Acute liver failure, also known as fulminant hepatic failure (FHF), includes a spectrum of clinical entities characterized by acute liver injury, severe hepatocellular dysfunction and hepatic encephalopathy. The objective of this study was to assess cerebral autoregulation (CA) in 25 patients (19 female) with FHF and to follow up with seventeen of these patients before and after liver transplantation.
Patients and MethodsThe mean age was 33.8 years (range 14–56, SD 13.1 years). Cerebral hemodynamics was assessed by transcranial Doppler (TCD) bilateral recordings of cerebral blood velocity (CBv) in the middle cerebral arteries (MCA).
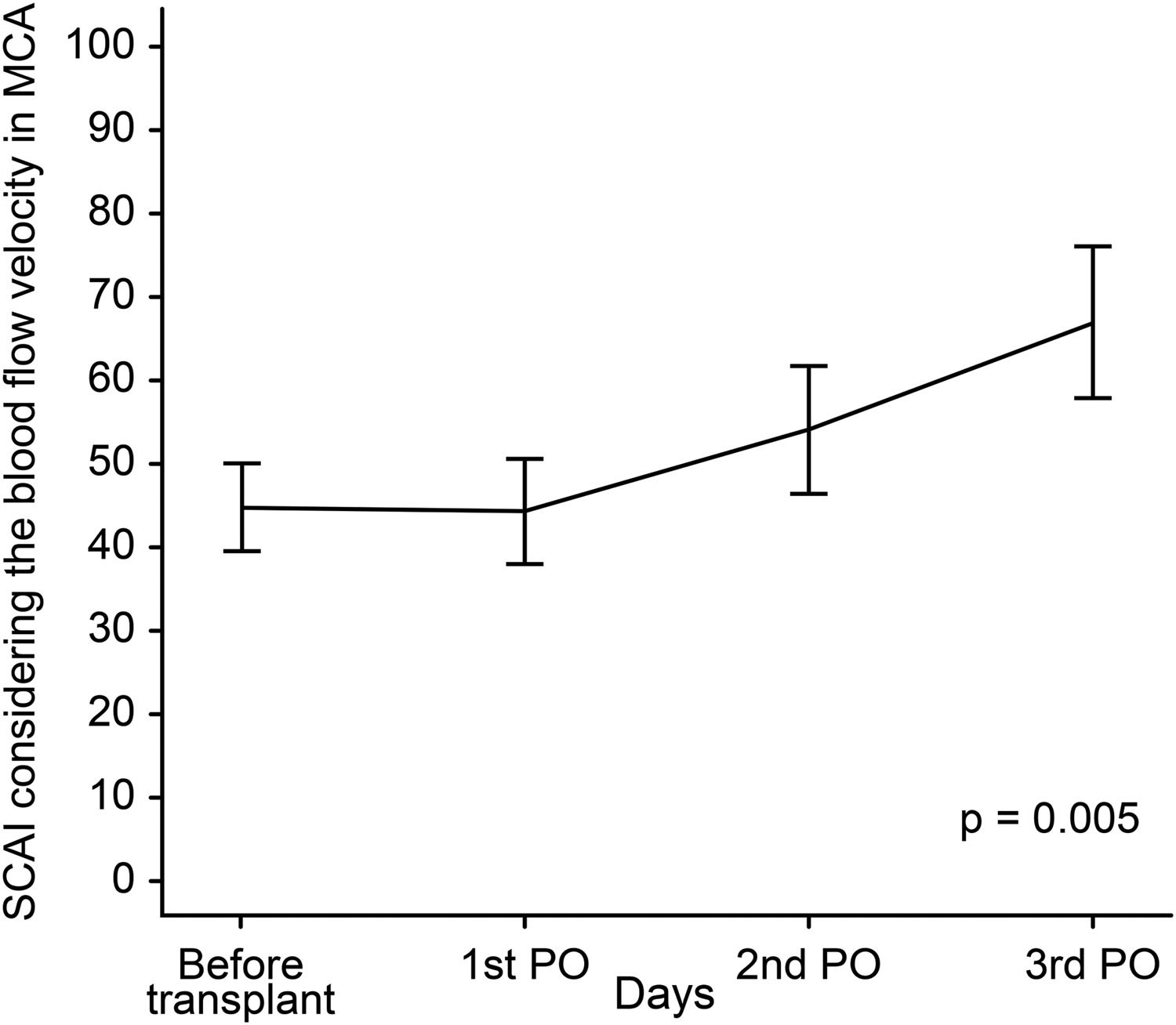
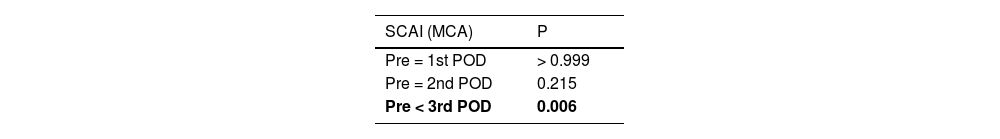
ResultsCA was assessed based on the static CA index (SCAI), reflecting the effects of a 20–30 mmHg increase in mean arterial blood pressure on CBv induced with norepinephrine infusion. SCAI was estimated at four time points: pretransplant and on the 1st, 2nd and 3rd posttransplant days, showing a significant difference between pre- and posttransplant SCAI (p = 0.005). SCAI peaked on the third posttransplant day (p = 0.006). Categorical analysis of SCAI showed that for most patients, CA was reestablished on the second day posttransplant (SCAI > 0.6).
