Chronic hepatitis B (CHB) may progress to more serious liver diseases and it is often accompanied by non-alcoholic fatty liver disease (NAFLD). NAFLD and CHB share risk factors for liver fibrosis and cirrhosis, but the influence of NAFLD on fibrosis progression is controversial. This retrospective study evaluated the prevalence of NAFLD in patients with CHB and investigated associations between NAFLD and liver fibrosis in a large multi-center cohort of hepatitis B patients submitted to liver biopsy.
Patients and MethodsTreatment-naïve patients with CHB who underwent liver biopsy were analyzed. Propensity score matching (PSM) was performed to adjust the confounders between patients with and without NAFLD.
ResultsA total of 1496 CHB patients were included. Two hundred and ninety (19.4%) patients were diagnosed with NAFLD by liver biopsy. The proportions of significant liver fibrosis (52.8% vs. 63.9%, P<0.001), advanced liver fibrosis (27.2% vs. 36.5%, P=0.003), and cirrhosis (13.4% vs. 19.7%, P=0.013) was considerably lower in CHB patients with NAFLD compared to those without NAFLD. 273 patients were included in each group after PSM adjusted for age, sex, hepatitis B envelope antigen status, and hepatitis B virus DNA. Liver fibrosis remained less severe in CHB patients with NAFLD than those without NAFLD (P<0.05) after PSM. The presence of NAFLD was considered an independent negative factor of significant liver fibrosis (odds ratio (OR) 0.692, P=0.013) and advanced liver fibrosis (OR 0.533, P = 0.002) in CHB patients.
ConclusionsNAFLD is not uncommon in CHB patients with the prevalence of 19.4%. The presence of NAFLD is associated with less severe liver fibrosis in CHB patients.
Registration No. of the study/trialNCT03097952.
Hepatitis B virus (HBV) infection is a leading cause of chronic liver disease (CLD), affecting approximately 240 million people globally [1]. Patients with chronic hepatitis B (CHB) may progress to severe CLDs, including cirrhosis and hepatocellular carcinoma (HCC) [2]. Non-alcoholic fatty liver disease (NAFLD) is an emerging and the most prevalent CLD due to the rising prevalence rates of obesity and metabolic syndrome [3]. The prevalence of NAFLD is up to 30% in both Western and Asian populations [4,5]. The annual rate of fibrosis progression in nonalcoholic steatohepatitis was 40.76% and the incidence of advanced fibrosis in nonalcoholic steatohepatitis was 67.95 in 1,000 person-years [3].
Chronic HBV infection and NAFLD are leading causes of severe liver manifestations, including liver cirrhosis, liver failure, and HCC [6–8]. It is estimated hepatic steatosis or fatty liver disease prevalence rate in CHB patients ranges from 14% to 70% [9–15]. Though, the potential interaction between CHB and NAFLD is not yet fully understood. Metabolic factors and high body mass index (BMI) values are strongly associated with NAFLD and are independent risk factors for liver fibrosis and cirrhosis in CHB patients [4,16,17].
However, the effect of NAFLD on the progression of liver fibrosis in CHB remains controversial. Concomitant NAFLD has been reported to exacerbate liver damage in patients with CHB [18]. Previously, a retrospective study showed that hepatic steatosis was positively associated with the progression of both significant liver fibrosis and advanced liver fibrosis in patients with CHB [14]. Concomitant NAFLD could significantly increase the risk of HCC development in patients with CHB [19]. However, different results have also been reported. Chen et al. [20] found that the five-year cumulative incidence of cirrhosis in patients with CHB with and without NAFLD was not substantially different. Bondini et al. reported that liver fibrosis severity was associated with the known host and viral factors rather than the presence of NAFLD [18]. According to Zheng et al., liver fibrosis was more severe in patients with CHB without NAFLD, than in those with NAFLD [21].
Despite the rising prevalence of NAFLD in patients with CHB, there is still a scarcity of information about how NAFLD affects liver fibrosis. The present multi-center retrospective study evaluated the prevalence of NAFLD in patients with CHB and investigated the effects of NAFLD on liver fibrosis.
