0.0.1, IBM SPSS statistics, New York, USA).
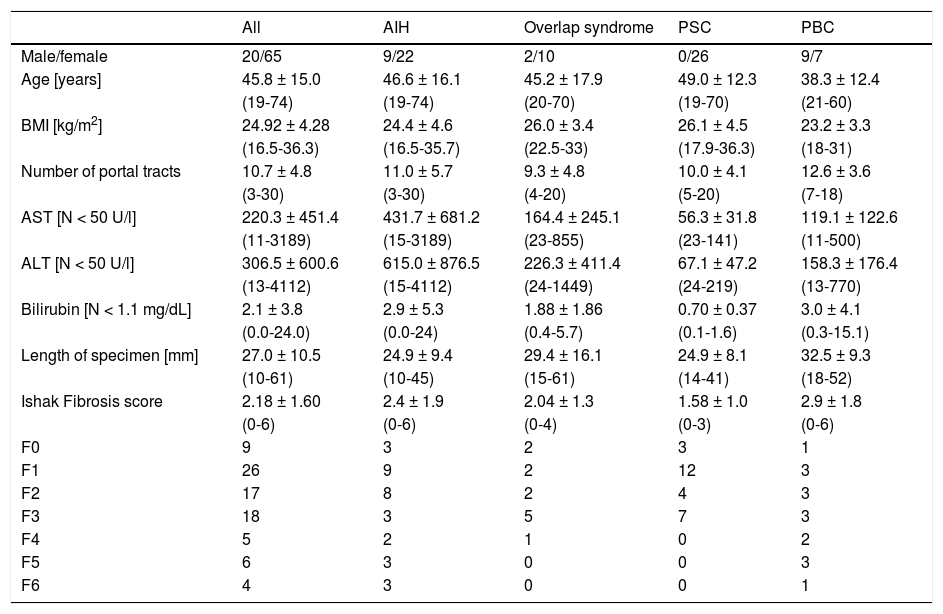
ResultsPatients’ characteristicsA total of 85 patients (65 female, 20 male, mean age 46 years) met the inclusion criteria. 31 patients suffered from AIH, 26 from PBC, 16 from PSC and 12 from overlap syndromes (3 with PSC-and 9 with PBC-AIH-overlap) (Table 1).
Patient data of all patients and of subgroups with the specific cholestatic liver diseases AIH, Overlap syndrome, PBC and PSC.
| All | AIH | Overlap syndrome | PSC | PBC | |
|---|---|---|---|---|---|
| Male/female | 20/65 | 9/22 | 2/10 | 0/26 | 9/7 |
| Age [years] | 45.8 ± 15.0 | 46.6 ± 16.1 | 45.2 ± 17.9 | 49.0 ± 12.3 | 38.3 ± 12.4 |
| (19-74) | (19-74) | (20-70) | (19-70) | (21-60) | |
| BMI [kg/m2] | 24.92 ± 4.28 | 24.4 ± 4.6 | 26.0 ± 3.4 | 26.1 ± 4.5 | 23.2 ± 3.3 |
| (16.5-36.3) | (16.5-35.7) | (22.5-33) | (17.9-36.3) | (18-31) | |
| Number of portal tracts | 10.7 ± 4.8 | 11.0 ± 5.7 | 9.3 ± 4.8 | 10.0 ± 4.1 | 12.6 ± 3.6 |
| (3-30) | (3-30) | (4-20) | (5-20) | (7-18) | |
| AST [N < 50 U/l] | 220.3 ± 451.4 | 431.7 ± 681.2 | 164.4 ± 245.1 | 56.3 ± 31.8 | 119.1 ± 122.6 |
| (11-3189) | (15-3189) | (23-855) | (23-141) | (11-500) | |
| ALT [N < 50 U/l] | 306.5 ± 600.6 | 615.0 ± 876.5 | 226.3 ± 411.4 | 67.1 ± 47.2 | 158.3 ± 176.4 |