ConclusionsThese results suggest that CA impairment pretransplant and on the 1st day posttransplant was re-established at 48–72 h after transplantation. These findings can help to improve the management of this patient group during these specific phases, thereby avoiding neurological complications, such as brain swelling and intracranial hypertension.
Fulminant hepatic failure (FHF) is a syndrome caused by sudden and massive destruction of hepatocytes, resulting in severe liver dysfunction [1,2]. The mortality rate in FHF is high, and most cases (50 %–80 %) culminate in brain swelling and intracranial hypertension (IH) [3–5]. These outcomes might be explained by the accumulation of glutamine, cytokines and liver necrosis products that could give rise to impaired cerebral autoregulation (CA) [4]. Under normal physiological conditions, CA protects the brain from large changes in arterial blood pressure (BP), but the loss of this mechanism can lead to brain hyperperfusion, edema and capillary damage.
There is scarce data on multimodal neurological monitoring in patients with FHF [3,6]; international society guidelines have recommended the routine monitoring of intracranial pressure (ICP) despite the invasiveness of this technique and the risk of intracranial hemorrhage [7,8]. However, to date, less-invasive methods have not been fully explored. Transcranial Doppler (TCD) ultrasound is a bedside method for evaluating cerebral hemodynamics that allows evaluation of cerebral autoregulation (CA) in patients with FHF. The study of CA in this subgroup could yield valuable information about the pathophysiology of brain edema and may also allow monitoring of possible treatments.
Therefore, the aim of the present study was to test the hypothesis that cerebral blood flow autoregulation in patients with FHF is improved by liver transplantation.
2Patients and Methods2.1Study designPatients with fulminant hepatic failure selected for liver transplantation were included in this observational, single-center, prospective (cohort) study.
Patients who underwent orthotopic liver transplantation were not sedated postoperatively. Spontaneous hepatic recovery was defined as improved prothrombin time on three consecutive measurements with clinical improvement. The average wait was 24–72 h to receive the transplant.
No patients received mannitol, thiopental or muscle relaxants during the study period. A catheter was inserted into a radial artery for blood sampling and monitoring of mean BP (mBP). All patients were mechanically ventilated. Arterial carbon dioxide tension and serum levels of hemoglobin were recorded during the study phases.
2.2Inclusion criteriaPatients of any age with the clinical diagnosis and laboratory criteria of FHF, as defined by the American Association for Study of Liver Diseases (AASLD), undergoing liver transplantation or otherwise (4).
2.3Exclusion criteriaImpossibility of performing TCD exam; pre-existing liver disease; and failure to diagnosis FHF.
2.4Clinical diagnosis of hepatic encephalopathyThe diagnosis of hepatic encephalopathy (HE) was based on neurological assessment and an encephalopathy grading scale using the West Haven criteria (proposed by Conn et al. in 1977) [9]. Mental grade status was determined by assessing behavior, intellectual function, neuromuscular function and alterations in consciousness. Grade I was defined as slight lack of awareness, shortened attention span, sleep disturbance, altered mood, slowed ability to perform mental tasks and asterixis exhibited on physical examination. Grade II was defined as lethargy or apathy, temporal disorientation, amnesia of recent events, impaired simple computations, inappropriate behavior, slurred speech and asterixis. Grade III was defined as somnolence, confusion, spatial disorientation, bizarre behavior, clonus, nystagmus and presence of the Babinski sign. Asterixis is usually absent in Grade III. Grade IV was defined as an unconscious, comatose patient [9,10]. The level of HE (grades I–IV) was clinically evaluated daily from the time of admission to its complete disappearance.
