Assessment of liver inflammation plays a vital role in the management of patients with autoimmune hepatitis (AIH). We aimed to establish and validate a nomogram to predict severe liver inflammation in AIH patients.
Patients and MethodsAIH patients who underwent liver biopsy were included and randomly divided into a training set and a validation set. Independent predictors of severe liver inflammation were selected by the least absolute shrinkage and selection operator regression from the training set and used to conduct a nomogram. Receiver characteristic curves (ROC), calibration curves, and decision curve analysis (DCA) were adopted to evaluate the performance of nomogram.
ResultsOf the 213 patients, female patients accounted for 83.1% and the median age was 53.0 years. The albumin, gamma-glutamyl transpeptidase, total bilirubin, red cell distribution width, prothrombin time, and platelets were independent predictors of severe inflammation. An online AIHI-nomogram was established and was available at https://ndth-zzy.shinyapps.io/AIHI-nomogram/. The calibration curve revealed that the AIHI-nomogram had a good agreement with actual observation in the training and validation sets. The area under the ROCs of AIHI-nomogram were 0.795 in the training set and 0.759 in the validation set, showing significantly better performance than alanine aminotransferase and immunoglobulin G in the training and validation sets, as well in AIH patients with normal ALT in the training set. DCA indicated that the AIHI-nomogram was clinically useful.
ConclusionsThis novel AIHI-nomogram provided an excellent prediction of severe liver inflammation in AIH patients and could be used for the better management of AIH.
Autoimmune hepatitis (AIH) is an inflammatory liver disease characterized by elevated levels of serum transaminase and immunoglobulin G (IgG), hypergammaglobulinemia, presence of autoantibodies, and interface hepatitis on liver histology [1–3]. Chronic and persistent liver inflammation is the main liver pathological feature in patients with AIH, which may lead to liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [4–6]. A majority of patients with AIH require immunosuppressive treatment [7]. Accurate measurement of liver inflammation may contribute to treatment initiation or modifications and is critical for the management of AIH patients in clinical practice [8].
Liver biopsy remains the gold standard to assess liver inflammation and fibrosis in AIH patients [9]. However, it is an expensive and invasive procedure. Many patients are unwilling to undergo a second liver biopsy [10,11]. Several noninvasive parameters such as serum alanine aminotransferase (ALT) and IgG are routinely used to assess the severity of liver inflammation in liver diseases. However, studies have demonstrated that the severity of inflammation is not always consistent with serum ALT and IgG levels in patients with AIH, and some AIH patients with normal ALT and IgG levels may have severe liver inflammation [12–16]. Therefore, developing a non-invasive model with good performance to assess the severity of liver inflammation in AIH patients is urgently needed. Patients with higher grades of inflammation are at greater risk of developing cirrhosis and should receive timely and appropriate intervention. The prediction of severe inflammation in patients with AIH has, however, received minimal attention. The noninvasive predictors for liver inflammation may contribute to assessing the severity of diseases, monitoring the disease progression, and more importantly indicating the histological remission after treatment for AIH patients.
This study aimed to establish and validate a novel nomogram for predicting severe liver inflammation in AIH patients.
2Patients and Methods2.1PatientsAIH patients who underwent liver biopsy between August 2011 and December 2020 in four medical centers (Nanjing Drum Tower Hospital; The Second Hospital of Nanjing; The Affiliated Infectious Diseases Hospital of Soochow University; The Fifth People's Hospital of Wuxi) were retrospectively included in the present study. The diagnosis of AIH was based on clinical, biochemical, serological as well as histopathological findings according to the diagnostic criteria of guidelines [8,17]. We excluded patients complicated with viral hepatitis or Epstein-Barr virus infection, drug-induced liver injury, nonalcoholic fatty liver disease (NAFLD), primary biliary cirrhosis, primary sclerosing cholangitis, or alcoholic liver disease.
2.2Data acquisitionThe medical records of each patient were reviewed. The demographic characteristics, laboratory results, and clinical information, including age, sex, blood routine test, liver function test, and coagulation function test were collected using a unified data frame. The upper limits of normal (ULNs) of ALT was defined as 40 IU/L.
2.3Liver histological assessmentAll selected patients underwent ultrasound-guided liver biopsy with at least 1 cm length, including six or more available portal tracts. The samples were evaluated by experienced pathologists who were blinded to the clinical characteristics of the subject. Liver inflammation activity was graded according to the Scheuer scoring system [18]. Grade (G) 0-1, G2 and G3-4 of liver inflammation were defined as no or mild inflammation, moderate inflammation and severe inflammation, respectively.
2.4Statistical analysisContinuous variables were shown as the median and interquartile range (IQR) and differences were compared using Student's t test or the Wilcoxon signed rank test. Categorical variables were presented as frequencies and percentages and were compared by the Chi-square test or Fisher exact test. Patients were randomly divided into a training set and a validation set in a ratio of 2:1. The least absolute shrinkage and selection operator (LASSO) regression was used in the training set to select predictors of severe liver inflammation. We built a predictive model by introducing the variables selected in the LASSO regression and presented the model in the form of an online dynamic nomogram (https://ndth-zzy.shinyapps.io/AIHI-nomogram/). Next, we evaluated the accuracy of the predictive model in the training set and validation set, respectively. The total scores of each patient were calculated based on the nomogram. We applied area under the receiver operating characteristic curve (AUROC) for measuring the discrimination. DeLong's test was used to compare AUROCs. The sensitivity, specificity, accuracy, positive predictive value (PPV), negative predictive value (NPV) and optimal cutoff value were calculated by the highest Youden's Index. To assess the degree of calibration of the risk nomogram, we conducted a calibration curve with 500 times bootstrap resampling and Hosmer-Lemeshow (HL) test. In addition, we performed decision curve analysis (DCA) to measure the clinical usefulness of nomograms based on the net benefit under threshold probability. The correlation between nomogram scores and liver inflammation grades was analyzed by the Spearman rank correlation. The difference at P < 0.05 (two-tailed) is considered statistically significant. All statistical analysis was performed using R software (version 4.1.3; R Foundation, Vienna, Austria; www.R-project.Org).