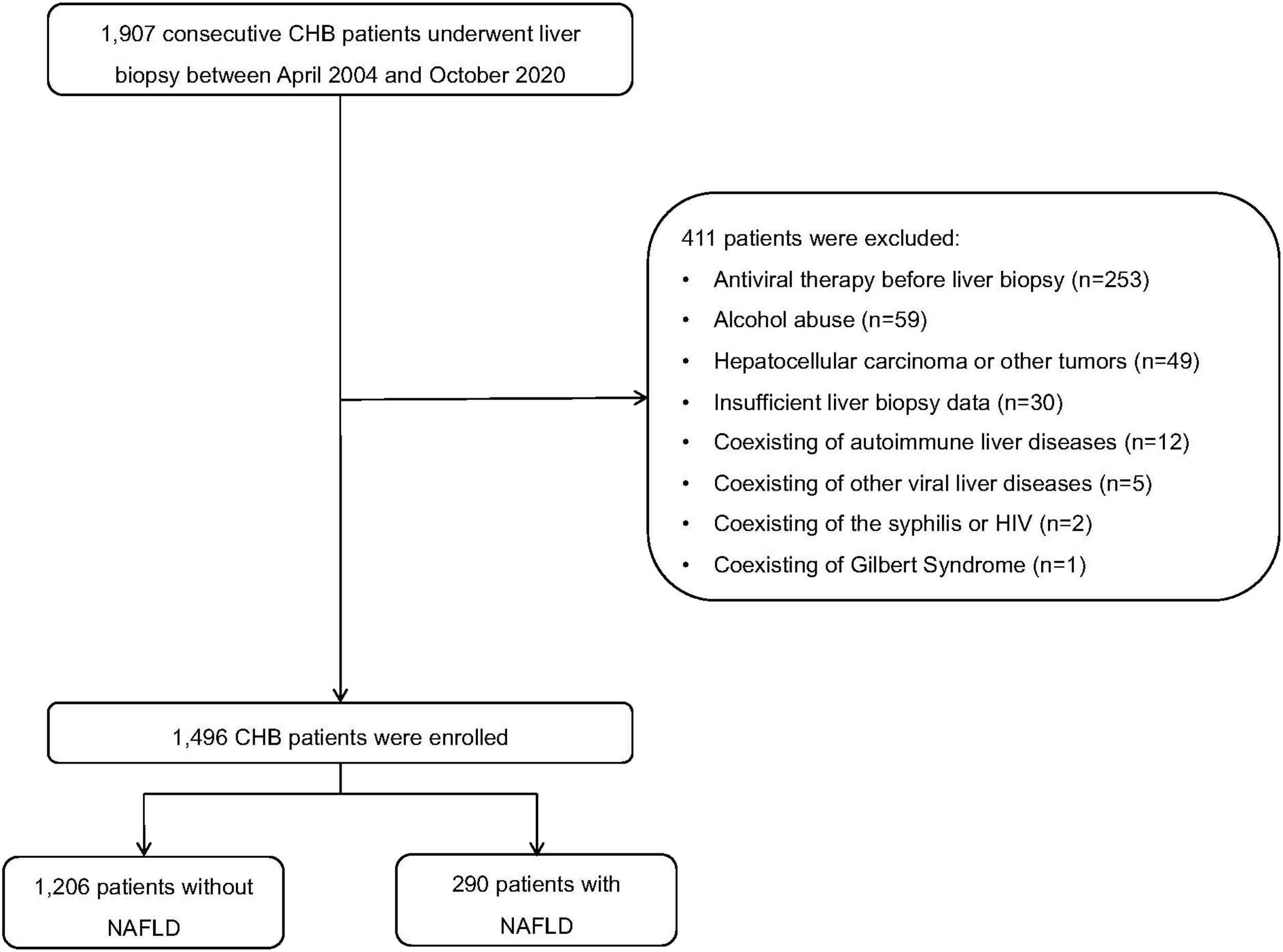
2Patients and Methods2.1Study populationPatients with CHB underwent liver biopsy between April 2004 and October 2020 at four medical centers (Nanjing Drum Tower Hospital, Nanjing, China; Huai'an No. 4 People's Hospital, Huai'an, China; Fifth People's Hospital of Wuxi, Wuxi, China; and Affiliated Infectious Diseases Hospital of Soochow University, Suzhou, China) were included in this study. All patients with CHB were positive for HBV surface antigens (HBsAg) for at least six months and were treatment-naïve. Patients with liver biopsy samples with a length of at least 1 cm and six portal tracts were included for the analysis. We excluded patients with other liver diseases, hepatocellular carcinoma, other types of cancers, and those who received antiviral therapy before liver biopsy, had incomplete clinical data or alcohol abuse report (≥ 30 and ≥ 20 g of alcohol per day for men and women, respectively) [22].
2.2Liver biopsy and histological assessmentThe decision for liver biopsy was made by the physician in charge of managing patients. Most of the decision of liver biopsy was conducted to assess the inflammation grades and fibrosis stages to assist in the determination to initiate antiviral treatment. Liver biopsy was performed under ultrasound guidance. The liver specimens were reviewed by two pathologists who were blinded to the liver function indicators.
The histological lesions of fibrosis were assessed according to the Scheuer scoring system [23]. Fibrosis was classified into five stages, F0 to F4, defined as follows: F0, no fibrosis; F1, enlarged, fibrotic portal tracts; F2, periportal or portal-portal septa, but intact architecture; F3, fibrosis with architectural distortion but no obvious cirrhosis; and F4, cirrhosis) [23]. The fibrosis stages F0-1, F2-4, F3-4, and F4 were summarized and categorized, respectively, as no/mild fibrosis, significant fibrosis, advanced fibrosis, and cirrhosis, respectively [23]. Since this is a retrospective study and the patients were included from four medical centers with a quite long period of time, the NAFLD activity scores (NAS) were not available.
According to the Brunt classification, steatosis was assessed and classified as four grades: S0, none; S1, up to 33%; S2, 33% to 66%; and S3, 66% or more [24]. S0 was designated as non-NAFLD and S1-3 was designated as NAFLD. Mild steatosis and moderate-severe steatosis were defined as S1 and S2-3, respectively [24]. The laboratory findings, including blood routine examination, liver function tests, and HBV serological indicators within two weeks before liver biopsy were collected and used for the analysis.
2.3Statistical analysisContinuous variables are expressed as median (interquartile) values, and categorical data are presented as percentages. The relevant continuous variables were compared using independent-group t-tests or the Mann-Whitney U tests. To compare categorical variables, the chi-squared test was applied. We used a balanced study approach based on propensity score matching (PSM) with a 1:1 ratio to account for potential bias between CHB patients with and without NAFLD. Four important demographic and laboratory parameters were matched: age, gender, HBeAg (hepatitis B envelope antigen) status, and HBV DNA. The risk factors for liver fibrosis were analyzed by binary logistic regression analysis and variables with P values < 0.05 in the univariate analysis were included in a multivariate input logistic regression analysis before and after PSM. The odds ratio (OR) with a 95% confidence interval (CI) was calculated. A two-tailed P-value less than 0.05 was considered statistically significant. All statistical analyses were conducted using the Statistical Package for the Social Sciences version 23.0 software program (IBM, Armonk, NY, USA).
2.4Ethical statementA waiver of informed consent was granted by the ethics committees due to a retrospective design and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethics Committee of The Nanjing Drum Tower Hospital (2008022).