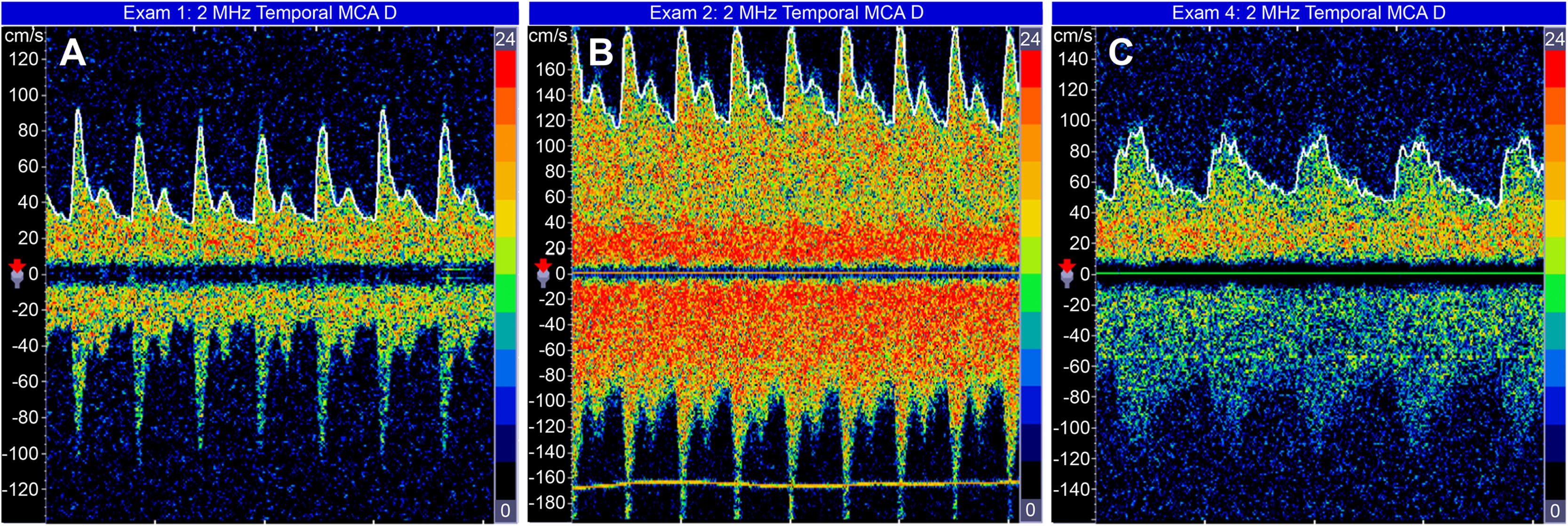
2.5Transcranial Doppler hemodynamic studiesTCD was performed using an ultrasound device equipped with a 2-MHz probe (Companion by EME/Nicoletࣨ). Blood velocities in the bilateral MCA were assessed through the temporal cranial windows using a standardized protocol described elsewhere [11–13]. The cerebral blood velocity (mean velocity and peak systolic velocity) and both the pulsatility index (peak systolic velocity – end-diastolic velocity / mean velocity) and resistance index (peak systolic velocity – end-diastolic velocity / peak systolic velocity) of each middle cerebral artery were recorded, and the correlating spectral waveforms were analyzed. The maximum cerebral blood velocity (CBv) of the two middle cerebral arteries (MCA) (Fig. 1) was used.
2.6Assessment of cerebral autoregulationThe evaluation of CA was based on studies by Tiecks et al. [14], who proposed the static autoregulation index (SCAI), a measure that considers the effects of BP elevation on CBv; the increase in ABP (20 mmHg to 30 mmHg) was induced by norepinephrine infusion at a dose of 5 µg for 15 min.