2.5Ethical statementsThe Ethics Committee of local hospitals authorized this study in conformity with the ethical principles of the Helsinki Declaration. All participants provided written informed consent before the liver biopsy.
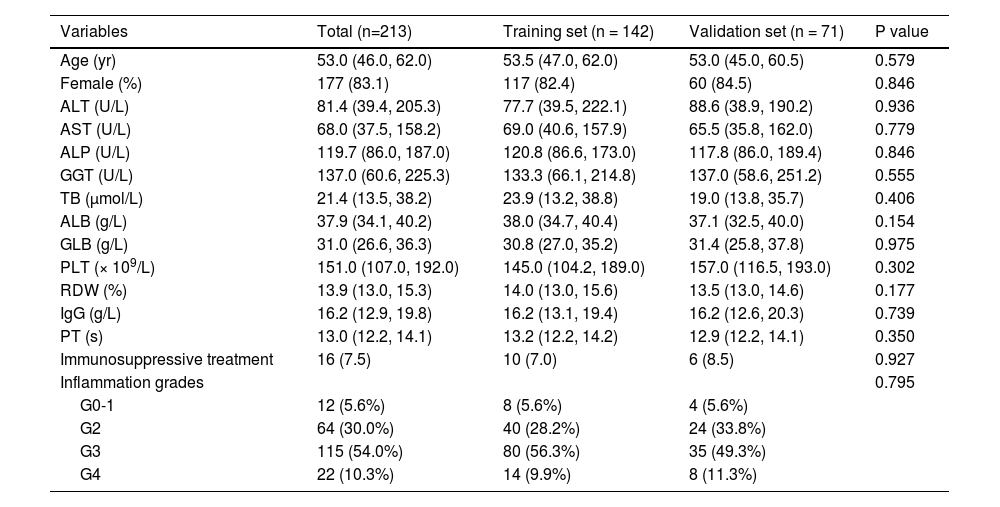
3Results3.1Clinical characteristics of patients with autoimmune hepatitisAmong the 277 patients with AIH who fulfilled with inclusion criteria, 64 patients with insufficient data were not included in the final analysis (Fig. 1). In a total of 213 patients with AIH, the proportion of female was 83.1% and the median age was 53.0 (IQR: 46.0-62.0) years. The median levels of platelets (PLT), red cell distribution width (RDW), ALT, gamma-glutamyl transpeptidase (GGT), and IgG were 151.0 (IQR: 107.0-192.0) × 109/L, 13.9 (IQR: 13.0-15.3) %, 81.4 (IQR: 39.4-205.3) U/L, 137.0 (IQR: 60.6-225.3) U/L, and 16.2 (IQR: 12.9-19.8) g/L, respectively (Table 1). Biopsies were taken after immunosuppressive treatment in 7.5% (16/213) of patients with AIH. The distribution of inflammation grades was as follows: G0-1, 12 (5.6%) patients; G2, 64 (30.0%) patients; G3, 115 (54.0%) patients; and G4, 22 (10.3%) patients (Fig. S1).
Characteristics for patients with autoimmune hepatitis.
| Variables | Total (n=213) | Training set (n = 142) | Validation set (n = 71) | P value |
|---|---|---|---|---|
| Age (yr) | 53.0 (46.0, 62.0) | 53.5 (47.0, 62.0) | 53.0 (45.0, 60.5) | 0.579 |
| Female (%) | 177 (83.1) | 117 (82.4) | 60 (84.5) | 0.846 |
| ALT (U/L) | 81.4 (39.4, 205.3) | 77.7 (39.5, 222.1) | 88.6 (38.9, 190.2) | 0.936 |
| AST (U/L) | 68.0 (37.5, 158.2) | 69.0 (40.6, 157.9) | 65.5 (35.8, 162.0) | 0.779 |
| ALP (U/L) | 119.7 (86.0, 187.0) | 120.8 (86.6, 173.0) | 117.8 (86.0, 189.4) | 0.846 |
| GGT (U/L) | 137.0 (60.6, 225.3) | 133.3 (66.1, 214.8) | 137.0 (58.6, 251.2) | 0.555 |
| TB (μmol/L) | 21.4 (13.5, 38.2) | 23.9 (13.2, 38.8) | 19.0 (13.8, 35.7) | 0.406 |
| ALB (g/L) | 37.9 (34.1, 40.2) | 38.0 (34.7, 40.4) | 37.1 (32.5, 40.0) | 0.154 |
| GLB (g/L) | 31.0 (26.6, 36.3) | 30.8 (27.0, 35.2) | 31.4 (25.8, 37.8) | 0.975 |
| PLT (× 109/L) | 151.0 (107.0, 192.0) | 145.0 (104.2, 189.0) | 157.0 (116.5, 193.0) | 0.302 |
| RDW (%) | 13.9 (13.0, 15.3) | 14.0 (13.0, 15.6) | 13.5 (13.0, 14.6) | 0.177 |
| IgG (g/L) | 16.2 (12.9, 19.8) | 16.2 (13.1, 19.4) | 16.2 (12.6, 20.3) | 0.739 |
| PT (s) | 13.0 (12.2, 14.1) | 13.2 (12.2, 14.2) | 12.9 (12.2, 14.1) | 0.350 |
| Immunosuppressive treatment | 16 (7.5) | 10 (7.0) | 6 (8.5) | 0.927 |
| Inflammation grades | 0.795 | |||
| G0-1 | 12 (5.6%) | 8 (5.6%) | 4 (5.6%) | |
| G2 | 64 (30.0%) | 40 (28.2%) | 24 (33.8%) | |
| G3 | 115 (54.0%) | 80 (56.3%) | 35 (49.3%) | |
| G4 | 22 (10.3%) | 14 (9.9%) | 8 (11.3%) |
ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; GLB, globulin; IgG, immunoglobulin G; PLT, platelets; PT, prothrombin time; RDW, red cell distribution width; TB, total bilirubin.
