The association of Non-Hodgkin lymphomas and Hepatitis C virus is well documented and antiviral treatments facilitate a virological and hematological response in the majority of HCV related Non-Hodgkin lymphomas. The recent years, direct acting antivirals have made cure possible almost for every HCV patient. Some concerns were raised as regards the frequency and the pattern of recurrence in HCV patients with HCC, treated with these agents. We present a patient with DLBCL, in remission after appropriate treatment, HCV cirrhosis that was cured with the new antivirals and shortly after SVR, he experienced a lethal lymphoma recurrence.
The association between hepatitis C virus (HCV) and non-Hodgkin's lymphoma (NHL) is common, especially marginal zone lymphoma, diffuse large B-cell lymphoma (DLBCL) and to a lesser extent follicular lymphoma [1]. HCV lymphomagenesis represents a fascinating model of malignant transformation involving several mechanisms, including chronic antigenic stimulation, interactions with the infected microenvironment and the inflammatory cytokines as well as direct transformation by virus proteins [2].
Among the HCV-associated aggressive NHL, DLBCL is the most frequent type. The first line treatment is the immunochemotherapy regimen R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). Antiviral treatment in combination or after the completion of immunochemotherapy, should also be recommended with the aim to eliminate the lymphoma trigger and potentially reduce the risk of lymphoma relapse [3]. In a large cohort study, sustained virological response (SVR) after antiviral treatment was shown to reduce the risk of B-cell NHL compared to untreated patients [4].
2Case reportWe present a 57-year old male who was referred for treatment of chronic hepatitis C (CHC), having had a history of B-cell NHL. He was diagnosed with DLBCL (stage IIA, Ann Arbor classification) following a biopsy of a supraclavicular lymph node, that showed infiltration by malignant cells CD20, CD10, Bcl-6 (40%), MUM1(30%) and BCL-2 positive, and Cyclin D1, CD5, CD23, CD3 negative. The Ki-67 was 50% positive (prognostic index at lymphoma diagnosis: IPS score: Low risk, R-IPS score: very good). The patient was treated with 6 cycles R-CHOP and achieved a complete response confirmed by whole body PET CT and bone marrow biopsy.
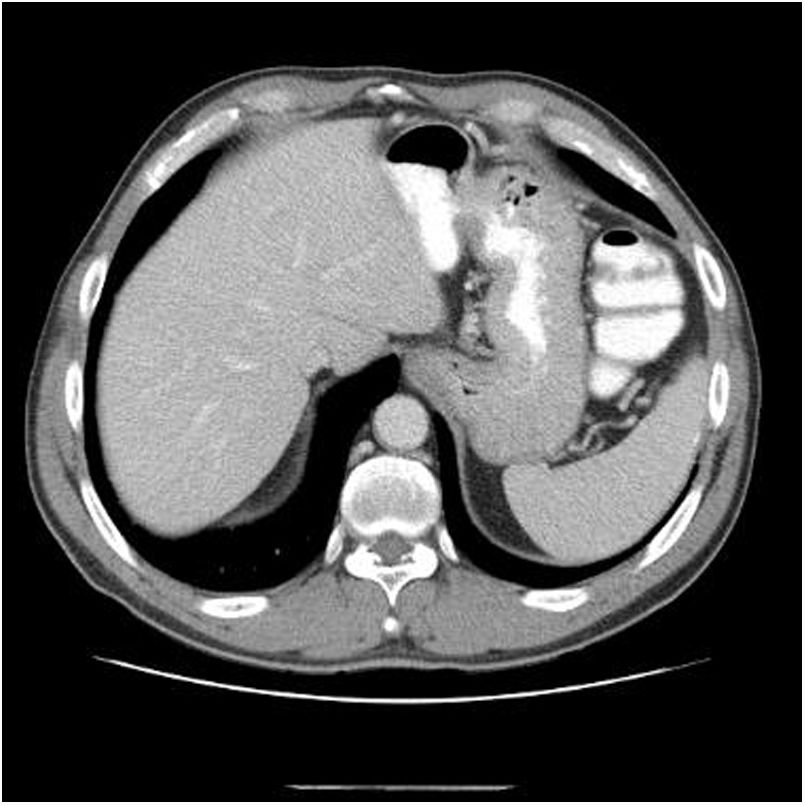
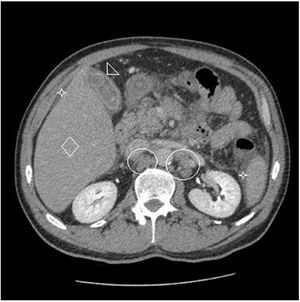
Eighteen months after completing chemotherapy, the patient was referred to the liver clinic for hepatitis C treatment. The clinical examination was unremarkable, the aminotransferases were mildly elevated (AST 46, ALT 66) the viral load was high (HCV-RNA 1.600.000IU/ml) and HCV genotype was 3a, whereas liver stiffness (evaluated by Fibroscan®) was 17.6kPa corresponding