Hepatitis B virus (HBV) related acute liver failure (ALF) is uncommon in our region, and there is limited HBV literature regarding the optimal management of these cases. In this article, we report two clinical cases of young men who have sex with men (MSM), both developed severe acute hepatitis caused by HBV, progressed to ALF and afterward required liver transplantation. Antiviral post-transplant treatment included entecavir without Hepatitis B Immunoglobulin (HBIG), and immunosuppression therapy with steroids, tacrolimus, and mycophenolate. Serologic follow-up showed early Hepatitis B surface Antigen (HBsAg) seroconversion, undetectable HBV viral load, and positive Anti-HBs titers. During later follow-up, Anti-HBs titers gradually fell (<10mUI/L after six months), with normal liver function.
DiscussionIn cases of HBV-related ALF, the liver develops a robust immune response, leading to, an early undetectable viral load and seroconversion, with loss of HBsAg, and the appearance of Anti-HBs as a result of the inflammatory response. The management varies depending on whether this is a de novo acute infection or a reactivation of a previous chronic infection. In both cases, the use of antiviral therapy is recommended, with entecavir or tenofovir, among others, but the use of specific HBIG is supported only in ALF related to chronic HBV infection. The optimal length of the antiviral therapy after liver transplantation is still under discussion.
ConclusionThese cases of HBV related ALF with an early HBsAg seroconversion demonstrates the relevance of requesting IgM antibody against hepatitis B core antigen (anti-HBc IgM) for the etiological study of ALF with negative HBsAg. Usage of HBIG does not seem essential during the post-transplantation period in these cases.
Infection due to hepatitis B virus (HBV) is the leading cause of acute hepatitis in eastern countries. The diagnosis of acute hepatitis B is based on the detection of Hepatitis B surface Antigen (HBsAg) and IgM antibody against hepatitis B core antigen (anti-HBc IgM). During the initial phase, the markers of replication HBeAg and DNA are present. The recovery phase consists in the disappearance of DNA, seroconversion of HBeAg to anti-HBe and HBsAg to anti-HBs. However, in cases of acute liver failure (ALF), HBsAg becomes negative, the clearance of the virus tends to be faster, and anti-HBc IgM could be the only marker of acute infection. Acute hepatitis infection can appear in the context of an acute de novo HBV infection, or in patients with chronic hepatitis B infection who develop an acute reactivation spontaneously or induced by immunosuppressors. The clinical, biochemical and serological features are similar between them. Medical records of HBV-associated chronic infection or family history of HBV infection may suggest reactivation, while high-risk sexual behavior and substance abuse, may suggest de novo hepatitis B. In acute infection, laboratory shows higher levels of anti-HBc IgM with lower levels of DNA viral load, HBeAg and HBsAg, than levels seen in reactivation [1]. The distinction between both situations is essential to differentiate their clinical courses, prognosis, and treatment [1].
In these cases, the prognosis is reduced, with a liver transplantation (LT) free survival ranging from 26 to 53% [2]. In patients with low risk of recurrence after LT, as in ALF cases, either no hepatitis B immunoglobulin (HBIG) or HBIG for a short period (5–7 days) combined with long-term antivirals, has been proven effective [3,4]. As HBV-related ALF is uncommon in our region, there is limited medical literature for its treatment. Two clinical cases, requiring LT, and management with antiviral drugs without HBIG, are described below.
2Case 1A 27-year old male, obese, with a history of being men who have sex with men (MSM), who presented jaundice, choluria and abdominal pain with progressive loss of consciousness, and was later hospitalized. He was diagnosed with ALF with hemodynamic instability and grade III hepatic encephalopathy, requiring ventilatory support. The initial etiological study was negative for HBsAg, Anti-Hepatitis C virus and HIV. The serological laboratory showed: antiHBc-IgM (+), HBeAg (−), AntiHBe (+), AntiHBs (+) and low HBV viral load (Table 1a); entecavir 1mg/day was initiated, within the first 18h of hospitalization and, the patient was transferred to a transplant center. In the following days, he had severe encephalopathy without cerebral edema nor focal lesions in the brain CT scan, but with a transcranial doppler ultrasonography measures suggesting of intracranial hypertension. The intracranial pressure (ICP) monitor, confirmed a 30mmHg pressure with good response to the initial medical treatment. The LT was performed 72h after the introduction of antiviral treatment. Immunosuppression induction therapy was with basiliximab, methylprednisolone and later treatment with tacrolimus, mycophenolate, and prednisone. Biopsy of explanted liver showed acute lobular hepatitis, with extensive necrosis of the parenchyma and 70% of macro and microvesicular steatosis.