The patients were randomly divided into a training set and a validation set in a 2:1 ratio. The clinical characteristics of patients in the two sets were presented in Table 1. The clinical features and liver pathology distribution were comparable between the training set and validation set.
3.2Comparison of biochemical and clinical features of patients with autoimmune hepatitis with and without severe inflammation in the training setPatients with severe liver inflammation accounted for 66.2% of the training set. As shown in Table S1, patients with severe inflammation had higher median levels of aspartate aminotransferase (AST) (79.4 U/L vs. 47.0 U/L, P = 0.002), GGT (146.1 U/L vs. 95.7 U/L, P = 0.021), total bilirubin (TB) (27.0 μmol/L vs. 14.6 μmol/L, P < 0.001), RDW (14.6% vs. 13.0%, P < 0.001), and prothrombin time (PT) (13.5s vs. 12.6s, P < 0.001) compared to patients with non-severe inflammation, while had lower median levels of albumin (ALB) (37.3 g/L vs. 39.0 g/L, P < 0.001) and PLT (124.5 × 109/L vs. 184.0 × 109/L, P < 0.001). However, the proportion of female and the median age were comparable between patients with and without severe inflammation.
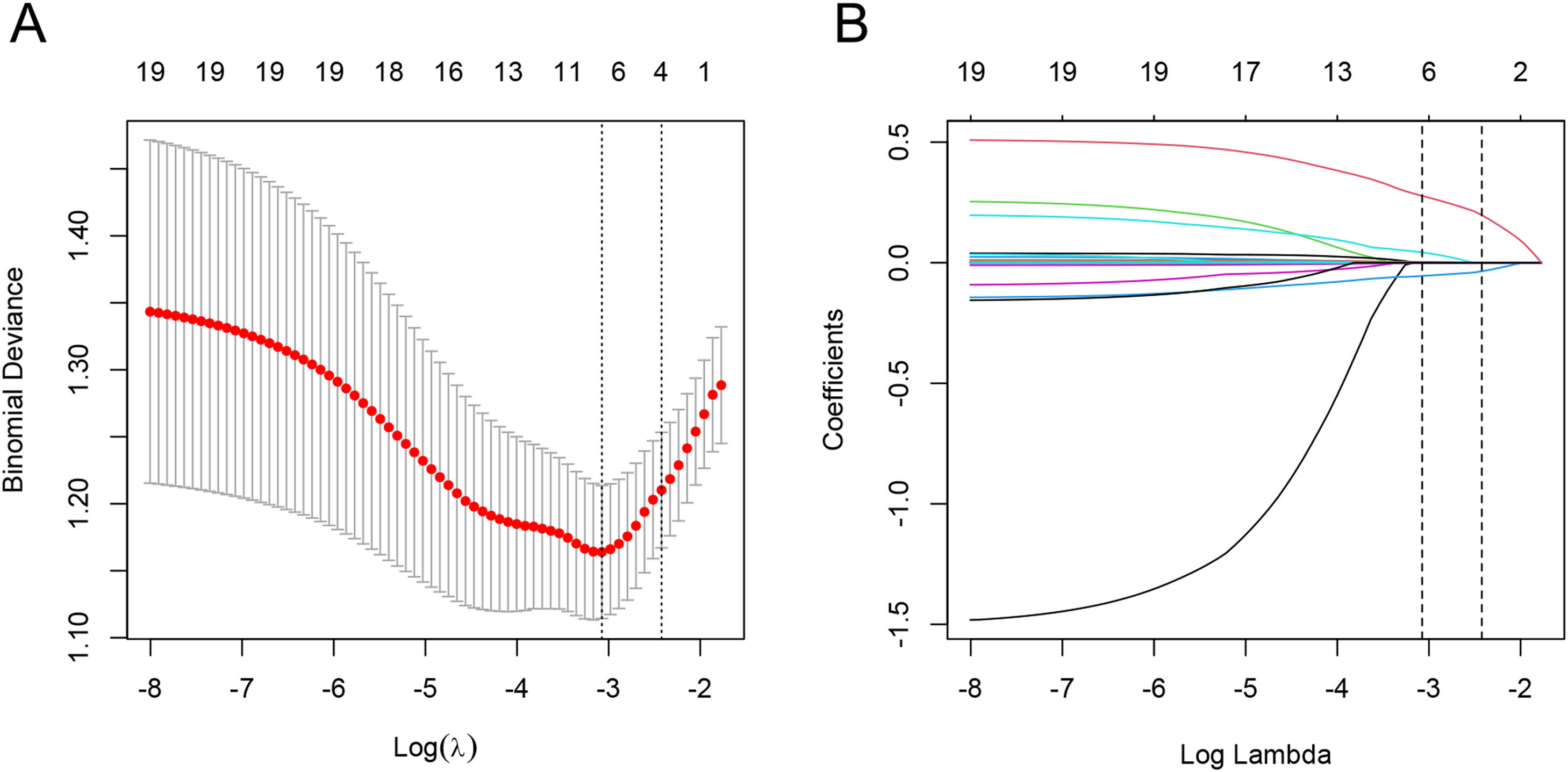
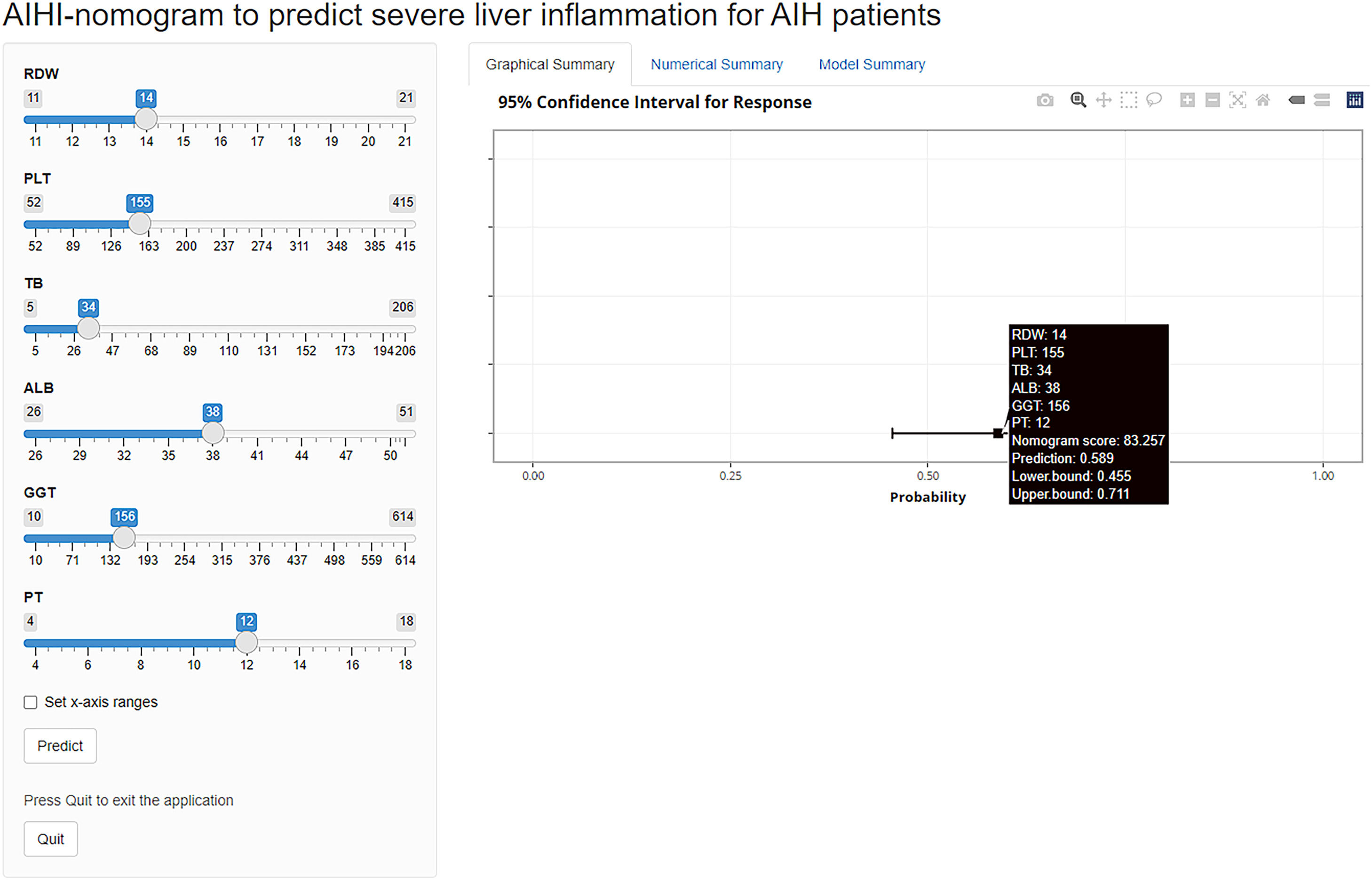
3.3Development of a nomogram estimating severe inflammationIn the process of variable selection for estimating severe inflammation, we utilized the LASSO method. Specifically, we applied LASSO regression analysis with tenfold cross-validation to the training set of 20 candidate variables. The tuning parameter (lambda) was selected based on deviance, using both the minimum criteria and the 1-SE criteria (Fig. 2A). Based on the minimum criteria, six variables with non-zero coefficients were selected and included in the final model (Fig. 2B). The variables included ALB, GGT, TB, RDW, PT, and PLT. These variables were utilized to build the prediction model and depicted as an AutoImmune Hepatitis Inflammation (AIHI)-nomogram which is freely available online at https://ndth-zzy.shinyapps.io/AIHI-nomogram/. To utilize this nomogram, by dragging data bar of the predictor variables and clicking the “predict” button, the total nomogram score and probability that a patient is predicted to be severe inflammation can be obtained. For example, an AIH patient with a PLT of 155 × 109/L, PT of 12 s, RDW of 14%, TB of 34 μmol/L, GGT of 156 U/L, and ALB of 38 g/L has a total nomogram score of 83.3 as well as a diagnosed probability of severe liver inflammation of 58.9%, according to the online AIHI-nomogram as a screenshot (Fig. 3).
