Ascites is the most common presentation of decompensated liver cirrhosis. It is treated with therapeutic paracentesis which is associated with several complications. The role of human albumin in patients with cirrhotic ascites remains elusive and has been extensively studied with conflicting results. Thus, in order to fully appraise the available data we sought to perform this systematic review and meta-analysis. Herein we included studies comparing the efficacy and safety of human albumin comparing with other volume expanders and vasoactive agents in patients undergoing paracentesis in cirrhotic ascites. Odds ratio (OR) and mean difference (MD) were used to estimate the outcome with a 95% confidence interval (CI). Albumin use reduced the odds of paracentesis induced circulatory dysfunction (PICD) by 60% (OR 0.40, 95% CI 0.27–0.58). While performing subgroup analysis, albumin use lowered the odds of PICD significantly (OR 0.34, 95% CI 0.22–0.52) in comparison to other colloid volume expanders, but did not lower the odds of PICD in comparison to vasoconstrictor therapy (OR 0.93, 95% CI 0.35–2.45). Albumin was associated with a statistically significant lower incidence of hyponatremia (OR 0.59, 95% CI 0.39–0.88). Albumin did not reduce the overall mortality, readmission rate, recurrence of ascites, mean arterial pressure, incidence of renal impairment, hepatic encephalopathy, and gastrointestinal (GI) bleeding. Thus, treatment with albumin in cirrhotic ascites reduced PICD and hyponatremia although there was no benefit in terms of mortality, readmission rate, recurrence of ascites, hepatic encephalopathy, and GI bleeding.
Ascites is the most common presentation of decompensated cirrhosis and is associated with complications like spontaneous bacterial peritonitis and 50% mortality at 2 years [1]. It produces symptoms including abdominal distension, abdominal discomfort, dyspnea, and spontaneous bacterial peritonitis often necessitating hospitalization [2,3]. Therapeutic paracentesis is the first line of treatment for patients with tense (i.e., grade 3) and refractory ascites [4]. Patients with liver disease have altered circulatory physiology, with splanchnic arteriolar vasodilation, hyperdynamic circulation, and decreased effective arterial blood volume. It is also associated with activation of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system (SNS), along with an increased antidiuretic hormone levels and sodium and water retention. Paracentesis substantially influences hemodynamics in an already altered physiological state [5]. Therapeutic paracentesis especially large-volume paracentesis (LVP) is associated with paracentesis-induced circulatory dysfunction (PICD) which is defined as a 50% increase in plasma renin activity (PRA) over the baseline on the sixth day after treatment, up to a value > 4 ng/mL per hour [6].
The role of adjunctive albumin infusion in preventing PICD after LVP has been studied since the 1980s [2]. Guidelines dealing with the management of ascites vary in their recommendations. The current European Association for the Study of the Liver (EASL) guideline (2016) recommends the infusion of human albumin after paracentesis, regardless of volume, although emphasizing the need for its substitution after a drained volume of 5 L or more. While the American Association for the Study of Liver Diseases (AASLD) guideline (2013) recommends albumin infusion after a paracentesis of 5 L or more, however, may not be necessary for a single paracentesis of less than 4–5L [7,8].
It is worthwhile to note the limited availability and a worldwide shortage of albumin. Albumin is extracted from human serum, although having fewer side effects, does carry a theoretical risk of transmission of diseases. Although the cost varies globally, considering the amount of albumin required after paracentesis to meet demands per guidelines (6–8 g/L of fluid removal) makes albumin therapy after paracentesis an expensive intervention. Cost-effective alternatives to albumins like artificial colloid volume expanders and vasoconstrictors have been investigated. Despite numerous randomized trials, it remains uncertain whether the effectiveness of such alternative treatments is comparable to that of albumin. This uncertainty, partly, reflects the limited statistical power of the randomized trials. Quantitatively combining results of all relevant trials by meta‐analysis could help in resolving the uncertainty. Most recently a meta-analysis by Kütting et al. (2016) failed to show benefit in clinical endpoints including mortality [9]. Furthermore, two large randomized controlled trials have been published since 2016 [10,11]. Thus, to fully appraise the available data, we sought to perform this systematic review and meta-analysis including 21 trials in 1584 patients with cirrhotic ascites.