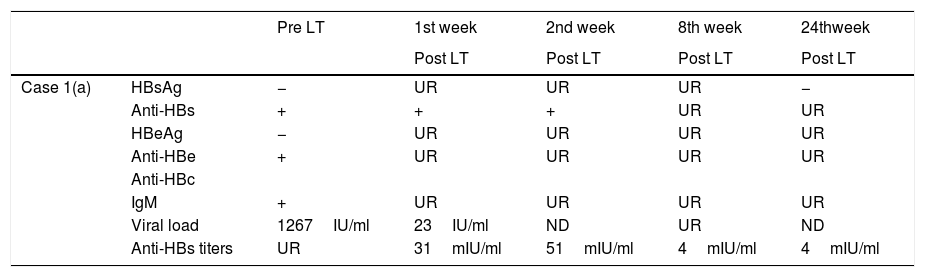
Evolution of HBV serological markers before the Liver Transplant (LT) and first six months after LT, for both patients.
| Pre LT | 1st week | 2nd week | 8th week | 24thweek | ||
|---|---|---|---|---|---|---|
| Post LT | Post LT | Post LT | Post LT | |||
| Case 1(a) | HBsAg | − | UR | UR | UR | − |
| Anti-HBs | + | + | + | UR | UR | |
| HBeAg | − | UR | UR | UR | UR | |
| Anti-HBe | + | UR | UR | UR | UR | |
| Anti-HBc | ||||||
| IgM | + | UR | UR | UR | UR | |
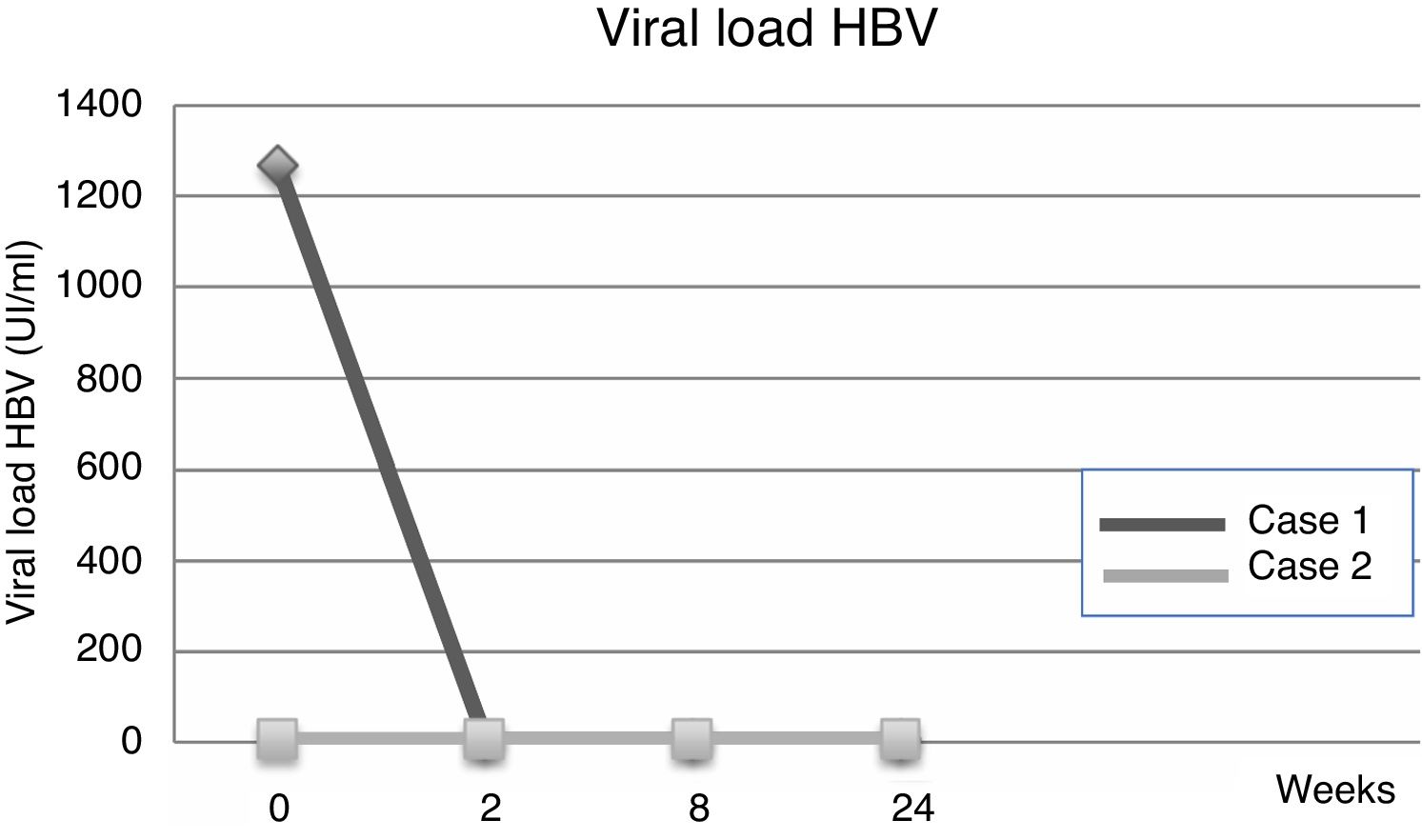
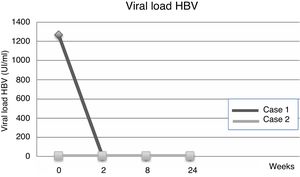
| Viral load | 1267IU/ml | 23IU/ml | ND | UR | ND | |
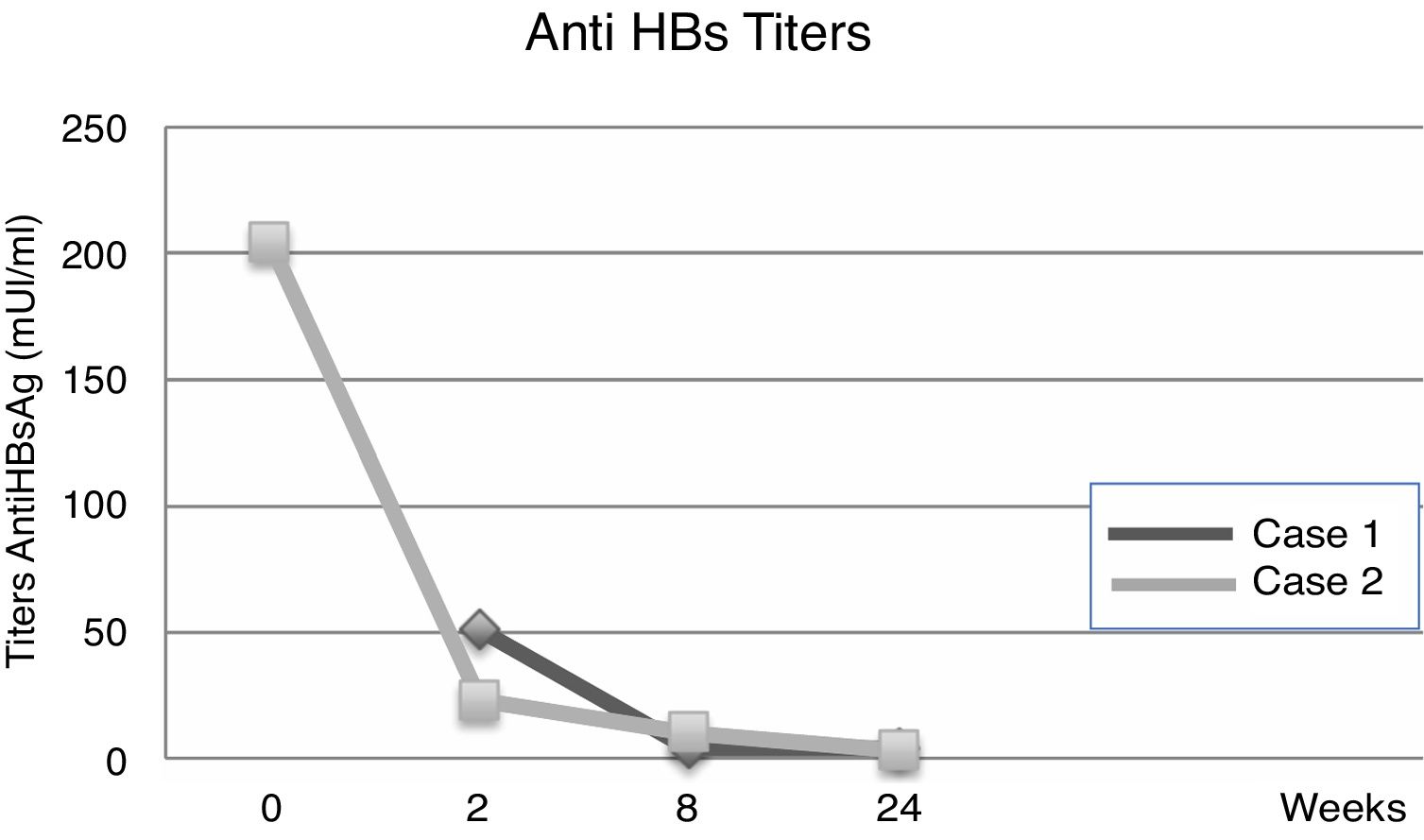
| Anti-HBs titers | UR | 31mIU/ml | 51mIU/ml | 4mIU/ml | 4mIU/ml |
| Pre LT | 1st week | 2nd week | 8th week | 24thweek | ||
|---|---|---|---|---|---|---|
| Post LT | Post LT | Post LT | Post LT | |||
| Case 2(b) | HBsAg | − | − | UR | UR | − |
| Anti-HBs | + | UR | + | UR | UR | |
| HBeAg | − | UR | UR | UR | UR | |
| Anti-HBe | + | UR | UR | UR | UR | |
| Anti-HBc | ||||||
| IgM | + | UR | UR | UR | UR | |
| Viral load | <6IU/ml | UR | UR | <6IU/ml | ND | |
| Anti-HBs titers | 204mIU/ml | UR | 23mIU/ml | 10mIU/ml | 3mIU/ml |
