Autoimmune hepatitis (AIH) is a prevalent noninfectious liver disease. However, there is currently a lack of noninvasive tests appropriate for evaluating liver fibrosis in AIH patients. The objective of this study was to develop and validate a predictive model for noninvasive assessment of significant liver fibrosis (S ≥ 2) in patients to provide a reliable method for evaluating liver fibrosis in individuals with AIH.
Materials and MethodsThe clinical data of 374 AIH patients were analyzed. A prediction model was established through logistic regression in the training set, and bootstrap method was used to validate the models internally. In addition, the clinical data of 109 AIH patients were collected for external verification of the model.The model was expressed as a nomogram, and area under the curve (AUC) of the receiver operating characteristic (ROC), calibration curve, and decision curve analysis were used to evaluate the accuracy of the prediction model.
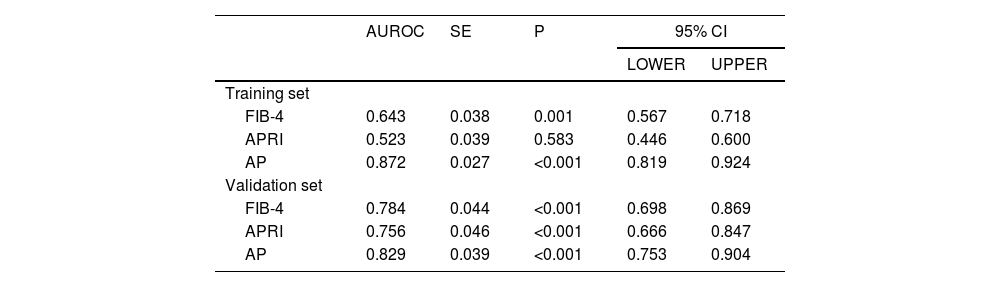
ResultsLogistic regression analysis revealed that age, platelet count (PLT), and the A/G ratio were identified as independent risk factors for liver fibrosis in AIH patients (P < 0.05). The diagnostic model that was composed of age, PLT and A/G was superior to APRI and FIB-4 in both the internal validation (0.872, 95%CI: 0.819–0.924) and external validation (0.829, 95%CI: 0.753–0.904).
ConclusionsOur predictive model can predict significant liver fibrosis in AIH patients more accurately, simply, and noninvasively.
Autoimmune hepatitis (AIH) is a liver inflammatory disease caused by immune abnormalities that lacks specific clinical symptoms and signs. It is marked by elevated circulating autoantibodies, increased concentrations of IgG, and unique histological features [1]. Clinically, it is believed to be caused by chronic and progressing inflammation of the liver, and in some cases, it eventually leads to liver inflammation, cirrhosis or failure and even death [2]. The diagnosis of this disease is challenging because the age of onset is wide, serological markers are diverse, and various clinical manifestations can range from silent disease to fulminant liver failure [3].
Liver fibrosis represents an inevitable stage in the progression from AIH to cirrhosis. Approximately 30% of patients with AIH have cirrhosis at diagnosis [1], and another 10% develop cirrhosis during follow-up [4]. Patients with untreated AIH progress to cirrhosis and may have complications such as liver failure, portal hypertension, and liver cancer. Although AIH patients achieve a biochemical response following treatment, due to the fluctuating progression of the disease, up to 40% of patients still have persistent histological activity, and fibrosis progression may still occur [5]. Therefore, patients with AIH require close, lifelong follow-up. Prednisone (or prednisolone) alone or in combination with azathioprine is considered the first-line treatment for AIH and can alleviate the symptoms of most AIH patients, achieve biochemical and histological remission, improve immediate and long-term survival, and prevent or reverse liver fibrosis [6]. In untreated patients, AIH progresses to cirrhosis at varying rates, with a subsequent risk of complications such as portal hypertension, liver failure, and hepatocellular carcinoma (HCC). Early treatment of liver fibrosis not only prevents the progression of fibrosis but also reverses it to a certain extent, resulting in a good long-term prognosis [7]. Therefore, the early diagnosis of hepatic fibrosis could have significant implications for the prognosis of AIH.
Liver biopsy is the gold standard for the diagnosis of liver fibrosis. Although the accuracy is extremely high, its repeatability is poor, it is not suitable for early liver fibrosis screening, and it is not suitable for regular monitoring as an invasive examination [8]. Traditional cortisol therapy is aimed at inhibiting inflammatory activity [9], and preventing or reversing liver fibrosis is not the main goal of treatment. At present, anti-fibrosis therapies are emerging [10,11], and in the development of anti-fibrosis interventions for autoimmune hepatitis, the first challenge is to determine the progression or regression of liver fibrosis during treatment in a reliable, noninvasive manner. Therefore, we aim to develop a repeatable and non-introgenic trauma method to assess the level of liver fibrosis in patients, which can be in determining the appropriate timing of treatment, treatment regimen, withdrawal time, etc. The currently proposed noninvasive serological models of liver fibrosis, such as AST to platelet ratio index (APRI), fibrosis-4 (FIB-4), etc., have shown poor accuracy in predicting liver fibrosis in AIH patients in existing studies [12–14]. FibroScan, a device used to detect liver fibrosis in clinical practice, is easily disturbed by liver inflammatory activity. Autoimmune hepatitis is a disease characterized by severe fluctuations in inflammatory activity, and FibroScan tends to overestimate liver fibrosis [15]. In addition, most of the existing serological diagnostic models were developed based on patients with viral hepatitis, and few noninvasive prediction models have been established based on liver fibrosis in patients with autoimmune hepatitis [12,16]. At present, no prediction models for significant liver fibrosis have been reported.
This study retrospectively collected the basic information and clinical data of AIH patients, aiming to establish a significant liver fibrosis diagnostic model suitable for AIH patients to evaluate liver fibrosis in patients with autoimmune hepatitis. At the same time, the accuracy of the model was tested by using DCA curve and calibration curve.
2Materials and Methods2.1Research objectPatients diagnosed with AIH who underwent liver biopsy at Zhejiang Provincial People's Hospital and the First People's Hospital of Xiaoshan District, Zhejiang Province, between January 2010 and October 2022 were enrolled. The diagnostic criteria for AIH were based on the simplified criteria recommended by IAIHG [17]. The exclusion criteria were as follows: (1) combined with viral hepatitis, alcoholic liver disease, nonalcoholic fatty liver disease, drug-induced liver injury, or other autoimmune liver disease; (2) hereditary and metabolic liver diseases, such as hepatolenticular degeneration and hepatic amyloidosis; (3) liver cancer and liver transplantation; (4) incomplete clinical data and laboratory test data; and (5) liver biopsy pathology that could not be used to stage liver fibrosis. In addition, the clinical data collected from Xixi Hospital, Affiliated with Zhejiang University, from January 2010 to May 2023, were used as the dataset for external independent validation.