| (13-4112) | (15-4112) | (24-1449) | (24-219) | (13-770) | |
| Bilirubin [N < 1.1 mg/dL] | 2.1 ± 3.8 | 2.9 ± 5.3 | 1.88 ± 1.86 | 0.70 ± 0.37 | 3.0 ± 4.1 |
| (0.0-24.0) | (0.0-24) | (0.4-5.7) | (0.1-1.6) | (0.3-15.1) | |
| Length of specimen [mm] | 27.0 ± 10.5 | 24.9 ± 9.4 | 29.4 ± 16.1 | 24.9 ± 8.1 | 32.5 ± 9.3 |
| (10-61) | (10-45) | (15-61) | (14-41) | (18-52) | |
| Ishak Fibrosis score | 2.18 ± 1.60 | 2.4 ± 1.9 | 2.04 ± 1.3 | 1.58 ± 1.0 | 2.9 ± 1.8 |
| (0-6) | (0-6) | (0-4) | (0-3) | (0-6) | |
| F0 | 9 | 3 | 2 | 3 | 1 |
| F1 | 26 | 9 | 2 | 12 | 3 |
| F2 | 17 | 8 | 2 | 4 | 3 |
| F3 | 18 | 3 | 5 | 7 | 3 |
| F4 | 5 | 2 | 1 | 0 | 2 |
| F5 | 6 | 3 | 0 | 0 | 3 |
| F6 | 4 | 3 | 0 | 0 | 1 |
In only 16/85 patients (19%), ARFI elastography of the liver was performed during a follow-up biopsy under immunosuppressive treatment (corticosteroids, azathioprine or cyclosporine). In particular, most of the patients with AIH (21/31, 68%) underwent hepatic ARFI and biopsy in the phase of initial diagnosis and had no treatment. The mean levels of AST were 220.3 U/l, with the group of AIH showing the highest levels and the PBC group showing the lowest levels. Predominantly females were affected by AIH or