2MethodsWe used PRISMA for the systemic review of available literature [12]. Our meta-analysis protocol was registered in Prospero (CRD42020223464) [13].
2.1Types of studiesAll randomized controlled trials (RCTs) published till 18 May 2021 comparing efficacy and safety of human albumin with other volume expanders and vasoactive agents following paracentesis in patients with ascites due to cirrhosis were included. Non-RCTs, Editorials, Commentaries, Viewpoint articles with no proper data of human albumin treatment in patients with ascites due to cirrhosis were excluded.
2.2Types of participantThe study included all the adult patients (≥ 18 years old) diagnosed with tense and/or refractory ascites due to cirrhosis.
2.3Types of interventionWe included studies where human serum albumin was administered following paracentesis in the patients with tense and/or refractory ascites due to cirrhosis.
2.4ComparatorComparators were placebo, standard care of management (diuretic therapy, and sodium restriction, and use of vasoconstrictors or other volume expanders after therapeutic paracentesis).
2.5Types of outcome measuresOur outcomes were incidence of paracentesis induced circulatory dysfunction, mortality, mean arterial pressure (MAP) at 3–4 days of treatment, hyponatremia, the reappearance of ascites, hospital readmission, and complications like hepatic encephalopathy, renal impairment, and gastrointestinal bleeding.
2.6Search methods for identification of studiesPublished papers were screened by two authors based on inclusion and exclusion criteria using Covidence [14]. Any possible conflicts were resolved by a third member.
2.6.1Electronic searchesWe have included the electronic search strategy in Supplementary file 1.
2.6.2Data collection and analysisDatabases like PubMed, PMC, Scopus, and Embase were searched using appropriate Boolean with no language restriction. We extracted the data onto a standardized form designed in Microsoft Excel-13.