UR: Unrequested/ ND: Non-detectable
Fourteen days after transplantation, the patient had an acute hepatic cellular rejection with a satisfactory response to standard therapy. At the time of discharge, the patient maintained treatment with entecavir indefinitely. Six months after transplantation, the patient presented HBsAg (−), HBV viral load (−), Anti-HBs titers below 10IU/L and normal liver function (Table 1a) (Fig. 1).
3Case 2A 28-year-old male, with a history of MSM, with multiple sexual partners, excessive alcohol consumption, and heavy smoking. He presented with a 5-day history of physical discomfort, abdominal pain, and jaundice. The initial etiological study was negative for HBsAg, Anti-Hepatitis C virus and HIV, being diagnosed with HBV-related ALF due to an Anti-HBc IgM (+) (Table 1b), initiating treatment within the first 24h of hospitalization with entecavir 0.5mg/day. On the following days, he had severe coagulopathy and progressive encephalopathy, requiring invasive ventilatory support. Nine days after starting the antiviral therapy, the patient required a LT. Explant showed a 578-g liver, with extensive panlobular necrosis, occasional remnants of periportal hepatocytes, without fibrosis or iron deposits, compatible with fulminant hepatitis. The immunosuppression induction received was basiliximab, and later treatment was with tacrolimus and prednisone. Entecavir treatment was maintained to avoid recurrence with no use of HBIG. Further follow-ups showed a progressive decrease of transaminases, with normal doppler ultrasound. At the time of discharge, the patient maintained entecavir therapy indefinitely. Six months after transplantation, he maintained HBsAg (−), HBV viral load (−), Anti-HBs titers under 10mUI/L, and normal liver function (Table 1b) (Fig. 2).
4DiscussionThe diagnosis of HBV-related ALF is characterized by acute hepatitis, associated with altered mental status and prolonged prothrombin time, with INR>1.5. Typical serology findings are HBsAg (−), Anti-HBs (+), Anti-HBc IgM (+), HBeAg (−), Anti-HBe (+) and DNA HBV (−), as a consequence of the aggressive immune response against HBV [5,6]. The classification according to the time of presentation between the onset of jaundice and development of hepatic encephalopathy is: hyper-acute (<7 days), acute (7–28 days) or sub-acute (from 4 to 26 weeks), each of them with different prognosis [7].