cholestatic liver diseases except for the PSC group with 9 men and 7 women. Moreover, the PSC group showed the lowest mean age with 38 years and the lowest BMI with 23.2 kg/m2 and the highest mean Ishak fibrosis score (indicating advanced disease).
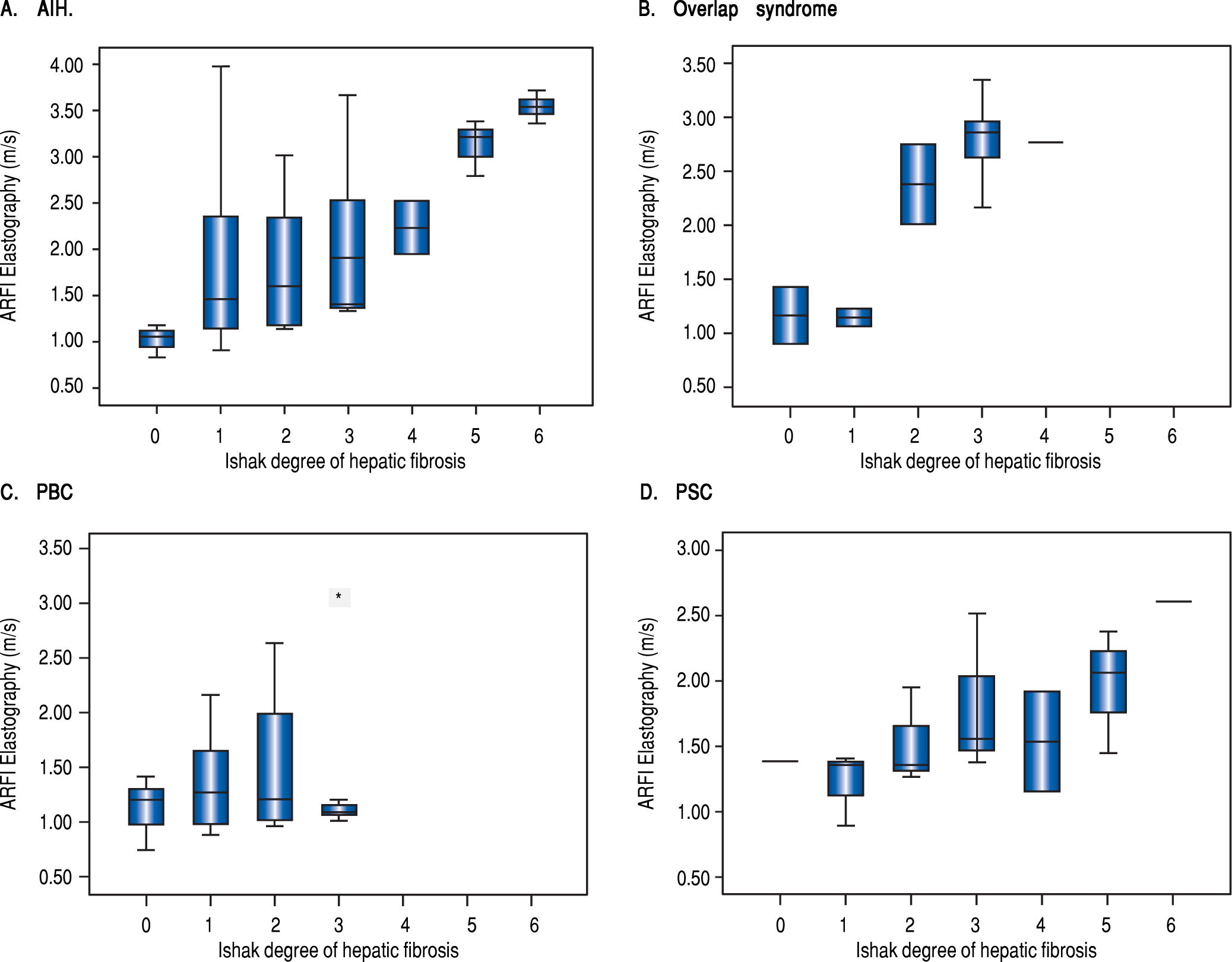
HistologyThe mean number of portal tracts was 10.7. Only 10/85 patients (11.8%) had a liver cirrhosis. The PBC group did not contain any cases with an Ishak score F > 4. The group with overlap syndromes included no complete or incomplete cirrhosis (Ishak F = 5 or F = 6). In the whole cohort, single data items were missing: 6x number of portal tracts, 4x length of biopsy specimen, 1x BMI and 3x ARFI measurement depth.
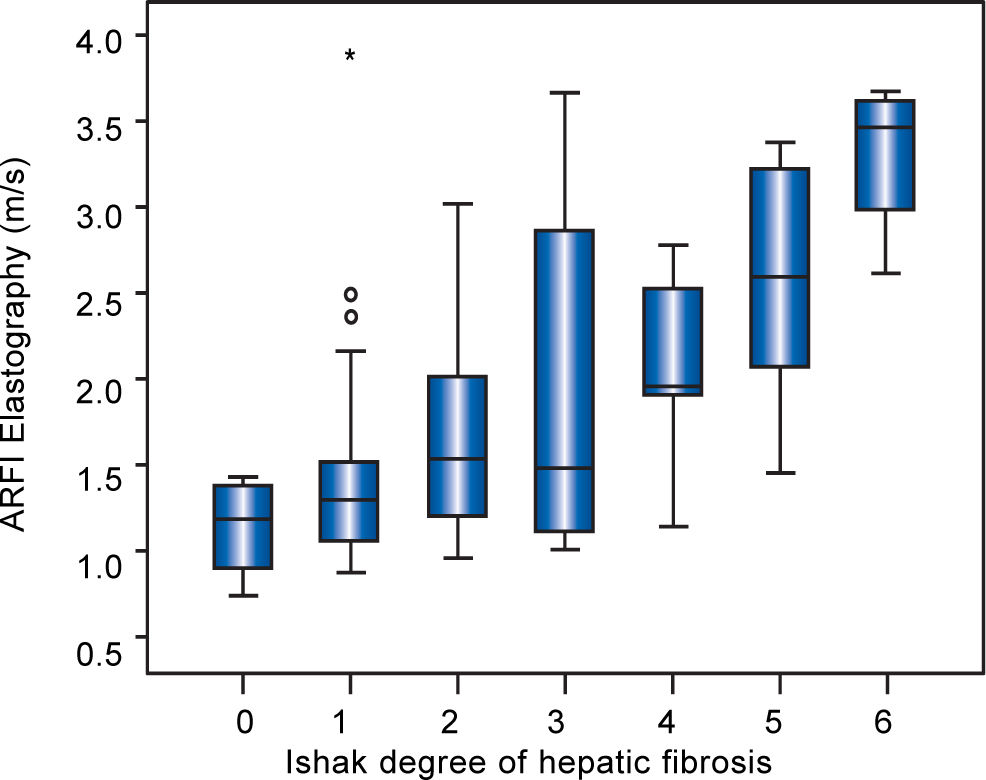
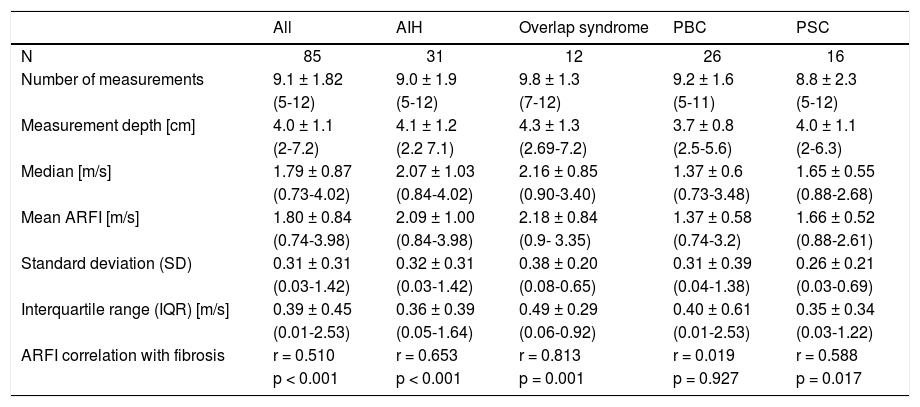
Point shear wave elastographyMean shear wave velocities of all 85 patients were 1.80 ± 0.84 m/s (0.74-3.98) and correlated with the degree of fibrosis (r = 0.510, p < 0.001) (Figure 1). The three outliers of fibrosis grade 1 with high elatography values (Figure 1) were all patients with AIH (only one of them with highly elevated transaminases > 2x ULN). The mean ARFI values of each fibrosis stage were increasing with the degree of fibrosis: 1.13 ± 0.26 m/s (F0), 1.48 ± 0.68 m/s (F1), 1.75 ± 0.68 m/s (F2), 1.98 ± 0.93 m/s (F3), 2.06 ± 0.63 m/s (F4), 2.54 ± 0.73 m/s (F5) and 3.30 ± 0.48 m/s (F6). The SD (r = 0.412; p < 0.01) and IQR (r = 0.339; p < 0.01) correlated with Ishak fibrosis score, respectively. A SD or IQR 30% of the ARFI mean as criterion of insufficient validity was observed in 9 or 15 of the patients, respectively, and was mainly found in patients with PBC or PBC-AIH-overlap syndrome.
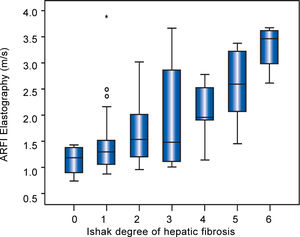
Table 2 shows mean ARFI values of the different diseases. In all patients overall and in patients with AIH or with overlap syndrome a significant correlation of hepatic fibrosis and ARFI shear wave velocities could be demonstrated. 20/31 (65%) of the AIH patients were examined before starting an immunosuppressive treatment. The subgroup of 26 patients with PBC containing only fibrotic stages F < 3 revealed no correlation of ARFI measurements with the Ishak fibrosis score (r = 0.019, p = 0.927) (Figure 2). In a subanalysis of those 44 patients only having F < 3 and no PBC, a correlation between fibrosis and ARFI could be revealed (r = 0.540, p < 0.001).
ARFI point shear wave elastography of the liver in patients with AIH, Overlap syndrome, PBC and PSC.