3Results3.1Clinical characteristics of patientsA total of 1907 patients with CHB were initially screening for this study (Fig. 1). Among them, 253 patients received antiviral therapy before liver fibrosis, and 158 patients met the further criteria for exclusion. Ultimately, 1,496 treatment-naïve CHB patients who underwent liver biopsy were included in the analysis. Among them, 19.4% (290/1496) patients had NAFLD. In the NAFLD group, the numbers and percentages of patients with steatosis grades S1, S2, and S3 were, respectively, 200 (69.0%), 67 (23.1%), and 23 (7.9%) (Table 1).
Clinical characteristics of the study population.
ALB, albumin; ALT, alanine aminotransferase; AST, Aspartate aminotransferase; BMI, body mass index; DM, diabetes mellitus; FBG, fasting blood glucose; GGT, gamma-glutamyl transpeptidase; Hb, hemoglobin; HBeAg, hepatitis B virus envelope antigen; HBV, hepatitis B virus; HDL, how-density lipoprotein; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; PLT, platelet; Tbil, total bilirubin; TC, total cholesterol; TG, triglyceride; UA, uric acid.
The median ages of patients between NAFLD and non-NAFLD groups were comparable (40.0 vs. 39.0 years; P=0.493). The proportions of diabetes mellitus (8.3%vs. 2.7%, P<0.001) and hypertension (10.7% vs. 4.1%, P<0.001) in the NAFLD