2.6.3Selection of studiesWe included studies comparing the efficacy and safety of human albumin treatment with outcomes of our interest in patients with ascites due to cirrhosis following paracentesis. We included studies that had both treatment and control groups. The treatment group was comprised of patients with liver cirrhosis and ascites who were treated with albumin after paracentesis and the control group was comprised of patients with liver cirrhosis and ascites who received paracentesis followed by standard medical treatments either placebo or vasoconstrictors or other volume expanders. Editorials, Commentaries, Viewpoint articles with no proper data of human albumin treatment in patients with ascites due to cirrhosis were excluded.
2.7Data extraction and managementWe evaluated the quality of studies and included the outcome in the qualitative and quantitative synthesis.
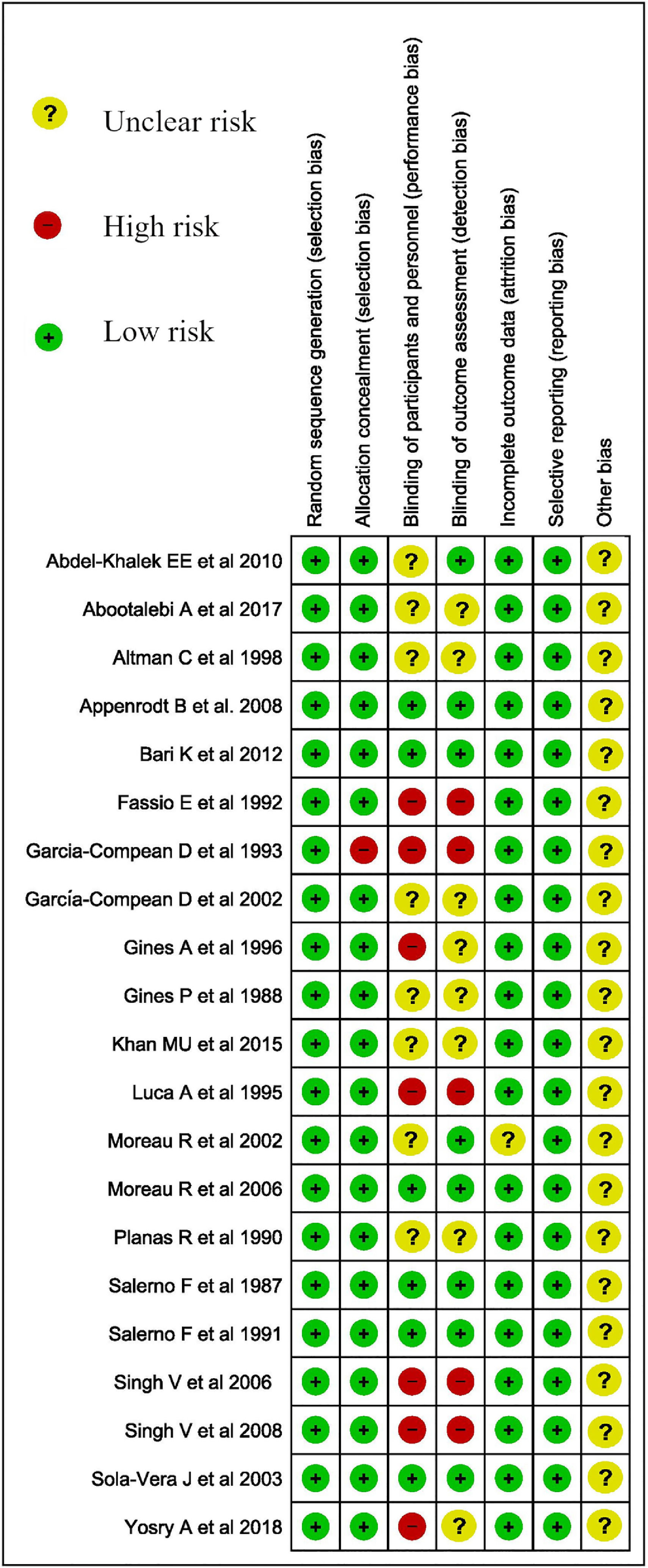
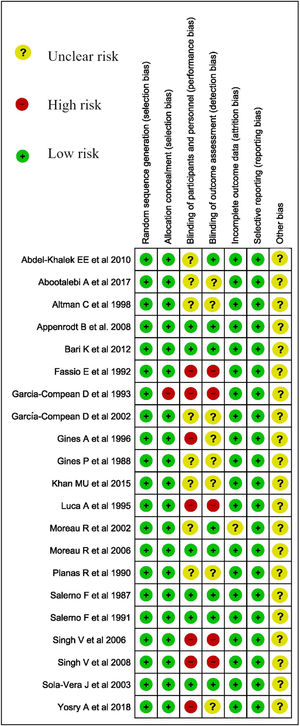
2.7.1Assessment of risk of bias in included studiesWe used Cochrane ROB 2.0 to assess the risk of bias for Trials (Fig. 1.) [15].
2.7.2Assessment of heterogeneityWe assessed the heterogeneity using the I-squared (I2) test. We used the Cochrane Handbook for Systematic Reviews of Interventions for interpretation of I2 test done as follows based on 0–40%: might not be important; 30–60% may represent moderate heterogeneity; 50–90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity [16]. The importance of the observed value of I2 depends on the magnitude and direction of effects and the strength of evidence for heterogeneity.
2.7.3Data synthesisRevMan 5.4 was used for data synthesis [17]. Odds ratio (OR) and mean difference was used to estimate the outcome with a 95% confidence interval (CI) where appropriate, and heterogeneity was measured using I²-test. We analyzed the mean difference between two groups, one receiving albumin and the other receiving other treatment for an outcome like mean arterial pressure after 3–4 days of treatment [18]. Further sensitivity analysis was performed based on time-frame (studies published before 2000 vs. after 2000) for main outcomes (PICD, mortality, MAP, and hyponatremia).