2.2Data collectionThe clinical data of the patients were collected, including age, sex and serological examination results, within a week before liver biopsy. Including serum biochemistry (TBIL, DBIL, IBIL, TP, ALB, GLO, A/G, ALT, AST, ALT/AST, GGT, ALP, LDH, TBA, and CHO), coagulation function test (PT, INR and FIB), serum immunoglobulin (IgA, IgG and IgM), blood routine blood examination (Hb, RDW, and PLT), serum autoantibodies (antinuclear antibody (ANA), smooth muscle antibody (SMA), antimitochondrial antibody (AMA), type 1 liver and kidney microsomal antibody (LKM-1) and soluble liver antigen/liver-pancreas antigen (SLA/LP).
2.3Pathological diagnosis of hepatic fibrosisLiver biopsy specimens were immersed in 10% neutral formalin and sent to the Department of Pathology. The diagnosis was performed by a pathologist with more than five years of clinical experience and the histopathological results of the patients were collected. These samples were evaluated by experienced pathologists who were blinded to the clinical characteristics of the subjects. According to the Scheuer scoring system [18], liver fibrosis was divided into five stages. The degree of liver fibrosis S0-S1 was defined as no significant liver fibrosis, and S2–4 was defined as significant liver fibrosis [19].
2.4Statistical analysisAll the statistical analyses were performed using SPSS 25.0 software and the R 4.2.3 programming language. Continuous variables are expressed as the mean ± standard deviation or median (quartile range), and categorical variables are described by frequency. Continuous variables were compared by the t-test or rank sum test. The chi-square (X2) test or Fisher's exact test was used to compare categorical variables. Univariate and multivariate logistic regression were used to quantitatively analyze the influence of each factor on the dependent variable in the training data, and the prediction model was constructed. The Bootstrap method was used to verify the validation dataset internally, and the validation dataset was used for external verification. The model was expressed as a nomogram, and AUC, calibration curve, and decision curve analysis were used to evaluate the predictive accuracy of the model. P < 0.05 was considered to indicate statistical significance.