group were significantly higher than that of non-NAFLD group. Regarding virology, the HBeAg positivity rate of NAFLD group was significantly lower than that of non-NAFLD group (37.5% vs. 44.5%; P=0.030). However, the median levels of HBV DNA were comparable between groups (4.3 log10 IU/mL vs. 5.0 log10 IU/mL; P=0.145).
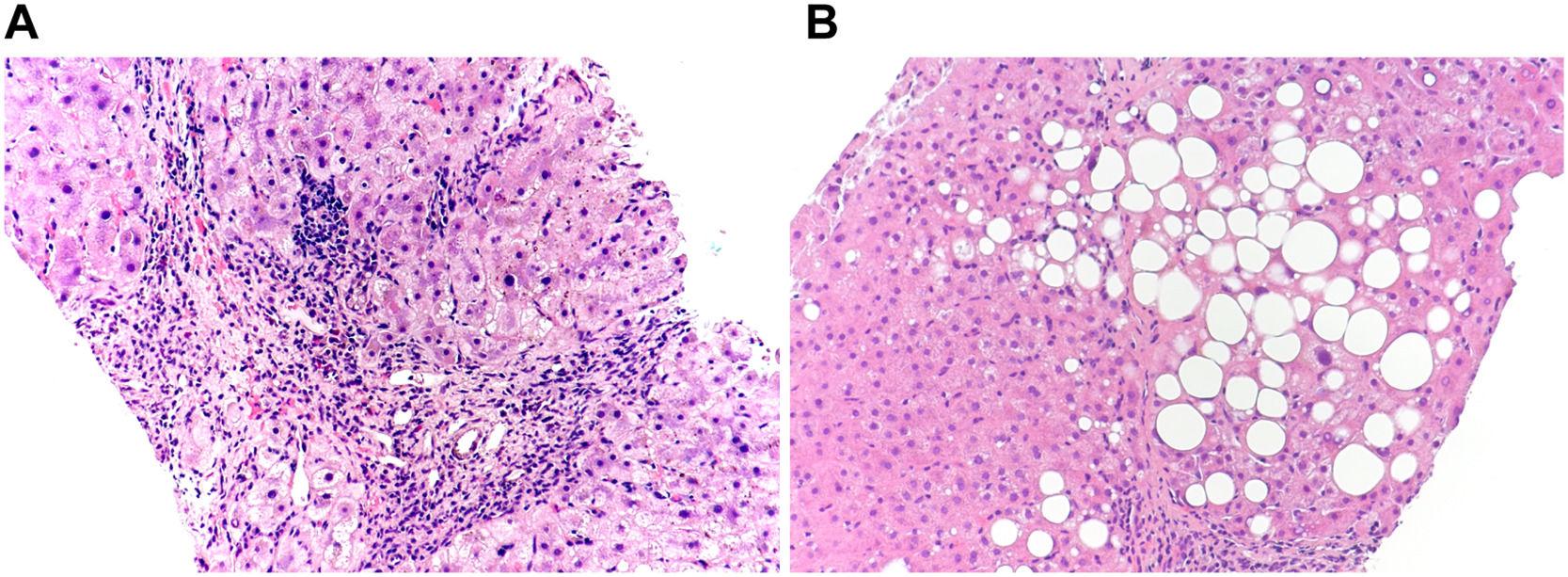
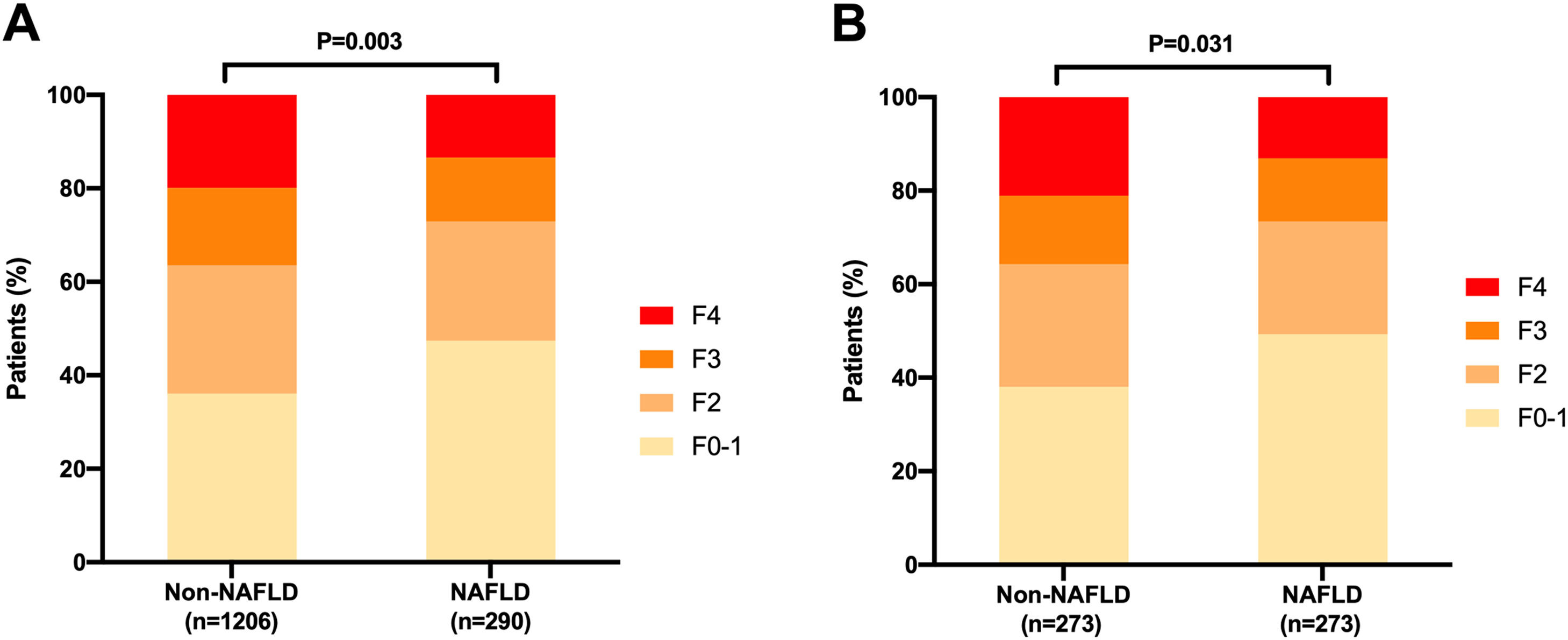
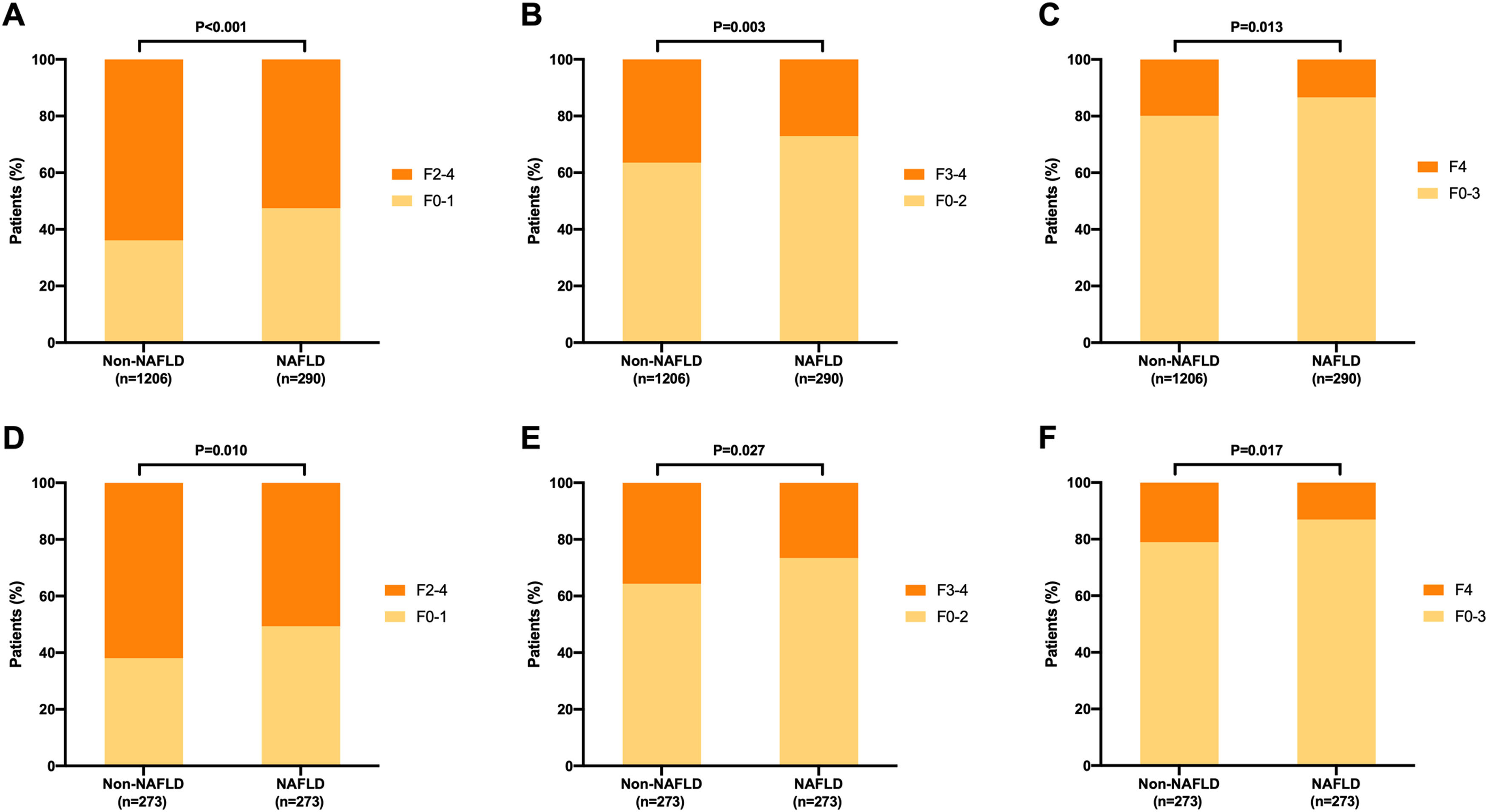
3.2Distribution of liver fibrosis stages before propensity score matchingRepresentative images of liver pathological changes in CHB patients with and without and NAFLD were presented in Fig. 2. In the NAFLD group, 137 (47.2%), 74 (25.5%), 40 (13.8%), and 39 (13.4%) patients were at fibrosis stage F0-1, F2, F3; and F4, respectively (Fig. 3A). In the non-NAFLD group, the prevalence of corresponding stage was 36.2%, 27.4%, 16.7% and 19.7%, respectively. The severity of liver fibrosis was significantly lower in the NAFLD group compared to non-NAFLD group (P=0.003). The percentage of patients in the non-NAFLD group who exhibited liver fibrosis, advanced liver fibrosis, and liver cirrhosis was significantly higher than that of the NAFLD group, respectively, 63.9% vs. 52.8% (P<0.001), 36.4% vs. 27.2% (P=0.003), and 19.7% vs. 13.4% (P= 0.0013) (Figs. 4A, 4B and 4C).