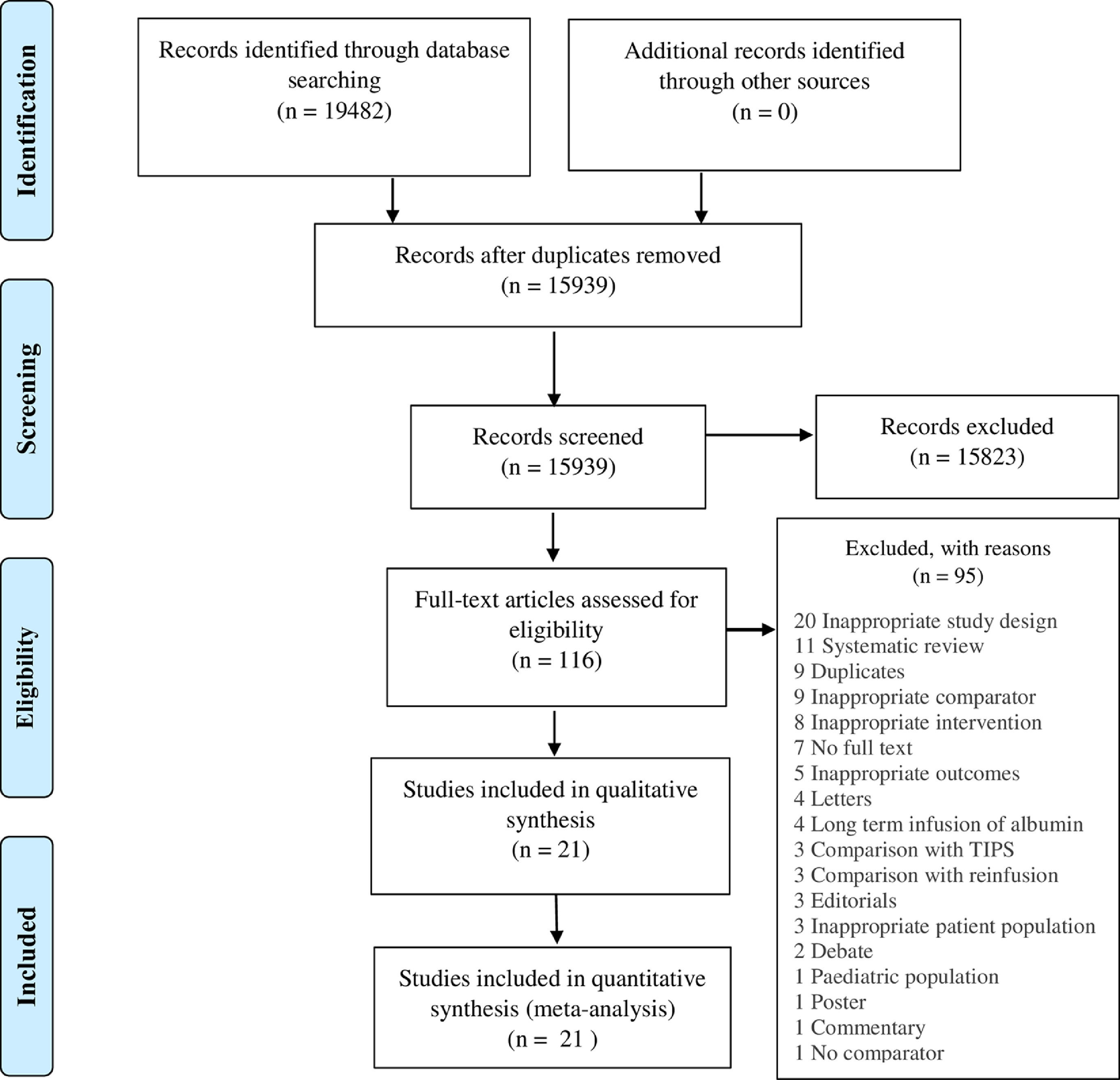
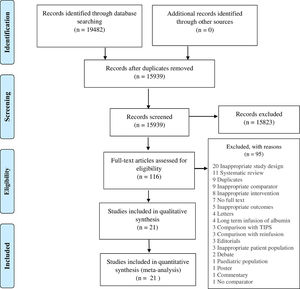
3ResultsWe identified 19482 studies after thorough database searching. After the removal of 3543 duplicates, we screened the title and abstract of 15939 studies and excluded 15823 studies. A total of 116 studies were assessed for full-text eligibility and we excluded 95 studies with definite reasons (Fig. 2). We included 21 studies in our qualitative and quantitative analysis (Table 1). Basic features of included studies are presented in Supplementary file 2 (Tables 1 and 2).