Feature selection using the LASSO regression analysis with ten-fold cross-validation. (A) Deviance-based tuning parameter (lambda) selection in the LASSO regression was performed using the minimum criteria (left dotted line) and the 1-SE criteria (right dotted line). (B) A coefficient profile plot against the log (lambda) sequence was generated, and feature selection was performed based on the minimum criteria (left dotted line), where 6 nonzero coefficients were selected.
Online AIHI-nomogram for the prediction of severe inflammation in patients with autoimmune hepatitis. To use the online AIHI-nomogram, first input the values of ALB, GGT, TB, RDW, PT, and PLT into the tool. Then, click the “Predict” button to generate a graphical summary that displays the total nomogram score and corresponding probability of severe liver inflammation of the patient. Additionally, the numerical summary provides the values of probability and 95% CI.
The calibration curve and HL test were used to evaluate this predictive model, the apparent curve (actual) and bias-corrected curve (500 times bootstrapped adjusted) were all very close to the ideal curve which showed good agreement in both the training set and validation set. In the training set, the HL test revealed a good accuracy (P = 0.279), indicating that the model fits well. The HL test statistics in the validation set likewise demonstrated no statistical difference between AIHI-nomogram prediction and actual observation (P = 0.056) (Fig. 4).
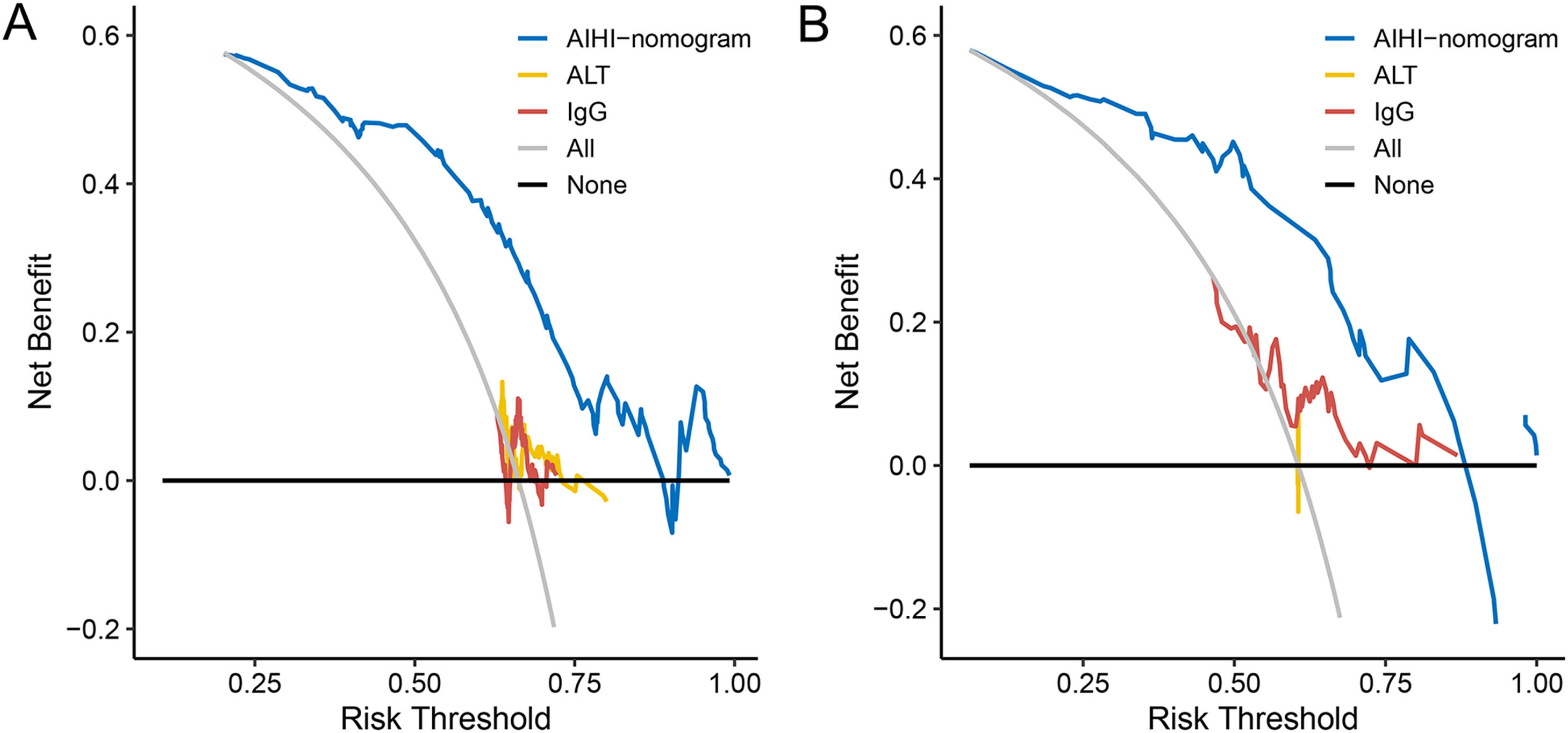
Fig. 5 showed the DCA for the AIHI-nomogram. The results demonstrated that the threshold probability range was 10–88% in the training set and 6–88% in the validation set for the AIHI-nomogram's ability to diagnose severe liver inflammation in patients with AIH. Therefore, with the broad range of threshold probabilities and high accuracy, the net benefit to patients using this model was significantly better than the “treat-all” or “treat-none” strategy as well as the ALT and IgG levels.