2.5Ethical statementThis study was approved by the local hospital's ethics committee and complied with the ethical guidelines of the Declaration of Helsinki(QT2023316). The researchers only analyzed anonymous data, so the Ethics Committee approved the waiver of informed consent.
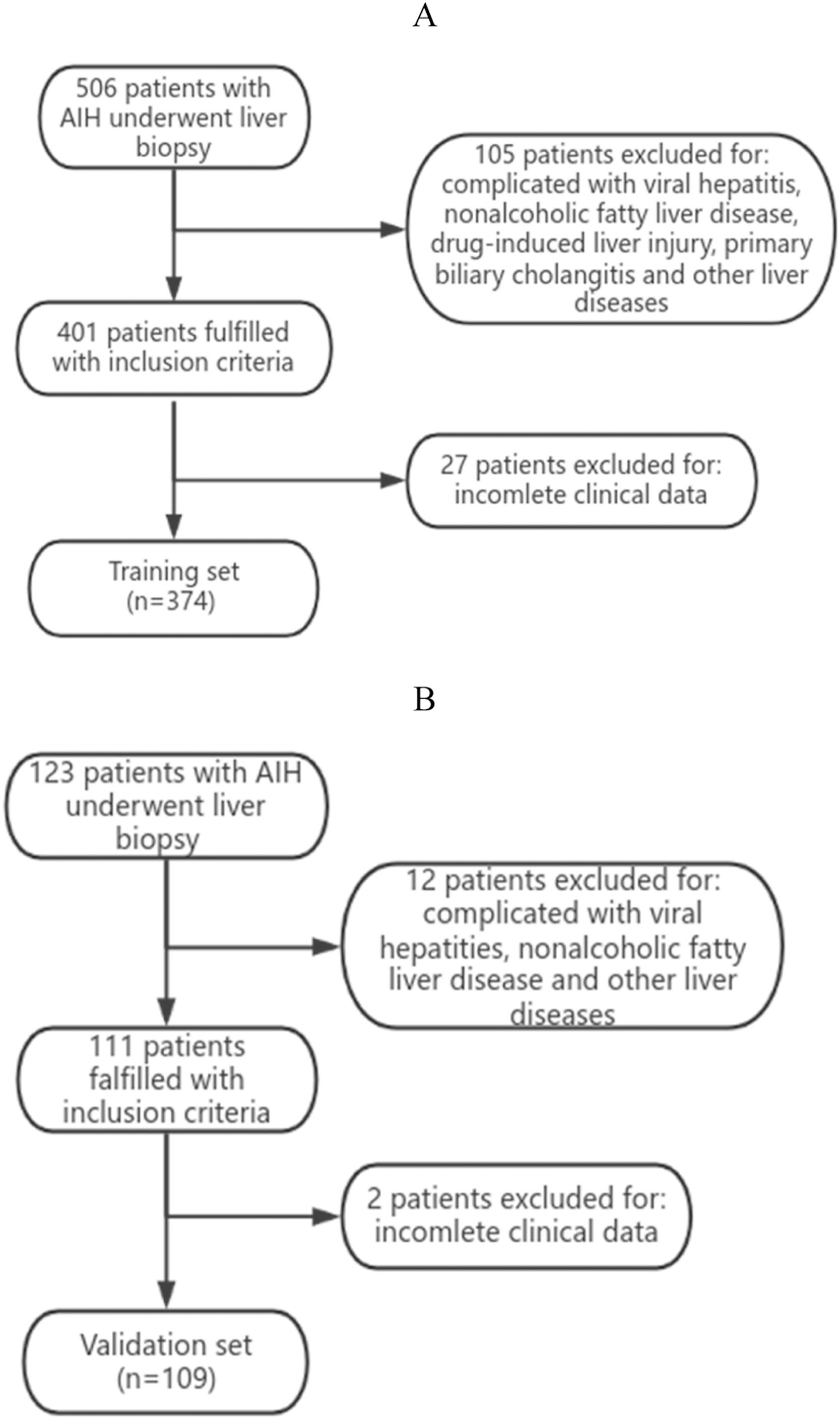
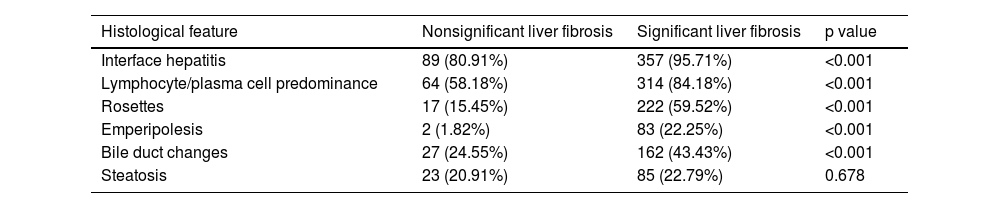
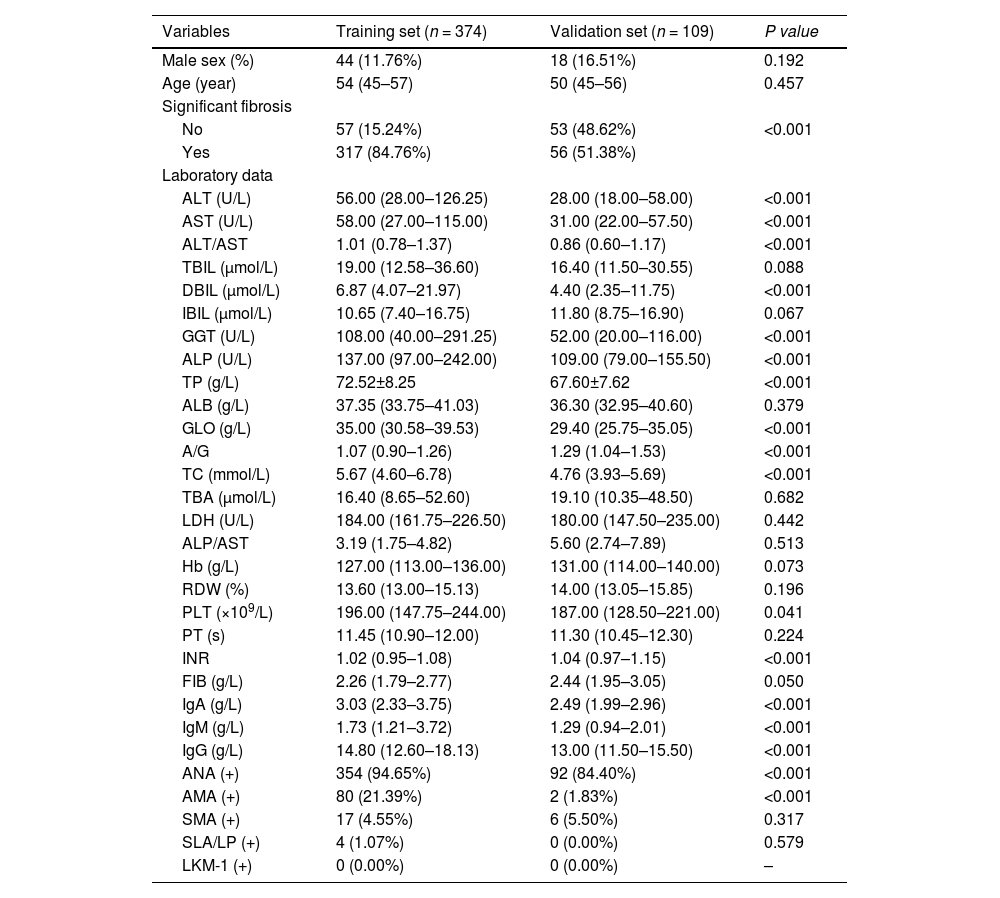
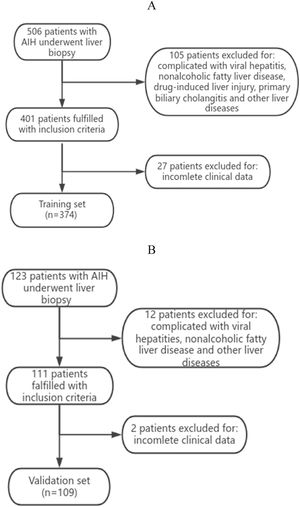
3Results3.1Analysis of the clinical laboratory parameters and histological characteristics of the patientsA total of 483 patients diagnosed with AIH met the inclusion criteria in this study (Fig. 1). A total of 62 male patients and 421 female patients were incleded. The age of the 483 patients ranged from 26 to 75 years old, with a median age of 53 years old. We summarized the histological features of AIH patients without significant liver fibrosis and significant liver fibrosis (Table 1). It can be seen that AIH patients with significant liver fibrosis have a higher probability of interface hepatitis, lymphocyte/plasma cell infiltration, rosettes, emperipolesis, and bile duct changes. A total of 374 patients from Zhejiang Provincial People's Hospital and the First People's Hospital of Xiaoshan District were used as the training group. A total of 109 patients from Xixi Hospital, affiliated with Zhejiang University, were included in the external validation group. The comparison of baseline information between the two groups is shown in Table 2.
Histological characteristics of the patients.
| Histological feature | Nonsignificant liver fibrosis | Significant liver fibrosis | p value |
|---|---|---|---|
| Interface hepatitis | 89 (80.91%) | 357 (95.71%) | <0.001 |
| Lymphocyte/plasma cell predominance | 64 (58.18%) | 314 (84.18%) | <0.001 |
| Rosettes | 17 (15.45%) | 222 (59.52%) | <0.001 |
| Emperipolesis | 2 (1.82%) | 83 (22.25%) | <0.001 |
| Bile duct changes | 27 (24.55%) | 162 (43.43%) | <0.001 |
| Steatosis | 23 (20.91%) | 85 (22.79%) | 0.678 |
Baseline comparison of patients in the training and validation groups.