| All | AIH | Overlap syndrome | PBC | PSC | |
|---|---|---|---|---|---|
| N | 85 | 31 | 12 | 26 | 16 |
| Number of measurements | 9.1 ± 1.82 | 9.0 ± 1.9 | 9.8 ± 1.3 | 9.2 ± 1.6 | 8.8 ± 2.3 |
| (5-12) | (5-12) | (7-12) | (5-11) | (5-12) | |
| Measurement depth [cm] | 4.0 ± 1.1 | 4.1 ± 1.2 | 4.3 ± 1.3 | 3.7 ± 0.8 | 4.0 ± 1.1 |
| (2-7.2) | (2.2 7.1) | (2.69-7.2) | (2.5-5.6) | (2-6.3) | |
| Median [m/s] | 1.79 ± 0.87 | 2.07 ± 1.03 | 2.16 ± 0.85 | 1.37 ± 0.6 | 1.65 ± 0.55 |
| (0.73-4.02) | (0.84-4.02) | (0.90-3.40) | (0.73-3.48) | (0.88-2.68) | |
| Mean ARFI [m/s] | 1.80 ± 0.84 | 2.09 ± 1.00 | 2.18 ± 0.84 | 1.37 ± 0.58 | 1.66 ± 0.52 |
| (0.74-3.98) | (0.84-3.98) | (0.9- 3.35) | (0.74-3.2) | (0.88-2.61) | |
| Standard deviation (SD) | 0.31 ± 0.31 | 0.32 ± 0.31 | 0.38 ± 0.20 | 0.31 ± 0.39 | 0.26 ± 0.21 |
| (0.03-1.42) | (0.03-1.42) | (0.08-0.65) | (0.04-1.38) | (0.03-0.69) | |
| Interquartile range (IQR) [m/s] | 0.39 ± 0.45 | 0.36 ± 0.39 | 0.49 ± 0.29 | 0.40 ± 0.61 | 0.35 ± 0.34 |
| (0.01-2.53) | (0.05-1.64) | (0.06-0.92) | (0.01-2.53) | (0.03-1.22) | |
| ARFI correlation with fibrosis | r = 0.510 | r = 0.653 | r = 0.813 | r = 0.019 | r = 0.588 |
| p < 0.001 | p < 0.001 | p = 0.001 | p = 0.927 | p = 0.017 |
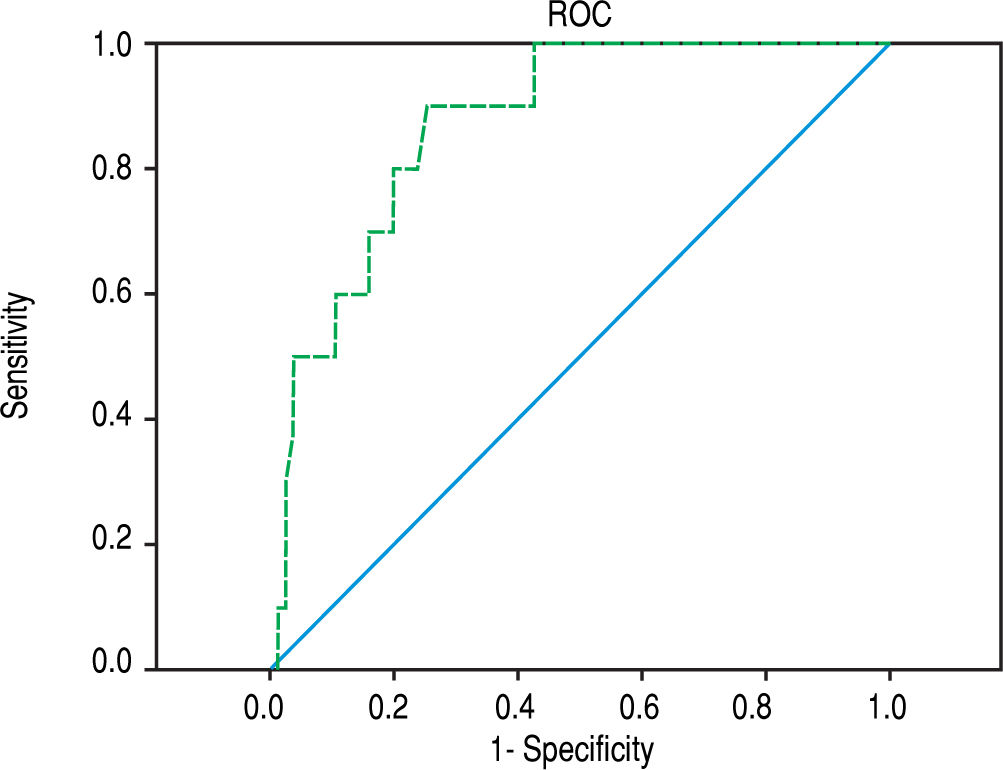
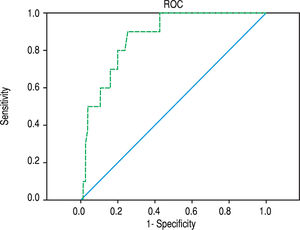
In all patients, ARFI elastography correlated with bilirubin (r = 0.450, p < 0.001) and with APRI (r = 0.324, p = 0.003). In PBC (r = 0.414; p = 0.036) but not in PSC patients (r = 0.417; p = 0.109) ARFI values correlated significantly with bilirubin. In all 85 patients, ARFI correlated with AST (r = 0.257, p = 0.018), but not significantly with BMI, patient age or measurement depth (p = 0.165, p = 0.242 and p = 0.484, respectively). Using a cut-off of 2.04 m/s for detecting cirrhosis (F > 5) resulted in a sensitivity of 90.0% and specificity of 74.7% (AUROC 87.2%, confidence interval 77.8-96.6%) - ROC curve Figure 3. The APRI score amounted to a mean of 3.4 ± 8.2 (0.1-60.9) and correlated with the Ishak fibrosis score (r = 0.341, p = 0.01). Excluding patients with an AST level above three times of the upper limit of normal, the remaining 64 patients (mean AST 61,3 U/l, Ishak fibrosis 2.1, ARFI 1.71 m/s) had still a significant but somewhat inferior correlation of ARFI elastography with fibrosis scoring (r = 0.487, p < 0.001). Furthermore, this subgroup of these 64 patients (AST level less than three times of the upper limit of normal) showed also a cut-off of 2.04 m/s for detecting the 7 patients with cirrhosis (F > 5). A sensitivity of 85.7% and specificity of 78.9% were calculated (AUROC 88.1%, confidence interval 77.5-98.7%).
In an additional analysis the 16 patients with immunosuppressive treatment (corticosteroids, azathioprine or cyclosporine) were excluded. The remaining subgroup of 69 patients (mean AST 250,7 U/l, Ishak fibrosis 2.25, ARFI 1.95 m/s) revealed a good correlation of ARFI elastography with Ishak fibrosis score (r = 0.520, p < 0.001).