Representative images of liver pathological changes in chronic hepatitis B patients with and without and nonalcoholic fatty liver disease (NAFLD). (A) Female, 53 years old, chronic hepatitis B; liver biopsy revealed stage 3 liver fibrosis, and no hepatic steatosis. (B) Female, 54 years old, chronic hepatitis B concurrent with NAFLD, liver biopsy revealed stage 1 liver fibrosis, and hepatic steatosis 33-66%. ×200 magnification, hematoxylin and eosin (H&E) stain.
Distribution of liver fibrosis stages in chronic hepatitis B patients with and without nonalcoholic fatty liver disease (NAFLD). (A) Distribution of liver fibrosis stages in patients with and without NAFLD before propensity score matching (PSM); (B) Distribution of liver fibrosis stages in patients with and without NAFLD after propensity score matching (PSM). Patient matching was performed with a 1:1 ratio for age, gender, HBeAg status, and HBV DNA levels which yielded 273 matched pairs in each group.
Comparison of liver fibrosis stages between chronic hepatitis B patients with NAFLD and without nonalcoholic fatty liver disease (NAFLD). A-C: Comparison of liver fibrosis stages between patients with and without NAFLD before propensity score matching (PSM); D-F: Comparison of liver fibrosis stages between patients with and without NAFLD after PSM. Patient matching was performed with a 1:1 ratio for age, gender, HBeAg status, and HBV DNA levels which yielded 273 matched pairs in each group.
In this study, PSM was applied to match individuals in the NAFLD and non-NAFLD groups, yielding 273 matched pairs. After PSM, the two groups were comparable regarding gender, age, HBeAg status, and HBV DNA levels (Supplemental Table 1). Among the patients with NAFLD, the number of patients with liver fibrosis stages F0-1, F2, F3, and F4 were 134 (49.1%), 66 (24.1%), 37 (13.6%) and 36 (13.2%), respectively. In the non-NAFLD group, the number of those with liver fibrosis stages F0-1, F2, F3, and F4 were 104 (38.1%), 72 (26.4%), 40 (14.6%), and 57 (20.9%), respectively (Fig. 3B). Liver fibrosis was also less severe among patients with NAFLD compared to those without NAFLD (P=0.031). Compared with the NAFLD group, the non-NAFLD group showed higher percentages of significant liver fibrosis (61.9% vs. 50.9%, P=0.010, Fig. 4D), advanced liver fibrosis (35.5% vs. 26.7%, P=0.027, Fig. 4E), and liver cirrhosis (20.9% vs. 13.2%, P=0.017, Fig. 4F).
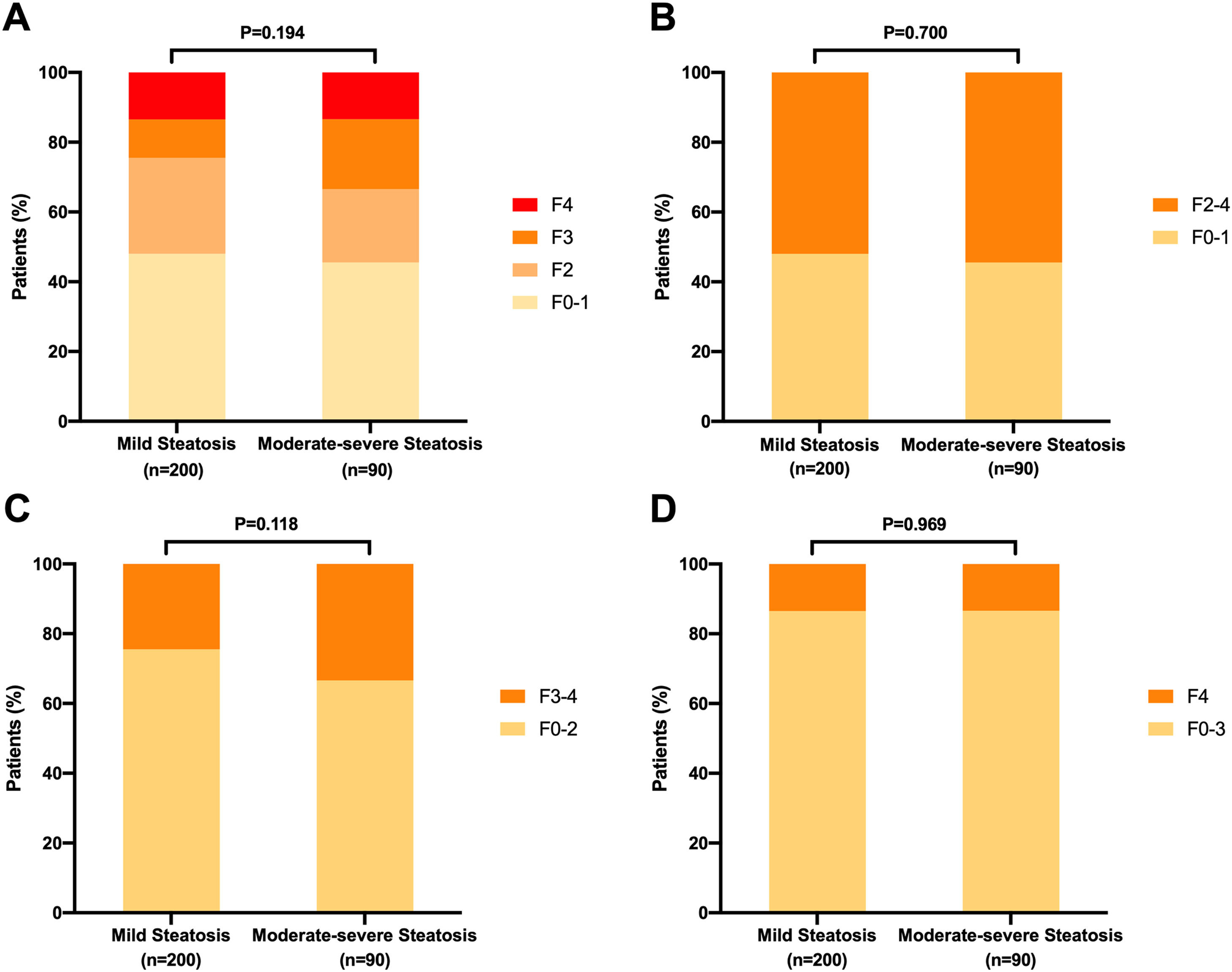
3.4Distribution of liver fibrosis stages among patients with mild steatosis and moderate-severe steatosis patientsThe clinical characteristics were comparable between patients mild steatosis (S1) and moderate-severe (S2-3) steatosis patients (Supplemental Table 2). For patients with mild steatosis, the number of patients at fibrosis stages F0-1, F2, F3, and F4 were 96 (48.0%), 55 (27.5%), 22 (11.0%), and 27 (13.5%), respectively. For those with moderate-severe steatosis, the corresponding number were 41 (45.6%), 19 (21.1%), 18 (20.0%), and 12 (13.3%), respectively (Fig. 5). The percentages of patients with significant liver fibrosis (52.0% vs. 54.5%, P=0.700), advanced liver fibrosis (24.5% vs. 33.4%, P=0.118), and liver cirrhosis (13.5% vs. 13.3%, P=0.969) were comparable between mild steatosis and moderate-severe steatosis groups.