| Variables | Training set (n = 374) | Validation set (n = 109) | P value |
|---|---|---|---|
| Male sex (%) | 44 (11.76%) | 18 (16.51%) | 0.192 |
| Age (year) | 54 (45–57) | 50 (45–56) | 0.457 |
| Significant fibrosis | |||
| No | 57 (15.24%) | 53 (48.62%) | <0.001 |
| Yes | 317 (84.76%) | 56 (51.38%) | |
| Laboratory data | |||
| ALT (U/L) | 56.00 (28.00–126.25) | 28.00 (18.00–58.00) | <0.001 |
| AST (U/L) | 58.00 (27.00–115.00) | 31.00 (22.00–57.50) | <0.001 |
| ALT/AST | 1.01 (0.78–1.37) | 0.86 (0.60–1.17) | <0.001 |
| TBIL (μmol/L) | 19.00 (12.58–36.60) | 16.40 (11.50–30.55) | 0.088 |
| DBIL (μmol/L) | 6.87 (4.07–21.97) | 4.40 (2.35–11.75) | <0.001 |
| IBIL (μmol/L) | 10.65 (7.40–16.75) | 11.80 (8.75–16.90) | 0.067 |
| GGT (U/L) | 108.00 (40.00–291.25) | 52.00 (20.00–116.00) | <0.001 |
| ALP (U/L) | 137.00 (97.00–242.00) | 109.00 (79.00–155.50) | <0.001 |
| TP (g/L) | 72.52±8.25 | 67.60±7.62 | <0.001 |
| ALB (g/L) | 37.35 (33.75–41.03) | 36.30 (32.95–40.60) | 0.379 |
| GLO (g/L) | 35.00 (30.58–39.53) | 29.40 (25.75–35.05) | <0.001 |
| A/G | 1.07 (0.90–1.26) | 1.29 (1.04–1.53) | <0.001 |
| TC (mmol/L) | 5.67 (4.60–6.78) | 4.76 (3.93–5.69) | <0.001 |
| TBA (μmol/L) | 16.40 (8.65–52.60) | 19.10 (10.35–48.50) | 0.682 |
| LDH (U/L) | 184.00 (161.75–226.50) | 180.00 (147.50–235.00) | 0.442 |
| ALP/AST | 3.19 (1.75–4.82) | 5.60 (2.74–7.89) | 0.513 |
| Hb (g/L) | 127.00 (113.00–136.00) | 131.00 (114.00–140.00) | 0.073 |
| RDW (%) | 13.60 (13.00–15.13) | 14.00 (13.05–15.85) | 0.196 |
| PLT (×109/L) | 196.00 (147.75–244.00) | 187.00 (128.50–221.00) | 0.041 |
| PT (s) | 11.45 (10.90–12.00) | 11.30 (10.45–12.30) | 0.224 |
| INR | 1.02 (0.95–1.08) | 1.04 (0.97–1.15) | <0.001 |
| FIB (g/L) | 2.26 (1.79–2.77) | 2.44 (1.95–3.05) | 0.050 |
| IgA (g/L) | 3.03 (2.33–3.75) | 2.49 (1.99–2.96) | <0.001 |
| IgM (g/L) | 1.73 (1.21–3.72) | 1.29 (0.94–2.01) | <0.001 |
| IgG (g/L) | 14.80 (12.60–18.13) | 13.00 (11.50–15.50) | <0.001 |
| ANA (+) | 354 (94.65%) | 92 (84.40%) | <0.001 |
| AMA (+) | 80 (21.39%) | 2 (1.83%) | <0.001 |
| SMA (+) | 17 (4.55%) | 6 (5.50%) | 0.317 |
| SLA/LP (+) | 4 (1.07%) | 0 (0.00%) | 0.579 |
| LKM-1 (+) | 0 (0.00%) | 0 (0.00%) | – |
ALT, alanine transaminase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin; GGT, -glutamyl transferase; ALP, alkaline phosphatase; TP, total protein; ALB, albumin; GLO, globulin; A/G, albumin-to-globulin ratio; TC, total cholesterol; TBA, total bile acid; LDH, lactate dehydrogenase; Hb, hemoglobin; RDW, red blood cell distribution width; PLT, platelet count; PT, prothrombin time; INR, national standardized ratio; FIB, fibrinogen; IgA, immunoglobulin A; IgM, immunoglobulin M; IgG, immunoglobulin G; ANA, antinuclear antibody; AMA, antimitochondrial antibody; SMA, smooth muscle antibody; SLA/LP, soluble liver antigen/liver-pancreas antigen; LKM-1, liver kidney microsomes type-1.
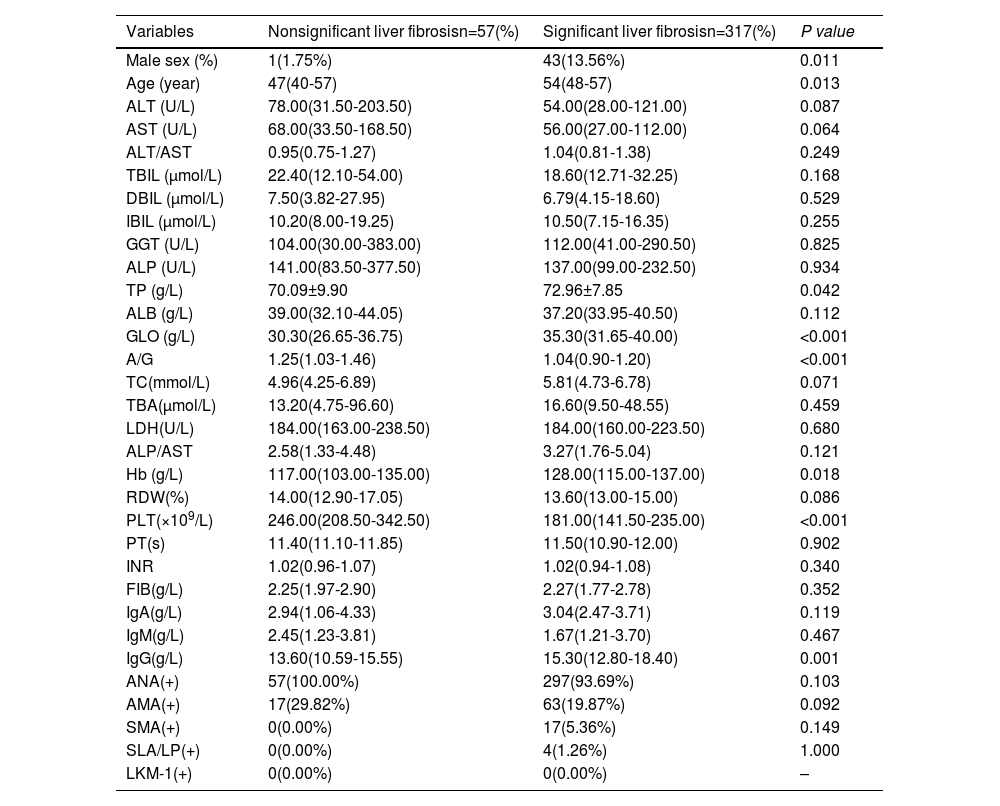
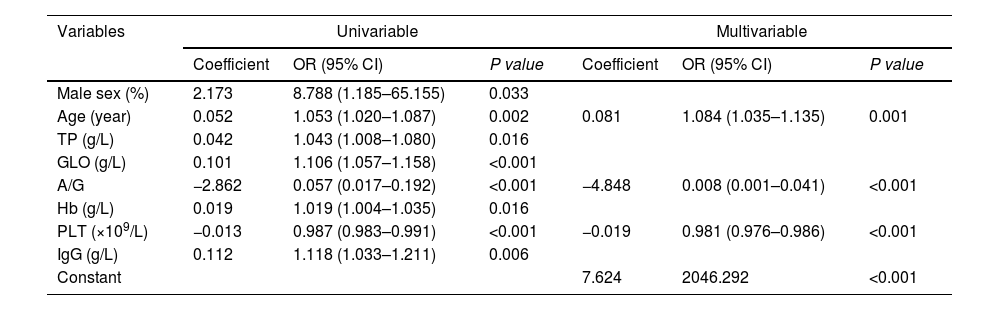
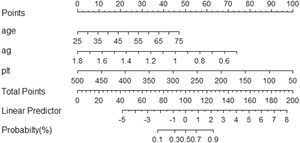
The Scheuer scoring system was used to classify liver fibrosis, and the 374 patients in the training group were then divided into non-significant liver fibrosis group (n = 57) and the significant liver fibrosis group (n = 317). There were statistically significant differences observed in sex, age, TP, GLO, A/G, Hb, PLT and IgG between the two groups (P<0.05) (Table 3). Univariate and multivariate logistic regression analyses showed that age, A/G and PLT were independent risk factors for significant liver fibrosis in AIH patients (P < 0.05) (Table 4). The corresponding prediction model was constructed with the above three indicators and named the AP model. The formula is as follows: AP = 7.624 + 0.081 × Age - 4.848 × A/G - 0.019 × PLT.