DiscussionARFI elastography is capable of detecting hepatic fibrosis, but has mainly been evaluated in chronic viral hepatitis.13 Elastographic data in patients with rare chronic liver diseases like AIH, PBC, PSC or overlap syndrome are sparse. Until 2013, these hepatic diseases were regularly analyzed in meta-analysis only in small subgroups compared with other liver diseases such as viral hepatitis or (non-)alcoholic liver disease.2,3 Most available data referring to transient elastography indicate that fibrosis and liver stiffness values themselves might be helpful for prognosis and risk stratification in chronic cholestatic diseases in order to establish an individual management.5’6 Our study shows a good correlation of ARFI elastography with hepatic fibrosis in AIH, PSC and overlap syndrome and a good diagnostic accuracy when testing for cirrhosis. The performance and applicability in patients with PBC and early stages of fibrosis remains questionable at least in our cohort.
In a previous study, ARFI elastography was evaluated in 9 patients with 2x AIH, 3x PBC and 1x PSC as well as in 11 healthy volunteers.7 Shear wave velocities were higher in patients with AIH and cholestatic liver disease than in healthy controls (1.51 ± 0.44 vs. 1.08 ± 0.1 m/s). A cut-off of 1.25 m/s was calculated to distinguish patients from controls (AUROC = 0.885; sensitivity 70.6%; specificity 95.5%). Patients had mainly METAVIR fibrosis score F2/3 and no cirrhosis. In another study, in 15 patients with treated AIH in remission, a liver biopsy was obtained on average 4.9 years after initial diagnosis. ARFI elastography could differentiate between no/mild fibrosis (n = 6) and significant fibrosis (F2-4, n = 9), showing 1.20 ± 0.24 m/s and 2.28 ± 0.68 m/s, respectively (p = 0.002).8 In contradiction to our results showing no correlation between ARFI and early hepatic fibrosis in PBC, a study by Zhang, et al.14 demonstrated a correlation between ARFI shear wave velocities and histological stages of fibrosis in 61 patients with PBC (r = 0.74; p < 0.001). A good diagnostic accuracy for detecting fibrosis was highlighted with a cut-off of 1.51 m/s (AUROC 0.83) for Ludwig F > 2 and of 1.79 m/s (AUROC 0.93) for fibrosis F > 3. For detecting hepatic cirrhosis in PBC (n = 9/61, 15%) a cut-off of 2.01 m/s with good diagnostic accuracy (AUROC 0.91) was found in accordance to our overall findings. In a different study measuring hepatic ARFI shear wave velocities in 120 cirrhotic PBC patients, shear wave velocity values were shown to increase in parallel with Child-Pugh stages A-C.15
Several observations declared that alcohol consumption or high levels of aminotransaminases lead to falsely high stiffness values notably in patients with viral hepatitis B and C.16-18 High levels of liver enzymes were observed in two cases with AIH and histologically proven necrosis having high stiffness values in transient elastography, which decreased 4 and 12 months after biopsy, respectively.19 Therefore some authors do not recommend elastography in case of high level of transaminases. In our study, ARFI shear wave velocities were still associated with the degree of fibrosis, although AST levels were sometimes higher than 10x upper limit mainly in patients with AIH.