Distribution of liver fibrosis stages in chronic hepatitis B patients with different steatosis stages. (A) Comparison of liver fibrosis stages between CHB patients with mild steatosis (S1) and moderate-severe steatosis (S2-3). Comparisonc of the proportions of significant fibrosis (B), advanced fibrosis (C) and cirrhosis (D) between CHB patients with mild steatosis (S1) and moderate-severe steatosis (S2-3).
According to the univariate logistic regression analysis, the associated factors of liver fibrosis included age, platelets, alanine aminotransferase (ALT), aspartate aminotransferase (AST), GGT, neutrophils, monocytes, the presence of NAFLD, HBeAg-positive status and HBV DNA levels at 5-7 log10 IU/mL. In the multivariate analysis, the independent risk factors of liver fibrosis included higher GGT levels (OR 1.007, 95% CI: 1.004-1.009; P < 0.001), and HBV DNA levels at 5-7 log10 IU/mL (OR 2.270, 95% CI: 1.469-3.506, P<0.001). Parameters that were negatively associated with significant liver fibrosis were higher platelet counts (OR 0.994, 95%CI 0.992-0.996, P<0.001) and concomitant NAFLD (OR 0.693, 95%CI: 0.518-0.928; P=0.014) (Table 2).
Univariate and multivariate analyses of factors associated with significant liver fibrosis in patients with chronic hepatitis B.
ALT, alanine aminotransferase; AST, Aspartate aminotransferase; BMI, body mass index; CI, confidence interval; GGT, gamma-glutamyl transpeptidase; HBeAg, hepatitis B virus envelope antigen; HBV, hepatitis B virus; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; PLT, platelet.
Further logistic regression analysis indicated that the independent risk factors of advanced liver fibrosis were higher GGT, BMI, HBeAg-positive status, and HBV DNA levels at 5-7 log10 IU/mL. Parameters that were negatively associated with advanced liver fibrosis were higher platelet counts, monocytes, the presence of NAFLD, and higher HBV DNA levels (Supplemental Table 3). Only platelets and GGT levels were associated with liver cirrhosis, while concurrent NAFLD was not (Supplemental Table 4).
4DiscussionThis multi-center, retrospective study with a large sample size investigated the prevalence of NAFLD in patients with CHB and the association between NAFLD and severity of liver fibrosis. In the present study, the prevalence of biopsy-proven NAFLD was 19.4%, similar to those reported in previous reports [14,18]. More importantly, we found that NAFLD concomitant with CHB was associated with less severe liver fibrosis.
Hepatic steatosis is commonly observed in patients with chronic hepatitis C and alcoholic liver disease [25]. NAFLD concomitant with CHB is also often encountered [15]. Previous studies showed that patients with CHB with NAFLD also present metabolic factors such as obesity, dyslipidemia, and diabetes mellitus [15,26]. The current results highlight the association between NAFLD and these metabolic factors. Moreover, previous studies reported that NAFLD was associated with long-term prognosis and antiviral therapy response in CHB [19,27]. A large retrospective study found that CHB patients with concomitant NAFLD were at higher risk of developing liver-related outcomes and death, compared to patients with CHB alone [27]. Concomitant NAFLD was also reported to be independently associated with increased risk of HCC by 7.3-fold in patients with CHB [19]. Moreover, concomitant NAFLD was also associated with failure of entecavir treatment [28] .