to cirrhosis (Child–Pugh A5, MELD 8). Upper endoscopy showed small esophageal varices whereas liver imaging (abdominal CT) was negative for any focal lesion or ascites (Fig. 1). He was treated according to the guidelines at that time with the direct acting antivirals (DAAs) sofosbuvir400mg/velpatasvir 100mg in combination with weight-based ribavirin (due to cirrhosis) for 12 weeks. The treatment was straightforward and uneventful. Three months after the end of treatment SVR was documented.
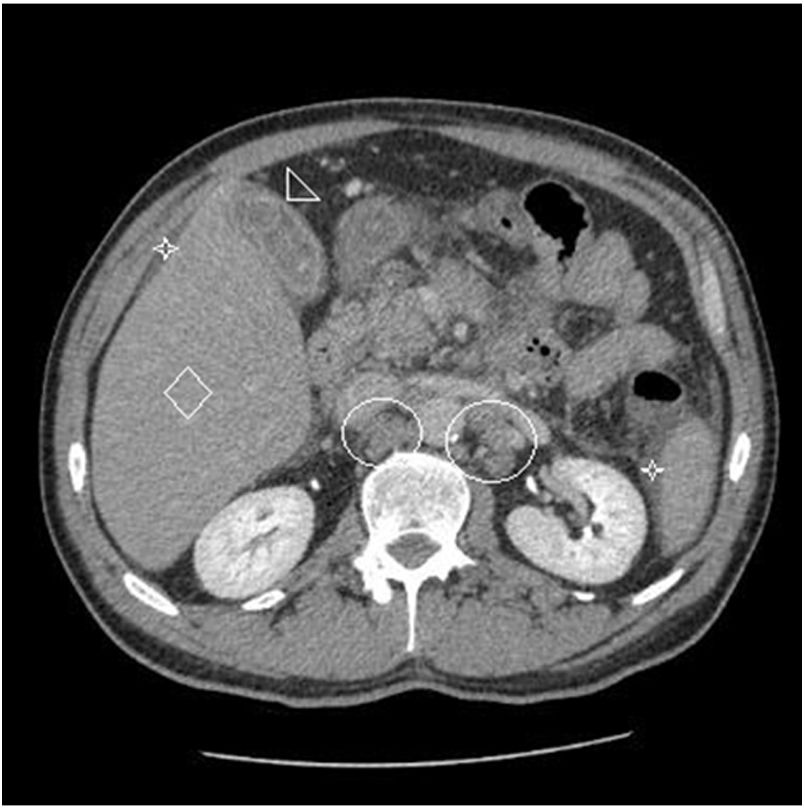
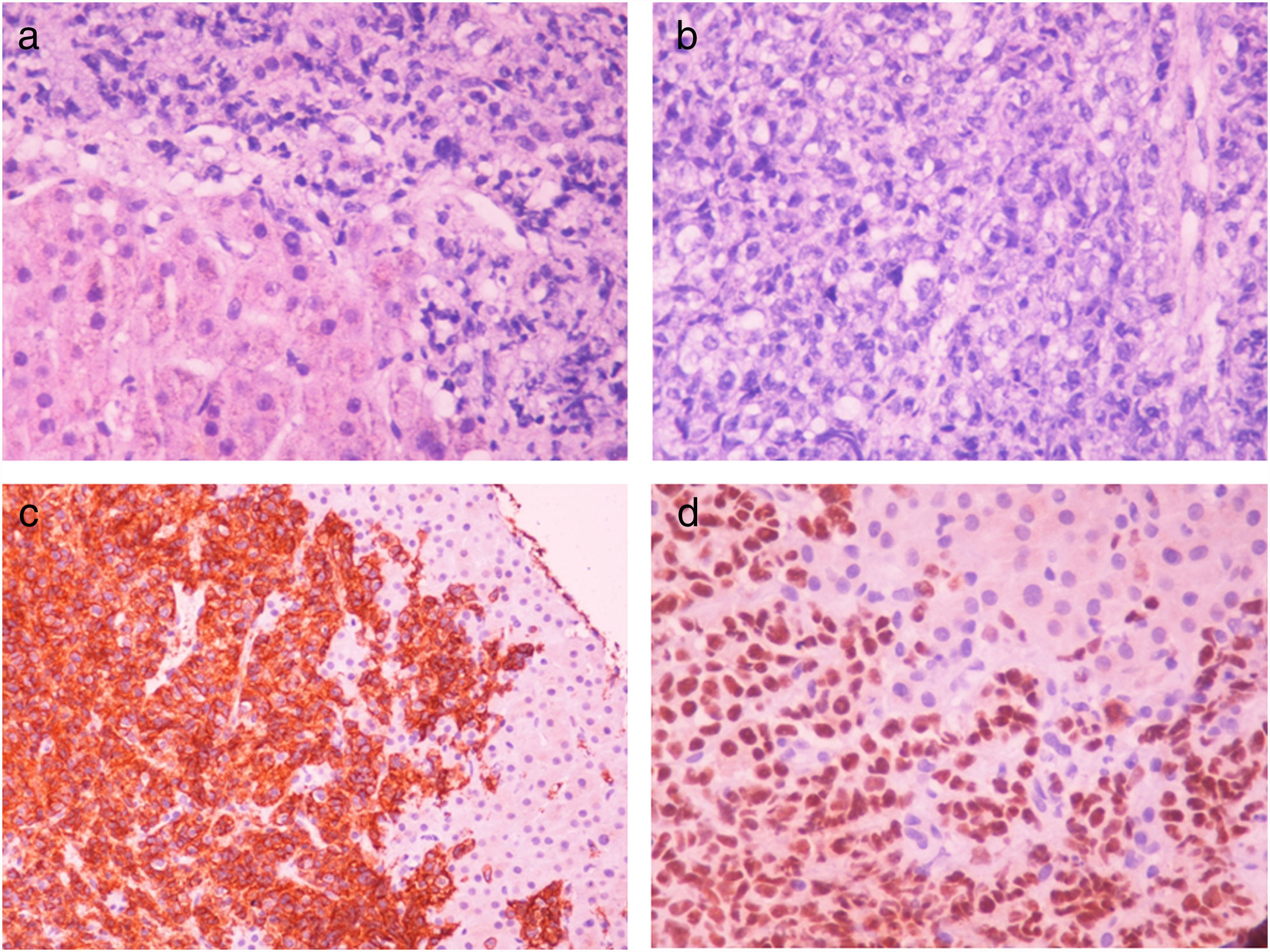
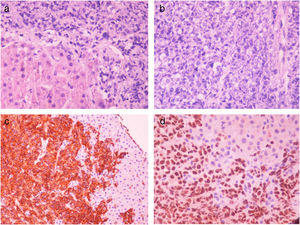
Two months after achieving SVR and despite a complete DLBCL remission being repeatedly documented (PET CT, hematological indices) for two consecutive years, the patient was admitted with right upper quadrant pain and deteriorating liver function with jaundice and INR prolongation. A CT scan showed generalized lymphadenopathy, abnormal thickening of terminal ileum, hepatomegaly and splenomegaly with ascites (Fig. 2). The liver biopsy showed (Fig. 3) extensive infiltration of liver parenchyma by DLBCL (Ann Arbor Stage IV at relapse, IPS score: high, R-IPS score: poor). The profile of the DLBCL in the liver was the same with the primary (CD20+, PAX-5+, CD3−, CD30−) that was originally treated. The patient succumbed from liver failure before receiving any chemotherapy.