Comparison of baseline characteristics and clinical data of patients in no significant and significant liver fibrosis group in the training group
| Variables | Nonsignificant liver fibrosisn=57(%) | Significant liver fibrosisn=317(%) | P value |
|---|---|---|---|
| Male sex (%) | 1(1.75%) | 43(13.56%) | 0.011 |
| Age (year) | 47(40-57) | 54(48-57) | 0.013 |
| ALT (U/L) | 78.00(31.50-203.50) | 54.00(28.00-121.00) | 0.087 |
| AST (U/L) | 68.00(33.50-168.50) | 56.00(27.00-112.00) | 0.064 |
| ALT/AST | 0.95(0.75-1.27) | 1.04(0.81-1.38) | 0.249 |
| TBIL (μmol/L) | 22.40(12.10-54.00) | 18.60(12.71-32.25) | 0.168 |
| DBIL (μmol/L) | 7.50(3.82-27.95) | 6.79(4.15-18.60) | 0.529 |
| IBIL (μmol/L) | 10.20(8.00-19.25) | 10.50(7.15-16.35) | 0.255 |
| GGT (U/L) | 104.00(30.00-383.00) | 112.00(41.00-290.50) | 0.825 |
| ALP (U/L) | 141.00(83.50-377.50) | 137.00(99.00-232.50) | 0.934 |
| TP (g/L) | 70.09±9.90 | 72.96±7.85 | 0.042 |
| ALB (g/L) | 39.00(32.10-44.05) | 37.20(33.95-40.50) | 0.112 |
| GLO (g/L) | 30.30(26.65-36.75) | 35.30(31.65-40.00) | <0.001 |
| A/G | 1.25(1.03-1.46) | 1.04(0.90-1.20) | <0.001 |
| TC(mmol/L) | 4.96(4.25-6.89) | 5.81(4.73-6.78) | 0.071 |
| TBA(μmol/L) | 13.20(4.75-96.60) | 16.60(9.50-48.55) | 0.459 |
| LDH(U/L) | 184.00(163.00-238.50) | 184.00(160.00-223.50) | 0.680 |
| ALP/AST | 2.58(1.33-4.48) | 3.27(1.76-5.04) | 0.121 |
| Hb (g/L) | 117.00(103.00-135.00) | 128.00(115.00-137.00) | 0.018 |
| RDW(%) | 14.00(12.90-17.05) | 13.60(13.00-15.00) | 0.086 |
| PLT(×109/L) | 246.00(208.50-342.50) | 181.00(141.50-235.00) | <0.001 |
| PT(s) | 11.40(11.10-11.85) | 11.50(10.90-12.00) | 0.902 |
| INR | 1.02(0.96-1.07) | 1.02(0.94-1.08) | 0.340 |
| FIB(g/L) | 2.25(1.97-2.90) | 2.27(1.77-2.78) | 0.352 |
| IgA(g/L) | 2.94(1.06-4.33) | 3.04(2.47-3.71) | 0.119 |
| IgM(g/L) | 2.45(1.23-3.81) | 1.67(1.21-3.70) | 0.467 |
| IgG(g/L) | 13.60(10.59-15.55) | 15.30(12.80-18.40) | 0.001 |
| ANA(+) | 57(100.00%) | 297(93.69%) | 0.103 |
| AMA(+) | 17(29.82%) | 63(19.87%) | 0.092 |
| SMA(+) | 0(0.00%) | 17(5.36%) | 0.149 |
| SLA/LP(+) | 0(0.00%) | 4(1.26%) | 1.000 |
| LKM-1(+) | 0(0.00%) | 0(0.00%) | – |
ALT, alanine transaminase; AST, aspartate aminotransferase.
Univariate and multivariate logistic regression analysis of factors affecting significant liver fibrosis in AIH patients in the training set.
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| Coefficient | OR (95% CI) | P value | Coefficient | OR (95% CI) | P value | |
| Male sex (%) | 2.173 | 8.788 (1.185–65.155) | 0.033 | |||
| Age (year) | 0.052 | 1.053 (1.020–1.087) | 0.002 | 0.081 | 1.084 (1.035–1.135) | 0.001 |
| TP (g/L) | 0.042 | 1.043 (1.008–1.080) | 0.016 | |||
| GLO (g/L) | 0.101 | 1.106 (1.057–1.158) | <0.001 | |||
| A/G | −2.862 | 0.057 (0.017–0.192) | <0.001 | −4.848 | 0.008 (0.001–0.041) | <0.001 |
| Hb (g/L) | 0.019 | 1.019 (1.004–1.035) | 0.016 | |||
| PLT (×109/L) | −0.013 | 0.987 (0.983–0.991) | <0.001 | −0.019 | 0.981 (0.976–0.986) | <0.001 |
| IgG (g/L) | 0.112 | 1.118 (1.033–1.211) | 0.006 | |||
| Constant | 7.624 | 2046.292 | <0.001 | |||
TP, total protein; GLO, globulin; A/G, albumin-to-globulin ratio; Hb, hemoglobin; PLT, platelet count; IgG, immunoglobulin G.
The cut-off value and Youden index of the model were calculated. In the training group, when the patients were higher than the cut-off value (>1.917), there was significant liver fibrosis, and when the patients were lower than the cut-off value (<1.917), there was no significant liver fibrosis.