In contrast, Li et al. [29] found that concomitant NAFLD had no influence on long-term complete viral suppression and biochemical response in patients treated for CHB. Fung et al. [30] observed that patients with CHB with concomitant NAFLD might experience a favorable effect regarding HBsAg seroclearance. Recently, fatty liver was significantly associated with lower liver cirrhosis and HCC risk, and higher HBsAg seroclearance, in patients with CHB [31]. Several studies have determined a negative association between HBV DNA levels and NAFLD in patients with CHB [11,15].
Although several studies have shown that hepatic steatosis accelerates the progression of liver fibrosis in chronic hepatitis C virus infected patients, whether hepatic steatosis accelerates the progression of liver fibrosis in CHB patients is still controversial. Concomitant NAFLD aggravates liver damage, according to a large cohort study involving 5,406 CHB patients [32]. However, in this investigation, NAFLD was diagnosed by abdominal ultrasound. Charatcharoenwitthaya et al. [14] reported that NAFLD was an independent risk factor for significant liver fibrosis and advanced liver fibrosis in a liver biopsy cohort of patients with CHB with a limited sample size. On the other hand, several studies reported no association between liver fibrosis severity and NAFLD in patients with CHB [13,18,33]. Negative associations between NAFLD and liver fibrosis in patients with CHB were also reported. Liver fibrosis was less severe in patients with CHB with NAFLD, compared to those without NAFLD in the study by Shi et al. [26]
The current study analyzed the effect of NAFLD on liver fibrosis in patients with CHB with liver biopsy. Consistent with previous studies, our study suggests that liver fibrosis was less severe in patients with CHB with NAFLD compared to patients without NAFLD [21,26]. A study comprising 1915 patients with CHB who underwent liver biopsy found that the patients with NAFLD had significantly less severe fibrosis than patients without NAFLD (39.6% vs. 53.5%) [26]. Another study also found that liver fibrosis was less severe in CHB patients with NAFLD compared to patients without NAFLD [21]. However, ALT and HBV DNA levels in CHB patients with NAFLD were lower than that of patients without NAFLD in these two studies [21,26]. These confounding factors might have biased the results of previous studies. Thus, the PSM method was used in the present study to adjust the confounding factors, such as gender, age, HBeAg status, and HBV DNA level, which confirmed that liver fibrosis remained less severe in patients with concomitant NAFLD. A summary of the impact of NAFLD on fibrosis in CHB patients were presented in Table 3 [11,13,14,26,31,34-41]. As conflict results were reported by different studies, more prospective studies with large sample sizes are needed to confirm the impact of NAFLD on fibrosis in patients with CHB.
Studies on the impact of nonalcoholic fatty liver disease on liver fibrosis in patients with chronic hepatitis B.
| Author, year | Location | Study design | Total cases | Concurrent with steatosis | Diagnosis of NAFLD | Assessment of liver fibrosis | Conclusions |
|---|---|---|---|---|---|---|---|
| Thomopoulos KC et al. 2005 [34] | Greece | Retrospective | 233 | 18% | Liver biopsy | Liver biopsy | No significant association between advanced fibrosis and the presence of steatosis in CHB patients. |
| Shi JP et al, 2008 [26] | MainlandChina | Retrospective | 1915 | 14% | Liver biopsy | Liver biopsy | Steatosis in CHB is not related to more advanced fibrosis stage. |
| Yun JW et al, 2008 [13] | Korea | Prospective | 86 | 51.2% | Liver biopsy | Liver biopsy | Hepatic steatosis is not associated with hepatic fibrosis in CHB patients. |
| Karacaer Z et al, 2016 [35] | Turkey | Retrospective | 254 | 11.4% | Ultrasonography | Liver biopsy | Hepatic steatosis is associated with advanced hepatic fibrosis in CHB patients. |
| Charatcharoenwitthaya P et al, 2016 [14] | Thailand | Prospective | 256 | 38% | Liver biopsy | Liver biopsy | Steatohepatitis was an independent predictor of significant fibrosis and advanced fibrosis in CHB patients. |
| Seto WK et al, 2017 [12] | Hong Kong | Prospective | 1606 | 40.8% | Transient elastography | Transient elastography | Severe steatosis was associated with severe fibrosis in treatment-naïve patients and in patients receiving treatment in in CHB patients. |
| Hui RWH et al, 2018 [11] | Hong Kong | Prospective | 1548 | 56.6% | Transient elastography | Transient elastography | Severe steatosis was associated with increased fibrosis in CHB patients. |
| Peleg N et al, 2019 [36] | Israel | Retrospective | 524 | 46% | Ultrasonography or liver biopsy | APRI, FIB-4, Liver biopsy | Liver steatosis was not significantly associated with advanced fibrosis in CHB patients. |
| Wong SW et al, 2020 [37] | Malaysia | Prospective | 614 | 47.9% | Transient elastography | Transient elastography | Hepatic steatosis is associated with advanced fibrosis in CHB patients. |
| Li J et al, 2020 [31] | USA and Taiwan | Retrospective | 6786 | 31.55% | Ultrasonography or CT | Ultrasonography or CT | Fatty liver was significantly associated with lower cirrhosis and HCC risk and higher HBsAg seroclearance in CHB patients. |
| Mak LY et al, 2020 [38] | Hong Kong | Prospective | 330 | 48.8% | Transient elastography | Transient elastography | Persistent severe hepatic steatosis was independently associated with fibrosis progression in CHB patients. |
| Chen YC et al, 2020 [39] | Taiwan | Retrospective | 672 | 50.9% | Liver biopsy | Liver biopsy | Significant or advanced liver fibrosis is not associated with hepatic steatosis in CHB patients. |
| Khalili M et al, 2021 [40] | USA | Prospective | 420 | 31.4% | Liver biopsy | Liver biopsy | Steatohepatitis was associated with advanced fibrosis in CHB patients. |
| Diao Y et al, 2022 [41] | Mainland China | Retrospective | 733 | 37.2% | Transient elastography | Transient elastography | Severe steatosis were independently associated with significant fibrosis in CHB patients. |
Whether the grades of steatosis are associated with fibrosis in CHB patients is important. Hui et al found that severe steatosis was associated with an increased percentage of severe fibrosis compared to mild/moderate steatosis [11]. However, liver fibrosis and steatosis were assessed by liver stiffness and controlled attenuation parameter measurements using transient elastography in that study. In the present study, we explored the impact of steatosis grades on liver fibrosis in CHB patients. The grade of liver steatosis and fibrosis was assessed by liver biopsy which is much more accurate than transient elastography. We found that the stage of liver fibrosis was comparable between patients with mild steatosis and moderate-severe steatosis. Further studies are needed to confirm the impact of steatosis grades on liver fibrosis in CHB.