The SCAI was calculated with the following equations:
This index expresses the change in cerebrovascular resistance (CVR) as a percentage of CA full capacity. The SCAI ranges from 0 (0 %) to 1 (100 %). A value of 0 indicates that autoregulation is entirely absent, while a value of 1 reflects perfect autoregulation. A normal value of 0.7 ± 0.2 has been suggested [15–20]. For the purposes of this study, values > 0.6 represented recovery of CA.
2.7Statistical analysisThe differences among hemodynamic parametric variables were measured with repeated measures analysis of variance (ANOVA) [21] at four timepoints: preoperative, 1st postoperative (PO) day, 2nd PO day and 3rd PO day. For significant results, this was followed by post hoc analysis with multiple comparisons by the Bonferroni method among timepoints. A significance level (α) of 5 % was adopted.
2.8Ethical statementWritten informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the for Medical Research of the University of São Paulo (permit number 0750/10).
3ResultsTwenty-five patients (19 female) were included in the study from 2009 to 2013. Patients had a mean age of 33 ± 13 years (range, 14–56 years).
The etiology of FHP was unknown in 19 (76.0 %) patients. The remaining patients had the following etiologies: Wilson's disease (two patients), “Daime Saint” tea (one patient) and medicines used as 1) phenytoin (one patient), 2) propylthiouracil (one patient), and 3) rifampin and isoniazid (one patient). Eight (32 %) patients died at pretransplant and another six (24 %) patients died within the three days after transplantation.’. The main causes were sepsis and mixed shock states. No patient was diagnosed with brain death during the evaluation period.
Seventeen patients underwent computed tomography (CT) scans: six (24.0 %) had signs of brain swelling, and there were no patients with cerebral hemorrhage.
Before transplantation, two (8.0 %) patients had Grade I encephalopathy, two (8.0 %) had Grade II, fourteen (56.0 %) had Grade III and seven (28.0 %) had Grade IV encephalopathy. After transplantation, encephalopathy grade was assessed in nine patients, six (66.7 %) of whom had Grade I and three (33.3 %) had Grade II. Two patients could not be classified because they were intubated and on mechanical ventilation at the time of the final evaluation. All patients without liver transplantation died.
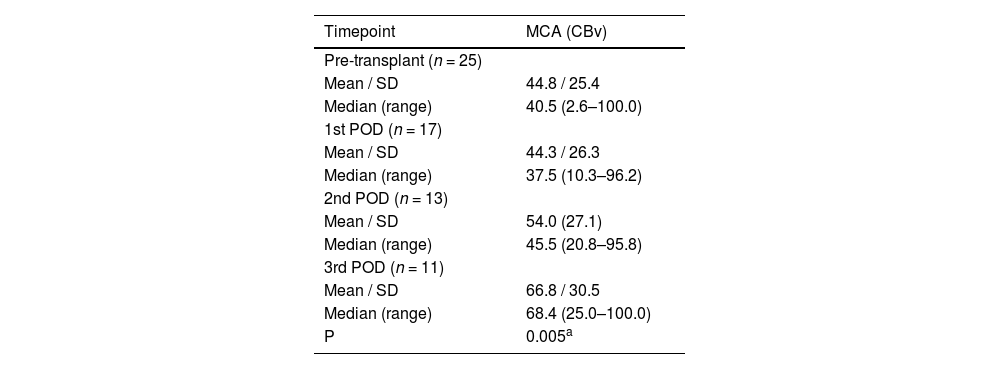
Regarding SCAI, a total of 17 patients were evaluated on the 1st PO day, 13 on the 2nd PO day, and 11 on the 3rd PO day. Table 1 and Fig. 2 depict the behavior of the SCAI considering the maximum CBv. The inferential results show the SCAI for the maximum MCA velocity (p = 0.005), revealing the same behavior across all timepoints (see Table 1). The SCAI was higher on the 3rd PO day than at pretransplantation (p = 0.006) (Table 2).
Summary of static cerebral autoregulation index measures for cerebral blood velocity in the middle cerebral artery by timepoint.
p value < 0.05 statistical significance; SD, Standard deviation; MCA, Middle Cerebral Artery; CBv, cerebral blood velocity; POD, postoperative.