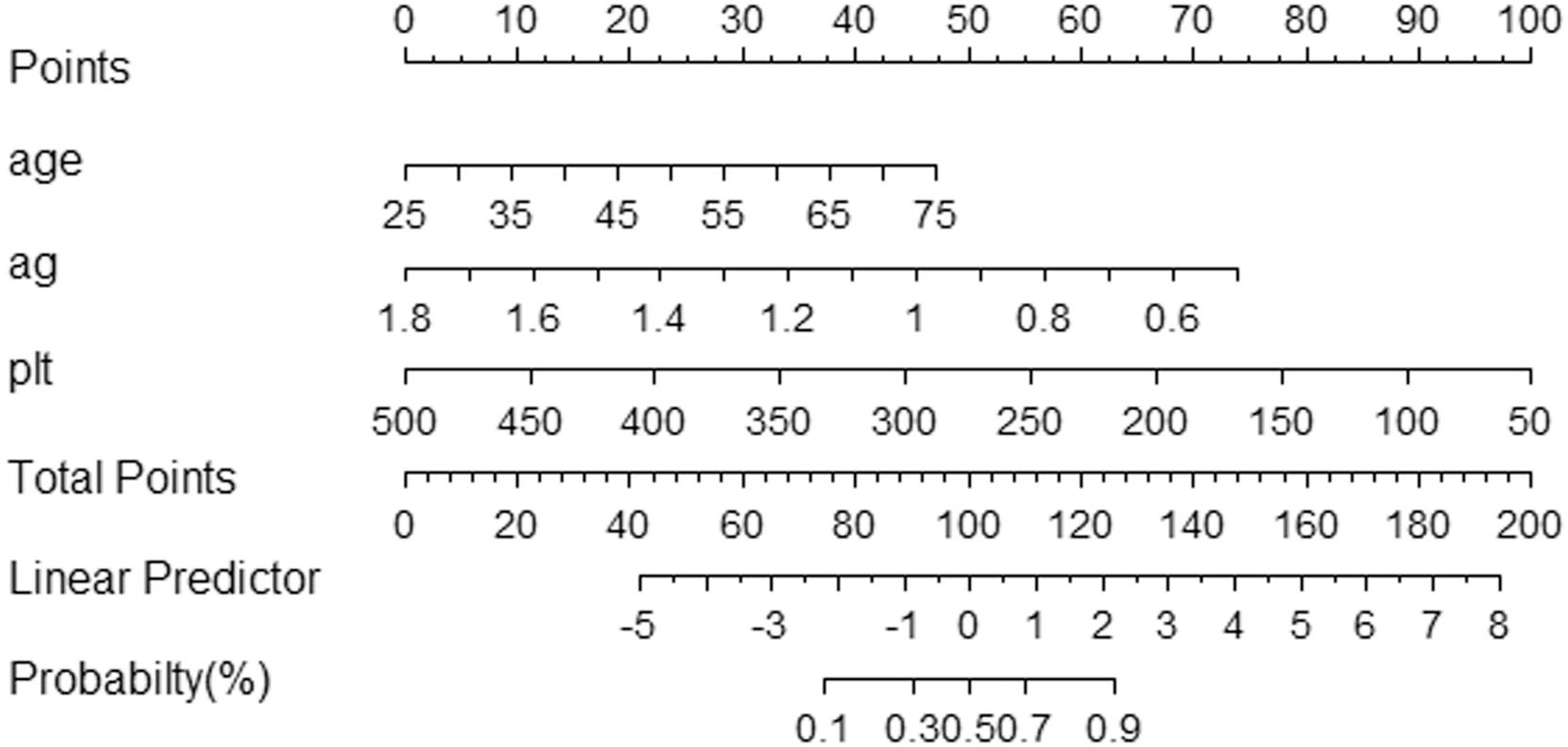
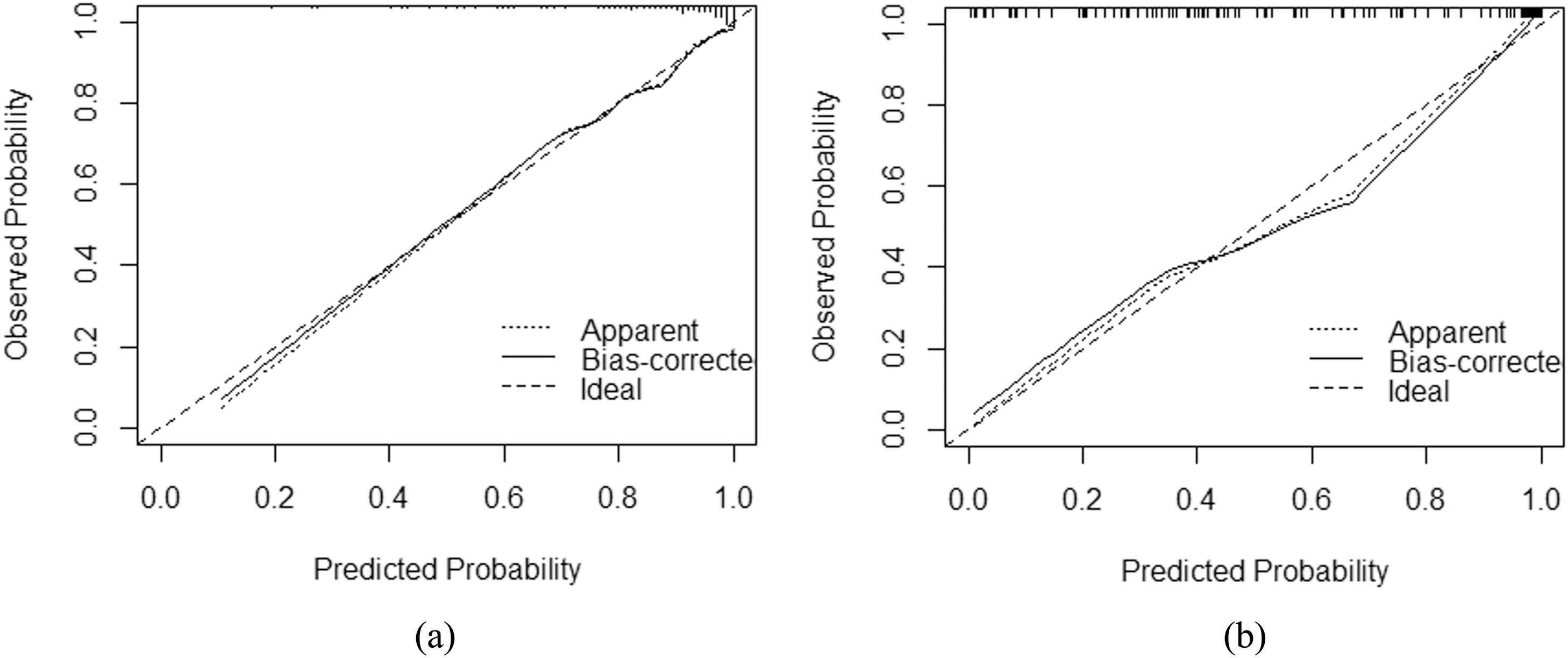
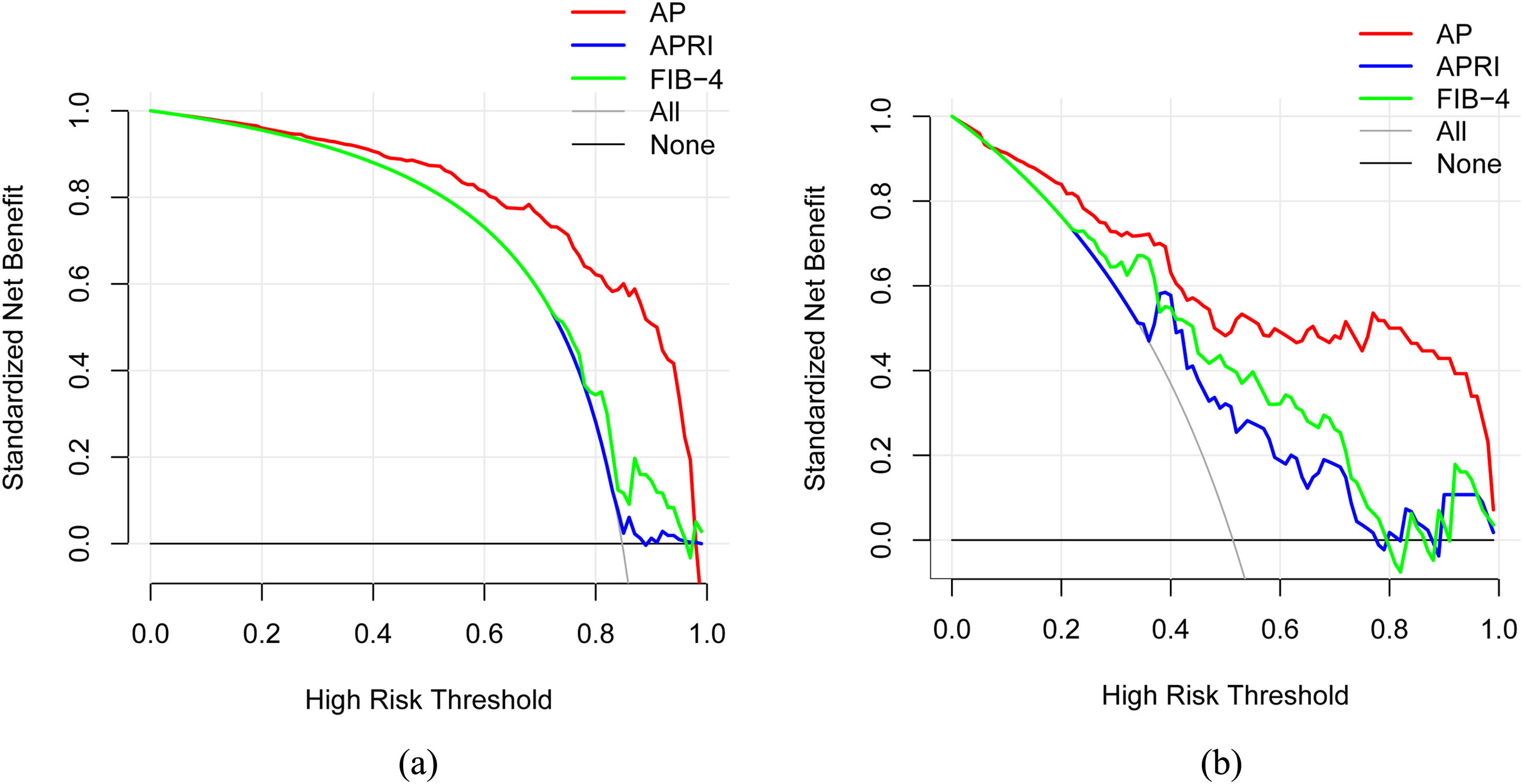
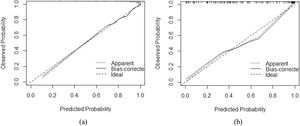
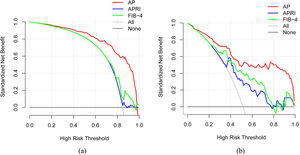
3.3Model comparison and verificationBased on the prediction model, we generated a clinical nomogram for model visualization and easy operation (Fig. 2). The training set was internally validated by bootstrap sampling 1000 times, and the AUCs were 0.872 (95% CI: 0.819–0.924) and 0.829 (95% CI: 0.753–0.904), which were better than FIB-4 and APRI. This suggests that the diagnostic performance of the established prediction model is higher than that of the above serological model (Table 5). In the calibration plot, the calibration line and, the ideal line apparent curve (actual) and the deviation correction curve (1000 bootstrap adjustments) are very close to the ideal curve and show good consistency in the training set and the verification set (Fig. 3). At the same time, we established a decision curve analysis (Fig. 4) to evaluate clinical utility. We can conclude that the model has obvious net benefits and high clinical application value.
AUROC values for comparing the different models.
| AUROC | SE | P | 95% CI | ||
|---|---|---|---|---|---|
| LOWER | UPPER | ||||
| Training set | |||||
| FIB-4 | 0.643 | 0.038 | 0.001 | 0.567 | 0.718 |
| APRI | 0.523 | 0.039 | 0.583 | 0.446 | 0.600 |
| AP | 0.872 | 0.027 | <0.001 | 0.819 | 0.924 |
| Validation set | |||||
| FIB-4 | 0.784 | 0.044 | <0.001 | 0.698 | 0.869 |
| APRI | 0.756 | 0.046 | <0.001 | 0.666 | 0.847 |
| AP | 0.829 | 0.039 | <0.001 | 0.753 | 0.904 |
FIB-4, fibrosis-4; APRI, the AST/platelet ratio index; AP, AP model.