Although the influence of HCV on the development of NAFLD is well-established, the mechanism of how HBV mediates hepatic steatosis remains unclear [42]. Several studies have shown that hepatic steatosis may worsen liver fibrosis in patients with CHB, while chronic liver inflammation is a typical fibrogenesis pathway [27]. The accumulation of fatty acids in hepatocytes results in excessive oxidative stress and the production of toxic lipid metabolites by activating nuclear factor–κB [43]. Ongoing liver damage mediated by chronic inflammation, oxidative stress, and apoptosis aggravates liver fibrosis induced by activated fibroblast and hepatic stellate cells [44].
However, numerous studies also determined a negative association between HBV DNA load and NAFLD risk in patients with CHB [11,15,30]. The potential mechanism behind this may be that fat accumulation in liver cells reduces HBV duplication by mediating hepatocyte necrosis [45,46]. In the present study, HBV DNA levels in patients with NAFLD were lower than in those without NAFLD. Patients with CHB with moderate-to-severe steatosis also presented lower HBV DNA levels than those with mild steatosis, while the difference was not significant. In addition, a large sample study that enrolled 3212 patients with CHB revealed that intrahepatic HBsAg-positive and hepatitis B core antigen-positive staining were lower in patients with NAFLD, compared with those without NAFLD [47]. These findings suggest that intrahepatic viral load is lower in patients with NAFLD than in patients without NAFLD. HBV DNA levels are a crucial factor influencing disease progression in patients with CHB. This may be one of the key reasons why the fibrosis was less severe in patients with CHB with NAFLD [48].
When compared to earlier investigations, the present study has some advantages. First, the sample size was larger, and patients were included from several centers. More importantly, the diagnosis of liver fibrosis and NAFLD was confirmed through liver biopsy. In addition, PSM was used to reduce the influence of confounding factors, and the results were consistent before and after PSM.
There are several limitations in the present study. First, this was a retrospective and cross-sectional analysis, and the findings require validation by further prospective and longitudinal investigations. Furthermore, the HBV genotype data were not available, and genotype may influence the development of NAFLD and liver fibrosis in patients with CHB. Third, this study did not consider the NAS scores. Thus, the association between NAS score and liver fibrosis in patients with CHB with NAFLD needs further investigation. Fourth, as a retrospective study, the alcohol consumption was assessed by reviewing the medical records of patients and the Alcohol Use Disorder Identification Test (AUDIT) scale was not used which might lead to bias. Fifth, due to the lack of long-term follow-up data, we could not able evaluate to the impact of NAFLD on the long-term outcomes of CHB patients. Finally, metabolic dysfunction-associated fatty liver disease (MAFLD) as a new definition was proposed in 2020. Different from non-alcoholic fatty liver disease (NAFLD), the diagnosis of MAFLD does not require the exclusion of other chronic liver diseases but needs the presence of metabolic disorders [49]. Recently, metabolic dysfunction-associated steatotic liver disease (MASLD) was proposed to replace NAFLD [50]. Both MASLD and MAFLD involve changes not only in terminology but also in definitions [50,51]. A recent study found that the discrepancy between MASLD and NAFLD is minimal and they concluded that it is reasonable to consider findings from NAFLD studies remain to be valid under the MASLD definition [52]. However, more studies are needed to explore the impact of MASLD on fibrosis in CHB patients.
5ConclusionsIn conclusion, concomitant NAFLD in patients with CHB was inversely associated with the progression of liver fibrosis in our study. However, more studies are needed to validate our findings and explore the potential mechanisms.
CRediT authorship contribution statementRenling Yao: Formal analysis, Investigation, Methodology, Software, Writing – original draft. Sufang Lu: Formal analysis, Investigation, Methodology, Software, Writing – original draft. Ruifei Xue: Formal analysis, Investigation, Methodology, Software, Writing – original draft. Jian Wang: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. Yuanwang Qiu: Investigation. Yuxin Chen: Investigation. Jiacheng Liu: Investigation. Li Zhu: Investigation. Jie Zhan: Investigation. Suling Jiang: Investigation. Shengxia Yin: Investigation. Xin Tong: Investigation. Weimao Ding: Investigation. Jie Li: Investigation. Chuanwu Zhu: Conceptualization, Data curation, Supervision, Writing – review & editing. Rui Huang: Conceptualization, Data curation, Supervision, Writing – review & editing. Chao Wu: Conceptualization, Data curation, Supervision, Writing – review & editing.