3.4Comparison between the AIHI-nomogram and other indexes for estimating severe inflammationWe calculated the scores of the AIHI-nomogram in different liver inflammation grades in the training set and validation set. The correlation analysis showed a positive correlation trend of nomogram scores with liver inflammation grades in the training set (r = 0.532, P < 0.001) and validation set (r = 0.440, P < 0.001) (Fig. S2).
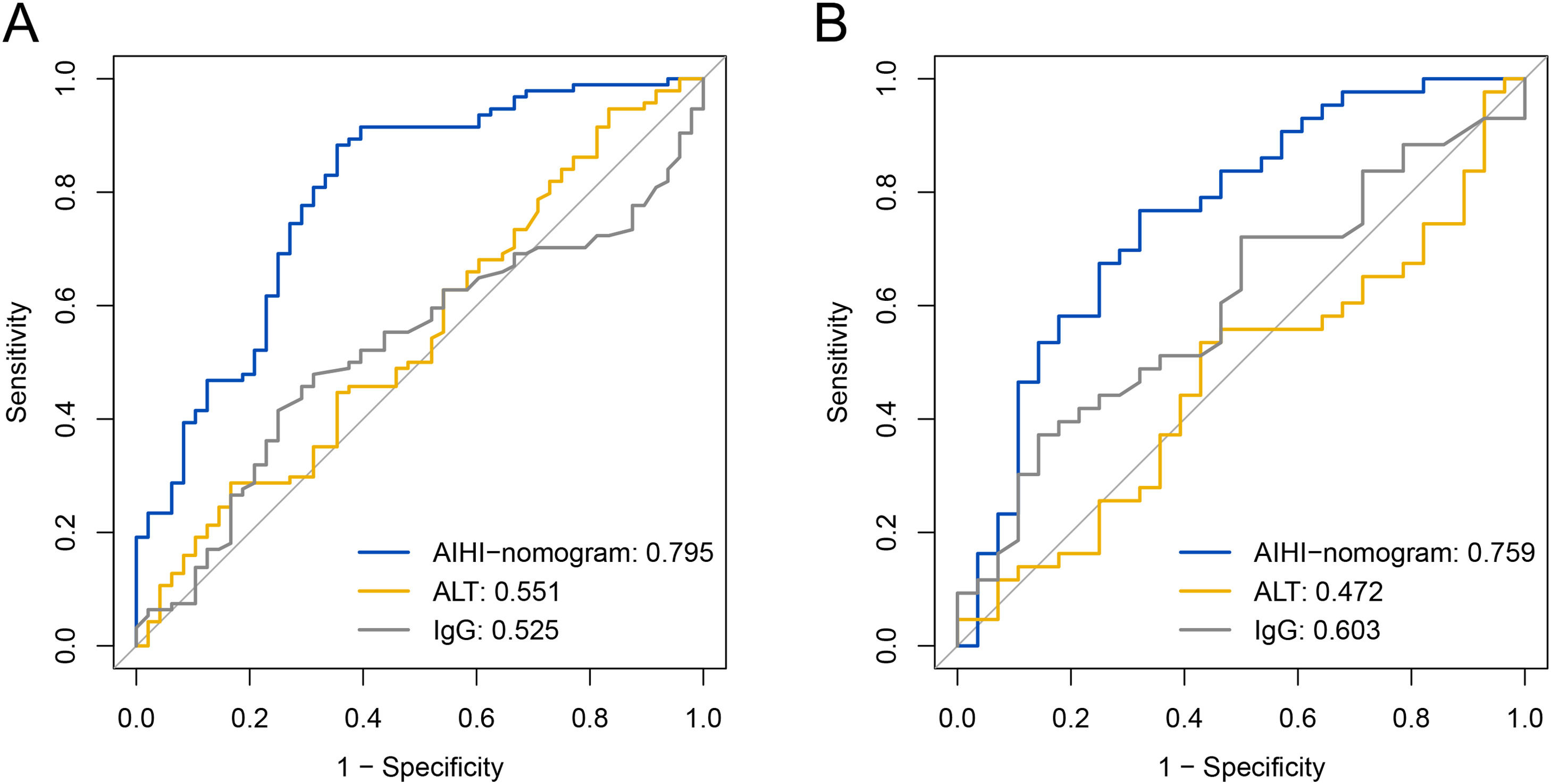
The ROC curves were conducted to evaluate the performance of the predictive model in identifying patients with severe liver inflammation (Fig. 6). In the training set, the AUROC of AIHI-nomogram was 0.795 (95% confidence interval [CI]: 0.715-0.876), and the optimal cut-off point was 130.109, with a sensitivity of 88.3% and a specificity of 64.6%. In the validation set, the AUROC of AIHI-nomogram was 0.759 (95% CI: 0.640-0.878), with a sensitivity of 76.7% and a specificity of 67.9%. As compared with other indexes, the predicting accuracy of AIHI-nomogram in severe inflammation was superior to serum ALT (AUC: 0.551, 95%CI: 0.449-0.652, P < 0.001) and IgG (AUC: 0.525, 95%CI: 0.428-0.623, P < 0.001) in the training set and was also superior to serum ALT (AUC: 0.472 95%CI: 0.333-0.610, P = 0.002) while nearly reaching statistical significance compared to IgG (AUC: 0.603, 95%CI: 0.469-0.736, P = 0.069) in the validation set (Table 2).