AIH is an incurable chronic inflammatory liver disease that can lead to cirrhosis, HCC, decompensation and death after immunosuppressive therapy. According to statistics, approximately one-third of patients with AIH already have signs of liver cirrhosis when they are first diagnosed, and AIH patients who receive immunosuppressive therapy still progress to liver cirrhosis at a rate ranging from 0.1 to 8.1% per year [20]. In clinical practice, it is impossible to use a sequential biopsy to regularly monitor the stages of liver fibrosis. Therefore, noninvasive assessment of fibrosis is of great significance. First, a small number of AIH patients showed mild elevations in transaminases and serum IgG levels at the time of first detection, while inflammatory activity and fibrosis progression in the liver may not be as easy as serological manifestations [21]. In these patients, the indications for starting immunotherapy are not obvious, but no treatment may also lead to the rapid progression of liver fibrosis. Secondly, after receiving immunosuppressive drugs for biochemical remission, normal transaminase levels do not rule out the activity of liver inflammation and progressive fibrosis [22]. Therefore, developing accurate, noninvasive methods to assess disease progression and guide treatment is crucial. We established a model of significant liver fibrosis in patients with AIH In order to predict early liver fibrosis in AIH.
First, we screened out AIH patients through simplified criteria. The inclusion criteria were determined by referring to the AIH diagnostic scoring system and previously reported factors related to liver fibrosis. Then, the model was established by univariate and multivariate logistic regression analysis. We found that three indicators, including age, A/G, and PLT were independent predictors of significant liver fibrosis in patients with autoimmune hepatitis. Age was a risk factor, while A/G and PLT were protective factors. These indicators are often routinely tested on admission and are very easy to obtain.