B-cell NHL is associated with chronic HCV infection and implicates chronic antigenic stimulation. Antiviral therapies may result in virological and hematological response in two thirds of patients with HCV-related NHL [5,6]. In a meta-analysis of 20 studies, the response rate with antiviral treatment was 73% and a strong correlation between SVR and lymphoma response was identified [7,8]. DAA treatment has been reported to reduce the frequency of the malignant B-cells in peripheral blood of patients affected by HCV-related lymphoproliferative disorders, however monoclonal populations can persist after viral eradication [9].
There is a lot of debate in the literature, on Hepatocellular Carcinoma (HCC) recurrence after DAAs treatment in patients with CHC and HCC. A number of reports have shown unexpected high rate and aggressive pattern of HCC recurrence in patients receiving DAAs treatment for HCV [10,11]. However, a recent systematic review and meta-analysis found no evidence that HCC occurrence or recurrence is different between interferon or DAAs based antiviral treatment [12].
Very few data exist on NHL recurrence after successful antiviral therapy of CHC in NHL patients. A recent study [13] reported the occurrence of NHL after achieving SVR in two patients treated with sofosbuvir/ledipasvir without prior history of hematological disease. A similar paper [14] reported the development of highly aggressive mantle lymphoma only one month after the completion of DAA treatment with sofosbuvir/ribavirin. Another series [15] reported five cases of HCV-related B-cell NHL following DAAs treatment, all but one achieving SVR and complete remission of NHL. Very recently, a similar to our patient's case was published from Japan where a patient treated for DLBCL, presented a relapse with fatal outcome 7 years after lymphoma treatment. The patient was treated with DAAs (sofosbuvir/ledipasvir for genotype 1b) and immediately after achieving SVR, presented with aggressive lymphoma recurrence [16].
The mechanism for the aggressive recurrence of HCC in patients with DAA-mediated HCV clearance is not clear yet. It has been hypothesized that this treatment restores frequency and function of certain immune cells, such as virus-specific CD8+ T cells and NK cells. Moreover, the lack of continuous interferon stimulation in the liver after elimination of the virus may also have a significant impact on intrahepatic immune responses. These alterations of the immunological balances in the liver have been related to HCC recurrence and progression [17].
There are no relevant data for patients with lymphoma recurrence as this is much rarer. Restoration of host T-cell and B-cell mediated immunity through HCV eradication, may result in dysregulated host immune response facilitating additional clonal evolution of NHL. Moreover, there is a suspicion that DAA might have a lower anti-lymphoma activity than IFN, as a percentage of patients presents early or late progression of the disease or hematologic relapse despite SVR; such events are much rarer with IFN which acts as immunological modulator. Another explanation for a possible lower efficacy of DAA on HCV-associated lymphomas is their lack of antiproliferative activity [18].
In cases of HCV-positive DLBCL, a crucial role for dysregulation of the microRNA network has been described and microRNA-26b (miR-26b) downregulation is involved in weakening tumor suppression [19]. A recent study [20] showed an inverse correlation of HCV viral load as well as lymphoma burden with miR-26b expression in peripheral blood mononuclear cells of HCV-NHL patients, which was proposed as potential biomarker to predict lymphoma response in HCV-NHL patients.
Our patient's case with DLBCL and successful treatment of HCV that shortly after SVR presented aggressive fatal relapse is a rarity in the literature. It highlights the need for close follow up of patients with HCV and NHL, in particular those with aggressive phenotypes even after excellent response following DAAs.AbbreviationsHCV hepatitis C virus hepatocellular carcinoma diffuse large B cell lymphoma Non Hodgkin's lymphoma sustained virological response direct acting antivirals gastrointestinal endoscopy interferon
Dr. Dimitrios N. Samonakis has received advisory board & consulting fee from Gilead sciences.