Analysis of the SCAI prior to liver transplantation revealed index values of > 0.6 in only one patient. By contrast, in the posttransplant phase, 12 of 17 patients had normal SCAI: 3 (25.0 %), 5 (41.7 %) and 4 (33.3 %) on the 1st, 2nd, and 3rd PO day, respectively. All patients evaluated before and after liver transplantation who showed improvement in brain autoregulation index occurred after transplantation, no patient had recovery without liver transplantation in the analyzed period.
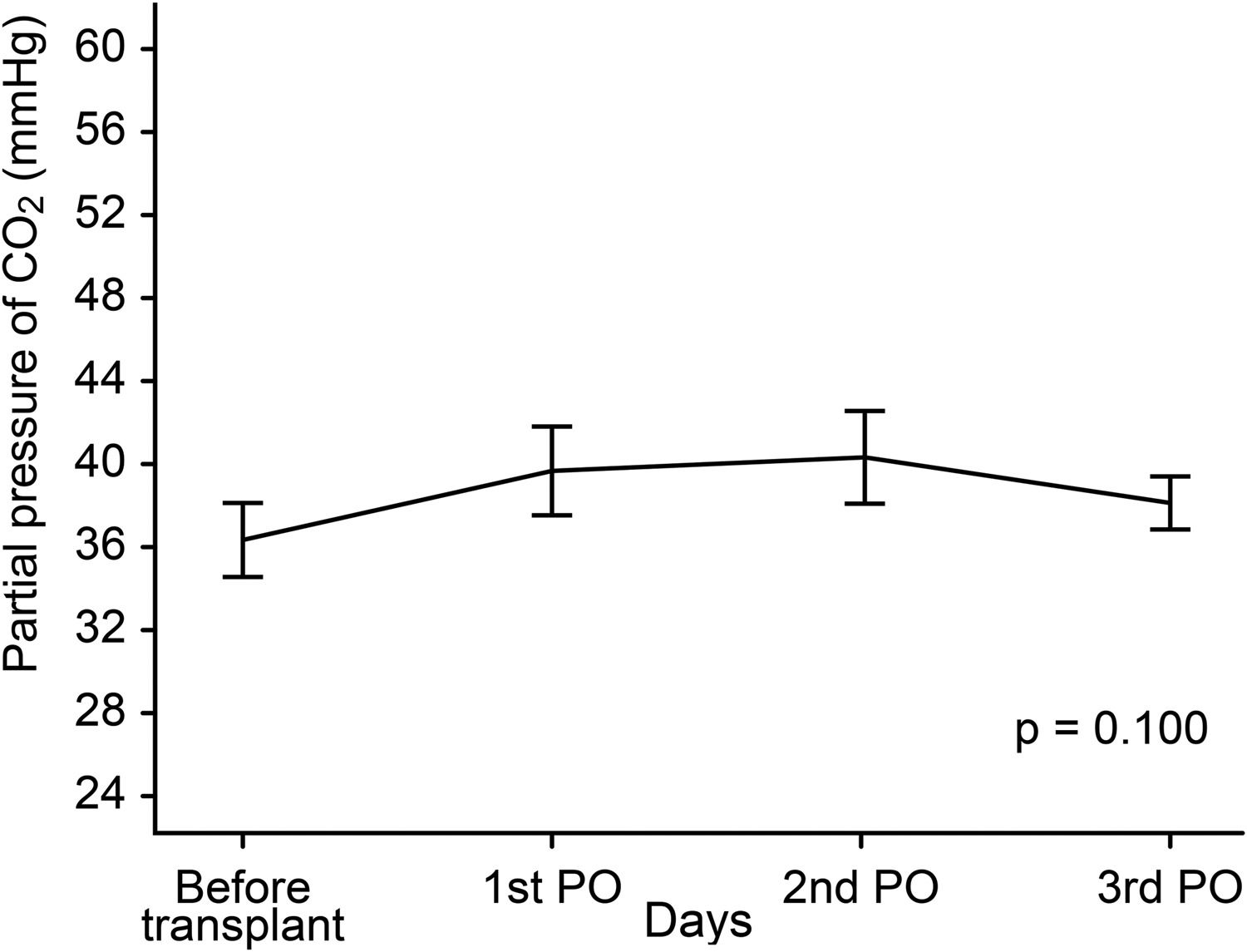
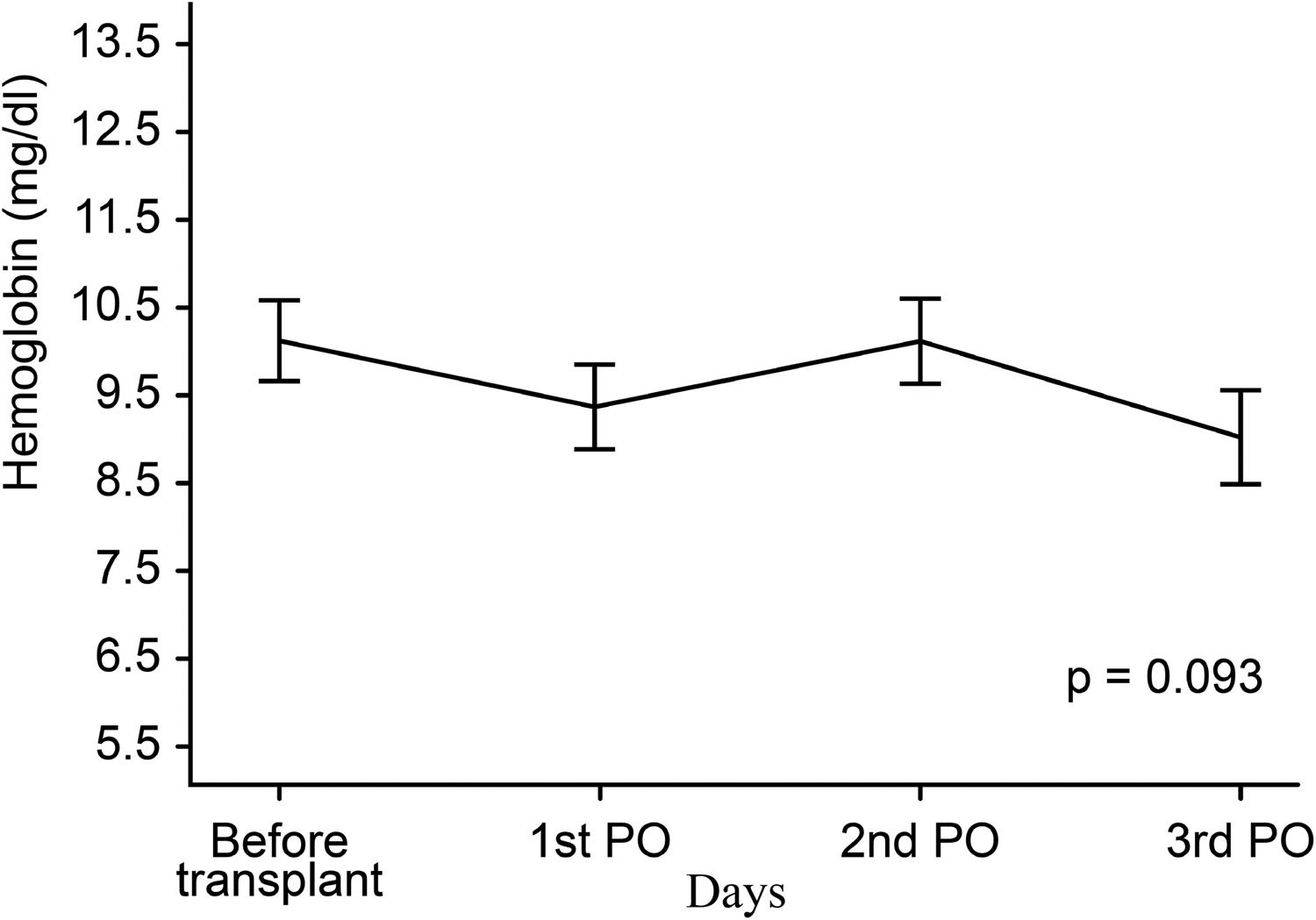
Figs. 3 and 4 show partial pressure of carbon dioxide (PCO2) levels and hemoglobin levels; each line representing the average profile over the timepoints. There were no statistically significant differences between levels of PCO2 (p = 0.100) or of hemoglobin (p = 0.093) at the timepoints.
CA may be compromised in FHF [22–24]; it appears to precede the development of elevated ICP [25,26]. Theoretically, CA impairment during FHF could be associated with substances released from necrotic liver and with the accumulation of toxic substances in the blood, including ammonia and glutamine that cannot be processed by the injured liver.