Qualitative summary of included studies.
| ID | Population | Intervention | Comparisons | Outcome | Follow Up Period |
|---|---|---|---|---|---|
| Abootalebi A [10] et al 2017 | N = 108 | Alb, 5 g/L of ascites removed | HES, 5 g/L of ascites removedAlb+ HES, 2.5 g/L Alb 2.5 g/L HES of ascites removed | At day 4, higher MAP in Alb group. No difference in weight, HR, UOP, laboratory values, and LOC. | |
| Abdel-Khalek EE [19] et al 2010 | N = 135 | 20% Alb, 8 g/L of ascites removed | HES 6%, 8 g/L of ascites removed | Postparacentesis transient hypotension significantly worse in HES group. At 6th day, no difference in renal or hepatic function, serum electrolytes, or complications. | 6-month follow up, no difference in readmissions, complications, or mortality. |
| Altman C [20] et al 1998 | N = 60 | Alb, 20 g if less than 2 L of ascites removed; 40 g if 2-5 L of ascites removed | HES group, 32.5 g if less than 2 L of ascites removed; 65 g if 2-5 L of ascites removed | Day 1, 3, and 15, no difference in development of renal failure or hyponatremia. | Up to 15 days, no difference in complications. |
| Appenrodt [6] | N = 24 | Alb, 8 g/L of ascites removed | Midodrine, 12.5 mg every 8 h for 2 days, six doses each | On day 6, PICD (>50% increase in pre-treatment renin), 31% (n = 4) Alb, 60% (n = 6) midodrine | |
| Bari K [21] et al. 2012 | N = 25 | Alb, 8 g/L of ascites removed | Octreotide long-acting release 20 mg intramuscularly to be repeated every monthMidodrine, 10 mg orally 3 times a day | No significant difference in time to recurrence of ascites.At day 6, no significant difference in PICD, MAP, HR, HRS | 10 months, no difference in mortality |
| Fassio E [22] et al 1992 | N = 41 | Alb, 6 g/L of ascites removed | Dextran, 6 g/L of ascites removed | At 24 hrs and 96 hrs, no significant difference in renal and liver function tests, MAP, or electrolytes. | Approximately 30 weeks, no different in re-admission and mortality. |
| Garcia-Compean D et al [23] 1993 | N = 35 | Alb, 5 g/L ascites removed | No albumin | At 12 hr, there was a significant decrease of CO in the no albumin group. At 6 and 24hrs, there was a significantly reduced PRA in the albumin group. At 1 and 6 hrs, there was a significantly reduced PAC in the albumin group. No difference in complications. | Frequency of complications after 24 hrs was no different between the groups. |
| García-Compean D [24] et al 2002 | N = 96 | Alb, 8 g/L of ascites removed | Dextran-40, 8 g/L of ascites removed | At 24 hour and 48 hrs, there was a significant decrease in MAP in both groups. Significant increase in UOP in both groups at 24 hrs, but only remained significant in the albumin group at 48 hrs. At 48 hrs, PRA was significantly higher in the Dextran-40 group. Significantly more PICD in Dextran-40 group. | Up to 44 months, no significant difference in complications, ascites recurrent rates, or mortality. |
| Gines P [2] et al 1988 | N = 105 | Alb, 40 g post para, 4-6 L ascites removed daily until resolution of ascites | No Alb, 4-6 L ascites removed daily until resolution of ascites | Day 6, no significant difference in standard renal function tests, PRA, and PAC. Paracentesis without albumin was associated with a significant increase in BUN, a marked elevation in PRA and PAC, and a significant reduction in serum sodium concentration. No difference in probability of survival. | Up to 40 weeks no significant reappearance of ascites, readmission or death. |
| Gines A [25] et al 1996 | N = 289 | Alb, 8 g/L of ascites removed | Dextran70, 8 g/L of ascites removedPolygeline, 8 g/L of ascites removed | At day 6, PICD occurred at a significantly greater frequency in the dextran and polygeline groups. Creatinine and PRA increase, and serum sodium decrease was significant in the dextran and polygeline when compared to the alb group. | Up to 30 months, no difference in readmission or death. |
| Khan MU [26] et al 2015 | N = 50 | Alb, 6 g/L and 125 ml/L fluid of ascites removed | Hemaccel 6 g/L and 125 ml/L fluid of ascites removed | At 6 days, no significant difference in creatinine, sodium, or MAP. | |