Relevant studies on liver stiffness evaluation focusing on AIH and cholestatic liver diseases were - up to now - mainly available for transient elastography. Transient elastography proved capable of evaluating fibrosis in PBC (n = 103).6 A good performance with both sensitivity and specificity > 90% for the diagnosis of severe liver fibrosis or cirrhosis (Metavir F > 3 and F = 4) was prospectively observed, but only 45% of PBC patients with intermediate fibrosis (Ludwig F = 2) could be correctly classified. High baseline or increasing values over time showed a significant minor prognostic outcome in a retrospective analysis. In a study by Corpechot, et al. 73 patients with PSC hepatic transient elastography was investigated in a follow-up-design.5 Stiffness measurements were linked to the stage of fibrosis (p < 0.0001). The diagnostic accuracy for detecting hepatic cirrhosis using a threshold of 13.4 kPa was 0.88 - comparable to our overall AUROC of 84.4%. Baseline liver stiffness and rate of progression were associated with adverse outcome such as death, liver transplantation, ascites, hepatic encephalopathy, gastrointestinal bleeding or hepatocellular carcinoma in patients with PSC.
In patients with AIH (n = 34 and n = 60),20 liver inflammation had an impact on liver stiffness measured by transient elastography in particular before and in the first three months of AIH treatment. A good correlation of transient elastography with hepatic fibrosis was shown after 6 months of immunosuppressive treatment - regardless of the residual hepatic inflammation. An excellent diagnostic accuracy for separating severe from non-severe fibrosis and for diagnosing cirrhosis (AUROC 0.95 with cut-off 16kPa) was found. A weakness of that study by Hartl et al. was the quite long interval between biopsy and transient elastography measurements (up to three and four months). Reliable data regarding 2D-SWE in AIH, PBC, PSC or overlap syndrome are still missing.
The power of our findings is impaired by certain weaknesses such as the retrospective character of the study and the fact that patients were not consecutively included over the whole study period which might have led to a certain selection bias. In addition, in some patients the mean number of measurements was somewhat lower than the recommended 10 measurements (11 patients (13%) had only 5 or 6 measurements). The group of PSC-/ PBC-overlap syndrome and PSC were quite small due to the rarity of disease. There were no cirrhotic livers either in the overlap or in the PBC group.
In conclusion, ARFI elastography correlates with fibrosis in AIH, PSC and overlap syndrome. Shear wave velocities in PBC should be interpreted with caution in early stages of fibrosis.
Abbreviations- •
AIH: autoimmune hepatitis.
- •
ALT: alanine transaminase.
- •
APRI: AST/platelet ratio index.
- •
ARFI: acoustic radiation force impulse.
- •
AST: aspartate transaminase.
- •
AUROC: area under the receiver operating characteristic.
- •
BMI: body mass index.
- •
CI: confidence interval.
- •
PBC: primary biliary cholangitis.
- •
PSC: primary sclerosing cholangitis.
- •
SD: standard deviation.
The authors declares that there is no conflict of interest regarding the publication of this article.
DisclosuresNo acknowledgmennts. No grants, no other financial support.