In the 1990s, numerous papers were published related to CA, especially stationary and dynamic TCD analysis [14,15]. During the same period, several studies investigating CA impairment in FHF patients were also published; however, they differed from the current study in that they involved a small number of patients or case reports and did not use the measurement of static autoregulation at several timepoints before and after liver transplantation. These differences may be explained by the severity of the patients with FHF and the difficulty in evaluating this phase [25,26].
4.1CA at various timepointsIn this study, the SCAI was increasingly higher after liver transplantation (Fig. 2). It is important to reinforce that SCAI was considered normal postoperatively, particularly on the 3rd POD, compared with the preoperative period. Similar results were reported in previous studies, indicating that CA may be re-established within a short timeframe either after liver transplantation (24–48 h) or after spontaneous liver regeneration (72–96 h) [26]. Therefore, SCAI normalization may be associated with both liver transplantation and resumed clearance of toxic substances from the blood. Another possibility is that impaired CA is the result of the release of toxic components of liver failure where total hepatectomy of the affected liver during transplant surgery may restore CA [27]. Paschoal-Jr et al. [28] studied the loss of CA in one patient who underwent liver transplantation. CA was impaired before transplantation and recovery was seen on the 3rd day after transplant. Unexpectedly, deterioration of CA was noted on the 5th day after transplantation, coinciding with hepatic artery thrombosis. The patient underwent liver retransplantation, and restoration of CA occurred 48 h later.
Dethloff et al. [29] studied CA and liver mass loss in an experimental study with rats. Each group represented different causes of acute hepatic insufficiency: 1) galactosamine poisoning, representing the hepatic necrosis group, 2) hepatectomy of 90 % of the liver, the mass loss group 3) portocaval anastomosis with shunting of toxins/blood into systemic circulation group, and 4) the control group. These groups simulated acute liver failure, yielding information on the additional effects of hyperammonemia. In two of these groups (galactosamine poisoning and hepatectomy of 90 % of the liver) the CA index indicated loss of autoregulation. On the other hand, CA was intact in the portocaval anastomosis group, despite the increase in ammonia, high concentration of glutamine and increases in both CBF and ICP. The authors concluded that the CA impairment was not caused by brain swelling /IH, and confirmed that portocaval anastomosis did not affect CA. However, massive hepatic necrosis and hepatic mass reduction were associated with a critical reduction in CA. These findings showed that CA impairment might be due to toxic substances released by necrotic liver in FHF [29,30]. In an experimental study, Larsen et al. also found that five rats with thioacetamide-induced FHF had CA impairment [22]. Later, Larsen et al. [23] demonstrated that CA was absent in patients with FHF and suggested that CBF could be maintained within the normal physiological range through control of BP, preventing hypoxia and/or intracranial hypertension induced by brain swelling.
Cardim et al. [31] retrospectively studied six patients undergoing orthotopic liver transplantation (OLT) with continuous monitoring of BP and CBv in the MCA. The main cerebral haemodynamic parameters assessed were non-invasive ICP, cerebral perfusion pressure, CA, pulsatility index, critical closing pressure and diastolic closing margin. Despite the small number of patients, their findings were in accordance with ours; they revealed marked alterations of these parameters in the OLT setting, which provide relevant clinical information when there is imminent risk of neurologic impairment.
Previous studies have shown that the value of the static CA index is considered normal within the range of 0.7 ± 0.2 (14–16), whereas other studies consider that under physiological conditions in humans (intact autoregulation), the normal value lies in the 0.85 to 0.95 range (18–20). In the present study, values > 0.6 were considered indicative of CA integrity, whereas (notably) this was not evaluated in previous studies of patients with FHF. In fact, the patients in the present study whose SCAI was > 0.6, including one patient before transplantation, had HE clinical statuses of grade I or II. However, when SCAI was < 0.6 HE, clinical status was grade III and IV. These results are consistent with those reported in the literature, showing an association between severe brain swelling and lower clinical grade [25,26,29,30]. Zheng et al. [32] performed a study of nine consecutive patients to assess CA using TCD and near-infrared spectroscopy (NIRS) during liver transplantation surgery and compared CA during the perioperative periods. CA was impaired in one patient during all phases of surgery, in two patients during the nonliver stage, and in one patient during the reperfusion phase. CA impairment was associated with convulsions and stroke in the postoperative period. It is important to point out that Zheng et al. also studied patients without FHF (32). An experimental study by De Lima Oliveira et al. [33], analyzing CA during Intracerebral Hemorrhage (ICH) induced in a nontraumatic experimental model, determining the effects of intracranial hypertension treatment on CA, observed an improvement in the index of static cerebral autoregulation after liver transplantation.