Age is the main factor of increased susceptibility to fibrosis [23]. The models currently reported, such as FIB-4 [24], HB-F [25], and King's score [26], all include age as a variable and a number of studies have shown that liver fibrosis is positively correlated with age [27–29]. Our study also confirmed that age is an independent risk factor for the development of significant liver fibrosis in patients with AIH. Some studies have found that [30] the age distribution of the onset of AIH is a double-peak distribution, and the two peaks are located around puberty and 40–60 years old. In this study, 74.6% of the patients with AIH were between 40 and 60 years old, which was consistent with the above conclusion. The reason for the bimodal distribution of age may be that when women enter puberty and menopause, a significant change in estrogen levels will lead to the occurrence of autoimmune diseases [31,32].
In this study, A/G is a protective factor for significant liver fibrosis in AIH patients. It has been reported in the literature that A/G levels may decrease with the progression of liver fibrosis [33,34]. The elevation of IgG and/or γ-globulins is one of the characteristic serological changes of AIH [35]. With the development of liver fibrosis, the liver synthesis function of patients with liver cancer is damaged; therefore, the synthesis of ALB is reduced, the production of GLO is increased, and the A/G ratio is gradually reduced. The A/G ratio is a good indicator for observing the progression of chronic liver disease [36–39]. Currently, there is no cohort study on AIH to verify the relationship between A/G and liver fibrosis. However, studies on viral liver and nonalcoholic fatty liver have shown that A/G levels are negatively related to the degree of liver fibrosis in patients with chronic hepatitis B and nonalcoholic fatty liver [25,33,34]. For example, Sripongpun et al. [34] reported a new fibrosis-8 score (FIB-8) model. In their model, A/G levels were a protective factor for significant liver fibrosis in patients with nonalcoholic fatty liver disease.
PLT is also an independent risk factor for significant liver fibrosis in AIH patients. Thrombocytopenia is a common complication associated with liver cirrhosis, which is exacerbated by decreased circulating platelet count due to an enlarged spleen, increased platelet consumption, and decreased synthesis and increased degradation of thrombopoietin (TPO) [40,41]. In addition to thrombocytopenia, qualitative platelet defects may occur, which may be due to decreased TXA 2 synthesis, decreased ATP and serotonin concentrations in dense granules, increased platelet inhibitor production, and impaired response to profibrotic agents [42], ultimately leading to PLT decline. Numerous studies have shown that PLT counts in patients with AIH are inversely correlated with the degree of fibrosis [43,44], which is consistent with our current study.
In this study, we constructed a new noninvasive model for predicting significant liver fibrosis in AIH, which was named AP model. The AUROC of AP model was superior to APRI and FIB-4 in internal validation (0.872,95% CI: 0.819–0.924) and external validation (0.829,95% CI: 0.753–0.904). In the training cohort, when the cut-off value was 1.917, the sensitivity of AIH was 75.7% and the specificity was 86.0%. This method successfully predicted significant fibrosis in 240 patients (75.71%) in the training set and 40 patients (71.43%) in the validation set. It shows that the model has good practicability in the noninvasive diagnosis of significant liver fibrosis in AIH.
This study also found that APRI and FIB-4 had poor accuracy in predicting significant liver fibrosis in AIH. The AUROC of APRI was 0.523(95%CI: 0.446–0.600) in the training group and 0.756 (95%CI: 0.666–0.847) in the validation group. The AUROC of FIB-4 was 0.643 (95%CI: 0.567–0.718) in the training group and 0.784 (95%CI: 0.698–0.869) in the validation group. The AUROC of the two models is smaller than that of the AP model. There was no strong correlation between APRI and FIB-4 scores and fibrosis stage in the training cohort. However, in the external validation cohort, these two scores have certain diagnostic values. The reason may be that the results are more heterogeneous because of the small sample size. It may also be because APRI and FIB-4 were originally developed based on the data of patients with chronic hepatitis C, and the diagnostic efficacy for AIH was poor [45–47]. In previous studies, the prediction accuracy of APRI and FIB-4 for AIH liver fibrosis also showed heterogeneity. From the meta-analysis of APRI and FIB-4 in the detection of AIH liver fibrosis by Bingtian Dong et al. [43], they believed that APRI (AUC: 0.66, 95%CI: 0.61–0.70) and FIB-4 (AUC: 0.75,95%CI: 0.71–0.79) were not ideal for the diagnosis of AIH liver fibrosis. The current number of published original articles on APRI and FIB-4 for assessing AIH liver fibrosis is limited, and the accuracy of APRI and FIB-4 requires large-scale and multi-center studies to further evaluate.
In addition, we also used a variety of methods to analyze the efficacy of the model. The calibration curve showed a strong consistency between the predicted value and the actual observed value. DCA showed that the model had high clinical applicability.
Our study has several advantages. First, according to current reports, this study established a noninvasive model to predict significant liver fibrosis in AIH patients for the first time [16,48-50]. Second, it was a multi-center and retrospective study, which reduces the differences in patient composition between different hospitals. Third, an independent cohort was recruited for external verification to provide a more convincing conclusion for the accuracy of predicting liver fibrosis. Fourth, the model is based on the relevant clinical data of AIH patients, which is more applicable to AIH patients. Fifth, the model was used to predict significant liver fibrosis in AIH patients, which is conducive to early intervention for liver fibrosis to obtain a better prognosis. Sixth, the variables in the model were determined by serological indicators, which are common laboratory tests and can be easily obtained at all levels of medical institutions. It is scientific to use the results of liver biopsy as an index to evaluate the degree of liver fibrosis. Therefore, the model could be used as a noninvasive indicator in primary hospitals to assess whether patients have significant liver fibrosis.
However, our research still has certain limitations. First, a total of 473 patients were included in this study, so the sample size was not particularly large. In future research, we will continue to train and verify our model through subsequent large-scale multi-center research and external verification studies. Second, the population included in this study is Chinese patients, so it is not clear whether the model is applicable to other races. Future studies need to evaluate the applicability of the model in a more diverse patient population.
5ConclusionsWe constructed a noninvasive, highly accurate model to distinguish whether AIH patients have significant liver fibrosis. This model is clinically beneficial to reduce the need for liver biopsy, helps the clinician to identify and treat these high-risk patients early and follow-up of AIH patients, and assists in formulating strategies and timing of immunosuppressive therapy in order to obtain a better prognosis and even reverse liver fibrosis.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributionsHongying Pan is the guarantor of the article. Specific author contributions: Hongying Pan and Jing Xu were responsible for project conceptualization; Hanzhu Chen: performed the research; Hanzhu Chen, Shouhao Wang and Chengan Xu: collected and analyzed the data; Hanzhu Chen wrote the manuscript; Wei Zheng, Qiaoqiao Yin, Fei Lv and Yue Zhao: critically reviewed the manuscript. All authors approved the final version of the article including the authorship list.