Diagnostic performances of the AIHI-nomogram, ALT and IgG in the training set and validation set.
| Variables | Training set (n=142) | Validation set (n=71) |
|---|---|---|
| AIHI-nomogram | ||
| AUROC (95%CI) | 0.795 (0.715-0.876) | 0.759 (0.640-0.878) |
| Cutoff value | 130.109 | 134.780 |
| Sensitivity/specificity (%) | 88.3/64.6 | 76.7/67.9 |
| Accuracy (%) | 80.3 | 73.2 |
| PPV/NPV (%) | 83.0/73.8 | 78.6/65.5 |
| ALT | ||
| AUROC (95%CI) | 0.551 (0.449-0.652) | 0.472 (0.333-0.610) |
| Cutoff value | 224.250 | 89.800 |
| Sensitivity/specificity (%) | 28.7/83.3 | 53.5/57.1 |
| Accuracy (%) | 47.2 | 54.9 |
| PPV/NPV (%) | 77.1/37.4 | 65.7/44.4 |
| IgG | ||
| AUROC (95%CI) | 0.525 (0.428-0.623) | 0.603 (0.469-0.736) |
| Cutoff value | 17.100 | 19.500 |
| Sensitivity/specificity (%) | 47.9/68.8 | 37.2/85.7 |
| Accuracy (%) | 54.9 | 56.3 |
| PPV/NPV (%) | 75.0/40.2 | 80.0/57.1 |
| P values of ROC contrast test | ||
| AIHI-nomogram vs. ALT | <0.001 | 0.002 |
| AIHI-nomogram vs. IgG | <0.001 | 0.069 |
AUROC, the area under the receiver operating characteristic curve; CI, confidence interval; NPV, negative predictive value, PPV, positive predictive value.
Thirty-six (25.4%) patients in the training set and 19 patients (26.8%) in the validation set had normal ALT, respectively. Among them, 61.1% (22/36) and 73.7% (14/19) of patients in the training set and validation set with normal ALT had severe inflammation, respectively. We next analyzed the predictive performance of AIHI-nomogram for severe liver inflammation in AIH patients with normal ALT (Fig. S3). For patients with normal ALT in the training set, the AUROC of AIHI-nomogram was 0.929 for predicting severe liver inflammation (95%CI: 0.831-1.000), and the optimal cut-off value was 106.428, with a sensitivity of 90.9% and a specificity of 92.9%. The predicting accuracy of AIHI-nomogram for severe liver inflammation was superior to serum ALT (AUC: 0.648 95%CI: 0.456-0.839, P = 0.011) and IgG (AUC: 0.563, 95% CI: 0.367-0.759, P < 0.001) for AIH patients with normal ALT in the training set. The AIHI-nomogram also showed higher predictive performance for severe liver inflammation with an AUC of 0.814 (95%CI: 0.615-1.000) compared to serum ALT (AUC: 0.571, 95% CI: 0.214-0.929) and IgG (AUC: 0.643, 95%CI: 0.375-0.911) for AIH patients with normal ALT in the validation set. However, the difference did not reach statistical significance (Table S2).
4DiscussionChronic and progressive liver inflammation is a crucial component of liver pathological injury in patients with AIH, which is associated with the development of liver fibrosis, cirrhosis, and HCC [5,19]. Severe liver inflammation accounted for 64.3% of AIH patients in the present study, suggesting that a majority of AIH patients had severe liver inflammation. Thus, accurately identifying liver inflammation is important for the assessment of disease severity and identifying individuals requiring more intensive care in patients with AIH. Moreover, the prediction of liver inflammation is important for the assessment of histological remission after treatment. In this study, we established and validated a novel AIHI-nomogram containing RDW, PLT, TB, ALB, GGT, and PT, which showed excellent predictive performance for severe liver inflammation. The accuracy of new AIHI-nomogram was higher in predicting severe liver inflammation than serum ALT and IgG in both training set and validation set.