Fluctuations in PCO2 and hemoglobin, common events in patients with FHF, can be associated with changes in CBF, hampering interpretation of TCD and distorting SCAI results. In the present study, values of hemoglobin and PCO2 were also recorded, and no significant differences were found along the four examination days. This finding may indicate that these variables did not interfere with CA results during the evaluation period. Macías-Rodriguez et al. [34] conducted a study employing TCD in individuals with liver cirrhosis and HE, no liver cirrhosis or HE, and controls. The authors assessed the microcirculatory integrity of reactivity to CO2 on TCD using the apnea test (breath holding test) by measuring pulsatility index (PI). The results showed higher PI in patients with decompensated cirrhosis than in individuals with compensated cirrhosis and control subjects. Individuals with cirrhosis and HE also had lower scores on the apnea test. The study showed that brain hemodynamics were altered in patients with liver cirrhosis and were strongly associated with disease severity and presence of HE [34].
4.2Study limitationsIn the present study, the sample comprised only a relatively small number of patients. Nevertheless, compared with previous studies, either experimental or in humans, our sample size was considerably larger. Unfortunately, there was a delay in performing liver transplantations in our hospital because of delays inpatient admissions, lack of donor organs, patient incompatibility and patient infections which contributed to reduce the number of patients in our study.
Static measurements evaluate the overall effect (efficiency) of the autoregulatory action, ie, the change in cerebrovascular resistance (CVR) in response to the manipulation of ABP, but they do not address the time in which this change in CVR is achieved (that is its ‘latency’). Measurement of the dynamic response, however, yields information about the latency as well, which may be relevant in certain clinical conditions including head injury [14].
For clinical purposes, however, it seems to be fair to assume that dynamic testing reflects most aspects of the autoregulatory response correctly. Thus, evaluation of dynamic CA by TCD may be useful for patient management. A variety of pathological conditions including brain trauma, subarachnoid hemorrhage, focal ischemia and FHF affect the autoregulatory abilities to a variable extent [14,17].
Another limitation was the lack of direct evaluation of ICP, which was not used because of the risk of intracranial hemorrhage. ICP could reinforce the TCD findings of an intact or nonintact CA mechanism and reveal those cases compatible with pseudoautoregulation [35,36].
5ConclusionsOur findings confirmed the occurrence of CA impairment in FHF and demonstrated that CA can be reestablished after liver transplantation. The most notable merit of our study is the accurate measurement to monitor autoregulation for detection of loss and recovery of cerebral autoregulation, as well as cerebral hemodynamic changes that affect FHF patients by using TCD. These ideas should be supported by larger clinical studies assessing safety and clinical validity to further elucidate the pathophysiology and to improve therapeutic planning in this phase of FHF.
CRediT authorship contribution statementFernando M Paschoal-Jr: Conceptualization, Data curation, Formal analysis, Software, Visualization, Methodology, Supervision, Writing – review & editing. Ricardo C Nogueira: Data curation, Formal analysis, Software, Visualization, Methodology. Karla de Almeida Lins Ronconi: Data curation, Formal analysis, Software, Visualization, Methodology, Project administration, Resources. Marcelo de Lima Oliveira: Data curation, Formal analysis, Software, Visualization, Methodology. Kelson James Almeida: Data curation, Formal analysis, Software, Visualization, Methodology, Project administration, Resources. Ivana Schmidtbauer Rocha: Data curation, Formal analysis, Software, Visualization, Methodology, Project administration, Resources. Eric Homero Albuquerque Paschoal: Data curation, Formal analysis, Software, Visualization, Methodology, Project administration, Resources. Joelma Karin Sagica Fernandes Paschoal: Supervision, Writing – review & editing. Luiz Augusto Carneiro D'Albuquerque: Supervision, Writing – review & editing. Manoel Jacobsen Teixeira: Supervision, Writing – review & editing. Ronney B Panerai: Supervision, Writing – review & editing. Edson Bor-Seng-Shu: Supervision, Writing – review & editing.