| Luca A [27] et al 1995 | N = 18 | Alb, 8g/L of ascites removed | No albumin | At 24 h, no significant difference in hemodynamics | |
| Moreau R [28] et al 2002 | N = 20 | Alb, 8 g/L of ascites removed | Terlipressin, total dose of 3 mg, administered as 1mg IV at the onset of paracentesis, 8 h and 16 h | At hospital discharge, no significant change in effective arterial blood volume assess by PRC | |
| Moreau R [29] et al 2006 | N = 68 | Alb, variable dosing | Synthetic colloid (3.5% polygeline), variable dosing | Follow up till 6 months, no significant difference in development of at least one liver-related complication or time-to-first complication.The total number of liver‐related complications adjusted to a 100‐day period was significantly lower in the alb group | |
| Planas R [30] et al 1990 | N = 88 | Alb, 8 g/L of ascites removed | Dextran-70 8 g/L of ascites removed | At day 6, no significant difference in renal and hepatic function or serum electrolytes.A significant increase in PRA and PAC was observed in the Dextran group compared to the Alb group. | Up to 40 weeks, no significant difference in readmissions or death. |
| Salerno F [31] et al 1987 | N = 41 | Albumin with repeated para | Diuretics alone | Ascites disappeared within 3 or 4 days with paracentesis, but only after 15 days with diuretics. No negative changes were induced in clinical and laboratory parameters of hemodynamic, hepatic and renal function after evacuation of the ascites. | Follow-up period for 19.8±2.6 weeks in Alb and 14±2.2 weeks in diuretics.No significant difference in complications or death. |
| Salerno F [33] et al 1991 | N = 54 | Alb, 20% 30mL/L of ascites removed | Hemaccel 3.5%, 150mL/L of ascites removed | At day 1, 3, and 6, hemodynamics, electrolytes, and hormone levels were not significantly different between the groups expect for albumin concentration; significantly higher in the Alb group at day 1, 3, and 6).No significant difference in complications. | Up to 172 weeks, probability of recurrence of massive ascites and probability of survival was similar between the two groups. |
| Singh V [33] et al 2008 | N = 40 | Alb | Midodrine | Plasma renin activity at 6 days after paracentesis did not differ in the two groups. Significant increase in 24-h urine volume and urine sodium excretion in the midodrine group. Midodrine therapy was cheaper than albumin therapy. | No difference in repeat para within 3 months of treatment. |
| Singh V [34] et al 2006 | N = 40 | Alb, 8g/L of ascites removed | Terlipressin, total dose of 3mg, administered as 1mg IV at the onset of paracentesis, 8 h and 16 h | No significant difference in development of PICD, renal impairment or hyponatremia at day 4–6 post intervention. | No difference in repeat para within 3 months of treatment. |
| Sola-Vera J [35] et al 2003 | N = 72 | Alb, 8 g/L of ascites removed | IV saline infusion, constant rate via a pump infusion device (170 mL of 3.5% saline solution / L of ascites removed at 999 mL/h) | The incidence of PICD was significantly higher in the saline group versus the albumin group, however not significant if less than 6L of ascitic fluid was removed. | No significant differences betweenclinical and laboratory data before second paracentesis between the two groups. |
| Yosry [11] A et al 2018 | N = 75 | Alb, 8 g/L of ascites removed | 2-day midodrine, 12.5mg q8hrs for 2 days30-day midodrine, 12.5mg q8hrs for 30 days | Significant increase in 24 h urine Na excretion and renal perfusion at 30 days in the 30-day midodrine group. Both midodrine groups were significantly lower in cost than Alb. No significant difference in development of renal impairment, hyponatremia, or mortality at day 6 and 30. | up to day 30 |
Abbreviations: Alb: Albumin, dl: Deciliter, g: gram, GI: Gastrointestinal, HE: Hepatic Encephalopathy, HES: Hydroxyethyl Starch, hr: Hour, HRS: Hepatorenal Syndrome, IV: Intravenous, L: Liter, MAP: Mean Arterial Pressure, ml: milliliter, N: Sample size, Na: sodium, PAC: Plasma Aldosterone Concentration, Para: Paracentesis, PCD: Paracentesis-induced Circulatory Dysfunction, PRA: Plasma Renin Activity, UOP: urine output.