HBV is one of the most important causes of ALF in Eastern countries. The condition can appear after an acute HBV infection, or in chronic carriers of the virus that develop a reactivation usually in an immunosuppressive environment [8–10]. Most immunocompetent adults, with de novo acute infection, recover spontaneously and treatment may not be required. Less than 4% of the acute hepatitis cases evolve with ALF [11].
Reactivation can occur in chronic HBV carriers with or without a positive HBsAg (being Anti-HBc positive). Diagnosis criteria are: detectable DNA viral load, whereas previously was undetectable, a rise in DNA viral load compared to historic values and, seroconversion from HBsAg negative/anti-HBc positive to HBsAg positive [4]. The clinical presentation depends on patient's comorbidities, use of immunosuppressive drugs, serological and immunological status, and viral factors such as genotype, and presence of mutations. Reactivation of chronic hepatitis B infection needs treatment to reduce the risk of hepatic decompensation and eventually development to ALF which in these cases have higher mortality than de novo acute infections [1,12,13]. If acute on chronic liver failure (ACLF) develops, the prognosis is poor, with a 3 month-mortality without LT of 50–55%, going as high as 90%, in cases of MELD score >30 [14].
It is hard to establish a differential diagnosis between those conditions since it is possible to be unaware of the patient's basal “HBV status” [15,16]. Patients that present with an HBV-related ALF may eventually have a chronic HBV infection, manifesting clinically for the first time as reactivation, making it difficult to distinguish the presence of cirrhosis [17].
From a pathogenic point of view, patients infected with HBV can show an intense immune response, which may lead to a quick elimination of the virus and the development of ALF. During the early stage of the infection, the HBV does not directly stimulate the immune system, but rather the immune system recognizes specific molecular patterns associated to the virus or the innate immune response of the hepatocytes detects the presence of HBV and inhibits its replication [18]. During the later stage, an innate immune response develops through a cytolytic immune mechanism, cytotoxic T CD8 lymphocytes attack the infected hepatocytes, recognizing the protein epitopes of HBV, especially the core antigen, and using another non-cytolytic T lymphocytes mediated mechanism, the gamma interferon and alpha tumoral necrosis factor is activated, because of their antiviral effects. This aggressive immune response induces the elimination of the virus, at the expense of generating severe liver damage. This is why, cases of HBV-related ALF have high levels of anti-HBc IgM, as well as low viral loads; as presented in our cases [19].
Molecular biology studies suggest that specific genotypes of the virus could predispose the HBV infected individual to develop ALF. A study carried out in the USA by the “Acute Liver Failure Study Group”, showed a high prevalence of the D genotype in acute severe HBV infections, versus patients with HBV chronic infection (32% vs. 16%, p=0.007) [2].
Moreover, some mutations have been more prevalent in HBV-related ALF and could induce retention of the virus inside the hepatocyte, with a higher capacity to replicate. Some of these mutations associated with ALF are Bj/B1 genotype and A1762T/G1764A, G1896A, G1899A, and A2339G mutations [20]. There is also a T1961V/C1962D mutation, which induces substitution of the HBV core protein [21].
There is no recommendation for the use of antiviral therapy for HBV during non- severe acute hepatitis; however, its use is widely supported for ALF cases, with the primary purpose of avoiding recurrence in patients requiring a LT [22].
As the prognosis of ALF worsens with the onset of encephalopathy, it is essential to enlist the patient for LT once the King's College's criteria (KCC) are fulfilled [2,22].
The KCC is based in a large study of 588 patients during fifteen years. It can be applied to acetaminophen-induced and non-acetaminophen induced ALF. For the latter, it has a positive predictive value of 96%, a negative predictive value of 82% and a predictive accuracy of 92% [23].
As for the Clichy criteria, they were developed upon a cohort of 115 patients with ALF due to HBV and has a positive predictive value of 96%, a negative predictive value of 50% and a predictive accuracy of 80% [24]. These criteria, mainly used in France, include grade 3 or 4 hepatic encephalopathy and factor V levels <20% in patients <30 years old and <30% in patients ≥30 years old. They were described initially in a cohort of viral ALF mainly due to HBV, but lately, it uses was expanded for other causes of ALF [24]. Later studies have shown less accuracy for the Clichy criteria than initially described, so the KCC are currently the most used in all etiologies of ALF [25].