Although liver biopsy is the gold standard for the assessment of inflammation degree, several shortcomings limit the widespread utilization of liver biopsy in clinical practice [10,11]. The levels of serum ALT and IgG have always been used to monitor liver inflammation activity. However, the association between these two indexes and histological activity in chronic liver diseases is still controversial. Gui et al. [20] reported that 25.4% of patients with chronic hepatitis B (CHB) with persistently normal serum ALT levels had significant inflammation and/or fibrosis. Our previous study also found that 24.8% of CHB patients with normal serum ALT levels presented significant liver inflammation [21]. The inconsistency of liver inflammation activity with serum ALT and IgG levels in patients with AIH was also reported. Lüth et al. [22] found that about half of patients with normal serum ALT and IgG showed histologic activity index scores of 4 or 5 in patients with AIH. The present study also revealed that the accuracy of ALT and IgG for predicting severe liver inflammation is unsatisfactory. Thus, establishing a non-invasive method for the assessment of liver inflammation severity for patients with AIH is urgently needed.
A nomogram is an intuitive graphical presentation of a complex statistical model, which can help physicians evaluate the likelihood of a clinical event and formulate individualized strategies for diagnosis and treatment [23]. For the construction of the nomogram, we examined routine clinical candidate predictors by shrinking the regression coefficients with the LASSO analysis. The AIHI-nomogram presented high discrimination and well-fitted calibration, indicating good reliability and generalizability of AIHI-nomogram. The training set and validation set showed consistent accuracy and better predictive performance of AIHI-nomogram than serum ALT and IgG. It should be noted that our results indicate that patients with a total AIHI-nomogram score greater than 130.109 are at high risk of severe inflammation and may require urgent treatment initiation or modification.
It is reported that a significant proportion of patients with AIH had histological disease activity despite normal serum ALT levels [24–26]. Our previous study showed that the proportion of severe inflammation was as high as 62.1% in AIH patients with normal ALT [27]. In the present study, 61.1% and 73.7% of patients with normal ALT in the training set and validation set had severe inflammation. Thus, predicting the severe inflammation of AIH patients with normal ALT is more important for the management of AIH patients. Our study showed that the AIHI-nomogram had a high predictive value of severe liver inflammation in AIH patients with normal ALT. The AIHI-nomogram showed higher predictive performance for severe liver inflammation as compared with serum ALT and IgG in the validation set. However, the difference did not reach statistical significance which might be interpreted by the small size of the validation set in which there are only 19 patients with normal ALT. Furthermore, there are only 19 patients in the training set and 11 patients in the validation set had normal ALT and IgG, respectively. Thus, we could not analyze the predictive performance of AIHI-nomogram for severe liver inflammation in AIH patients with normal ALT and IgG. Hence, the predictive values of AIHI-nomogram for severe inflammation in AIH patients with normal ALT and IgG deserve further investigation and validation.
In terms of indexes in this AIHI-nomogram, independent predictors for severe liver inflammation were RDW, PLT, TB, ALB, GGT, and PT, which were commonly used in clinical practice. The RDW is an indicator reflecting the uniformity in the volume of red blood cell, which is tested routinely as a part of complete blood cell counts [28]. Numerous studies have demonstrated the association between RDW level and severity in patients with chronic liver diseases [29–31]. Our previous study also showed the RDW level was positively associated with the degrees of liver inflammation in patients with AIH [29]. The PLT, as one of the most commonly used indicators for the severity of liver diseases, was verified to be associated with severe liver inflammation in this study. TB was a predictor of liver injury in many acute and chronic liver diseases. Wang et al. [32] established a nomogram for assessing liver inflammation activity based on TB and other indexes in patients with chronic drug-induced liver injury. The ALB level is closely related to the synthetic function of hepatocytes, which suggests that liver inflammation activity may affect albumin synthesis. Xu et al. [33] reported that ALB level was lower in patients with severe liver inflammation than in those with no or mild inflammation, and they demonstrated that a low level of ALB was a risk factor for severe liver inflammation in a cohort of patients with CHB. The GGT is a kind of microsomal enzyme secreted from hepatocytes and bile duct cell [34]. Elevated GGT was reported to be associated with liver inflammation activity in chronic liver diseases [21,35]. The relationship between PT and liver inflammation activity has been verified in previous studies [32,36]. The prolongation of PT is caused by a reduction in coagulation factors and fibrinogen synthesis, suggesting the synthetic capacity of hepatocytes is decreased with liver injury [37].
Generally, the AIHI-nomogram would improve the identification capability of severe liver inflammation in patients with AIH. Indexes contained in this nomogram can be easily obtained and it would be convenient and easy to use for physicians in clinical practice, especially in source-limited regions. However, several limitations in this study should be acknowledged. First, this is a retrospective study. Nevertheless, it should be noted that the inclusion of patients from multiple centers enhances the representativeness of our findings. Second, since this is a retrospective study, only 16 AIH patients received liver biopsy after immunosuppressive treatment were included. We acknowledge that the predictive values of our nomogram for liver inflammation deserve validation in AIH patients after immunosuppressive treatment in the future. Third, the AIHI-nomogram was only validated in one internal set and all the patients were Asian. Thus, the validation of AIHI-nomogram in prospective multicenter studies and other ethnicities is warranted.
5ConclusionsIn summary, a novel AIHI-nomogram for the excellent prediction of severe liver inflammation in patients with AIH was developed and validated. The easy-to-use online AIHI-nomogram provided a convenient and highly accurate tool for clinicians to identify AIH patients with a high risk of severe liver inflammation and requiring urgent treatment initiation or modification, especially for those with normal serum ALT levels. However, more studies are needed to validate the predictive accuracy of this AIHI-nomogram for liver inflammation in AIH patients after treatment.