Post-transplantation recurrence risk factors described in individuals with HBV chronic infection are: elevated viral load before transplant (≥105copies/ml) with a hazard ratio (HR) of 5, development of resistance to lamivudine, post-transplantation Anti-HBs title, maintenance of prednisone beyond three months post-transplantation (HR 2), co-infection with HIV, and presence of hepatocellular carcinoma in the explant [26].
It is not entirely clear which antiviral would be the best option. In a study carried out by Tillmann et al., a 20% improvement in the survival rate was seen in historical control patients, vs. 82.4% in patients with HBV-related ALF, treated with lamivudine [27]. Likewise, other reports in the available literature support the use of lamivudine in these cases [28,29]. A study carried out by Yu et al., support the efficacy of lamivudine, comparing 40 patients who received lamivudine and 40 control patients, concluding that treatment with lamivudine significantly decreases mortality for HBV-related ALF, with a rapid decrease in viral load - one of the favorable predictors of good response to treatment [30].
Resistance to lamivudine can develop due to mutations in the reverse transcriptase region of the HBV, the most common being the mutation in the C dominium of HBV polymerase [31]. This problem would not be present in cases of acute infection due to HBV and could even represent some benefit when compared to other drugs, such as a fast response and low rate of HBV mutations [28]. Similar effects have been seen with entecavir and tenofovir when managing HBV-related ALF; with no superiority in the clinical prognosis and both drugs showing a high genetic barrier to resistance [32].
The AASLD guidelines for HBV recommend lamivudine, telbivudine, tenofovir or entecavir as alternatives for short-term treatment [33]. Otherwise, clinical practice guidelines from EASL recommend using entecavir or tenofovir only [34].
Some studies have validated the use of post-transplant schemes free of HBIG. In a meta-analysis of 1400 transplanted patients, antiviral monotherapy is compared to antiviral+HBIG combined therapy; this study showed the advantage of the combined treatment in diminishing recurrence in those patients with positive viral load at the time of transplantation, a benefit that was not evident in patients with a negative viral load. Monotherapy with potent antiviral drugs post-transplantation seems to be sufficient to avoid recurrence in patients with a negative viral load before LT [35].
It is feasible to individualize the therapy based on viral and host factors before and after transplantation. Prophylaxis with a potent antiviral (entecavir/tenofovir), without using HBIG, to avoid HBV recurrence must be considered for patients with a reduced virological risk, such as those with a low or undetectable viral load (<105copies/ml). This was the strategy used in our two cases. This approach eliminates the very high financial costs and potential medical complications derived from using HBIG [23,36]. Even in chronic hepatitis infection, Fung et al. demonstrated that long-term entecavir monotherapy is highly effective in preventing HBV reactivation after LT [37].
Currently, there is no consensus regarding the duration of antiviral drugs treatment in HBV-related ALF, or whether the use of HBIG would be recommended in those cases as we explained.
Before the year 2000, patients with LT due to HBV had lower acute cellular rejection (ACR) events attributable to HBIG use [38]. HBIG anti-inflammatory activity prevents immunological graft damage improving graft and patient's survival [39,40]. During the following years, HBIG dose were reduced and eventually suspended in HBV ALF cases, and ACR incidence did not change. A multivariate analysis from Veerappan et al., showed that HBV is an independent factor of reduced ACR incidence, with no statistically significant difference between HBIG vs. no HBIG administration. These findings may be due to the innate immunosuppressive properties of chronic HBV infection [41].
In case number 1, our patient did not receive HBIG and developed ACR after LT. He was treated with high dose steroids and, antiviral drugs having a favorable outcome, overcoming the rejection and keeping an undetectable viral load. This outcome is presumably due to the patient's immune status, which is not majorly affected in ALF setting, where HBV is rapidly eliminated precisely because of a potent immune response.
5ConclusionIn summary, HBV-related ALF is not a frequent condition in our region; being more common in the Middle East and Eastern Asia. The two clinical cases described above had negative HBsAg at the time of diagnosis and positive anti-HBsAg. This should highlight the importance of requesting a full etiological HBV study, including anti-HBc IgM, used to diagnose ALF caused by HBV, when the host's immune response exceeds the damage done by the virus itself.
AbbreviationsHBV Hepatitis B virus acute liver failure men who have sex with men Hepatitis B immunoglobulin Hepatitis B surface Antigen antibody against hepatitis B core antigen liver transplantation acute on chronic liver failure King's College's criteria hazard ratio acute cellular rejection
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors have no conflicts of interest to declare.
None.