A total of 21 RCTs in 1584 patients were included in the analysis.
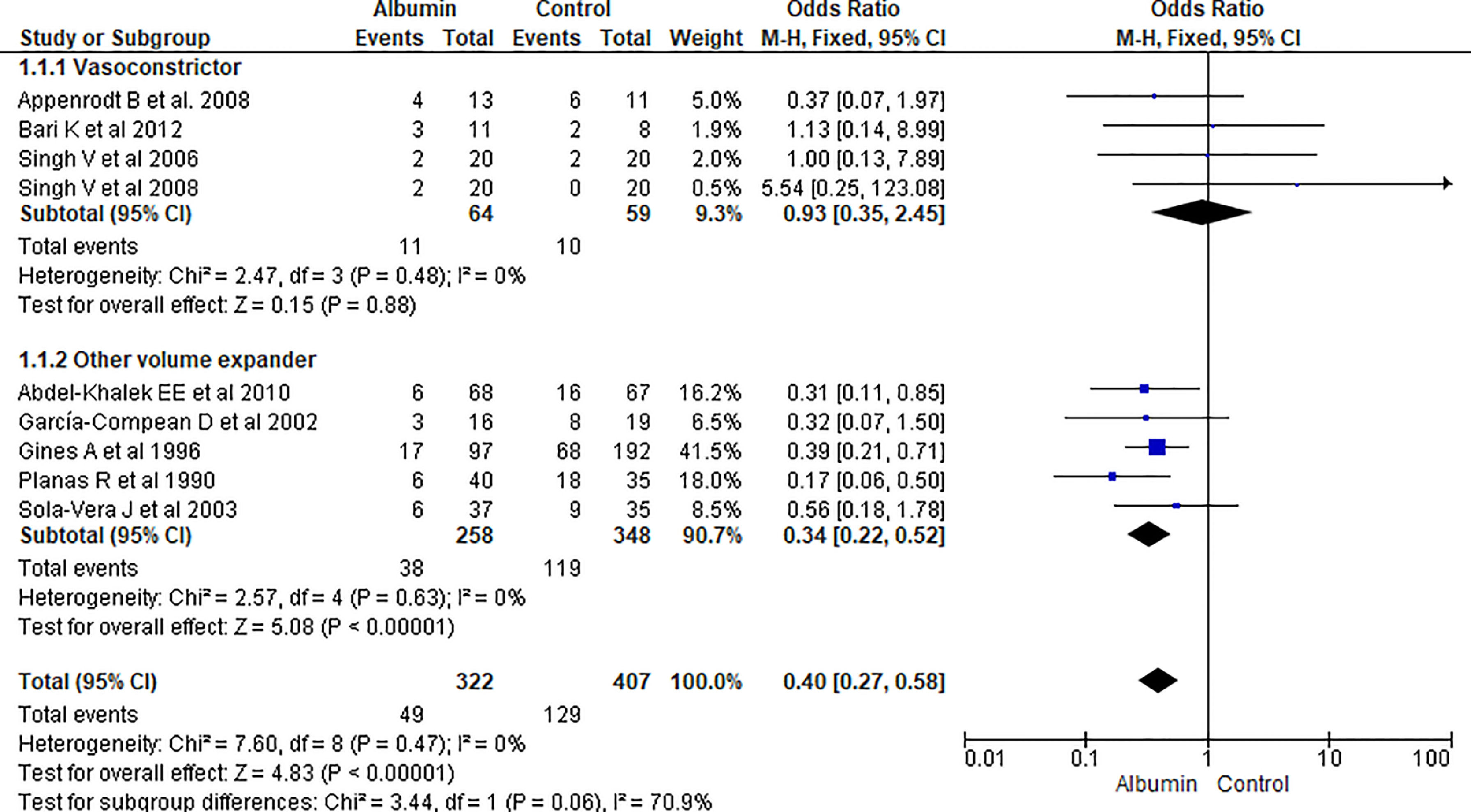
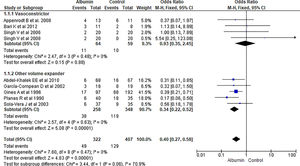
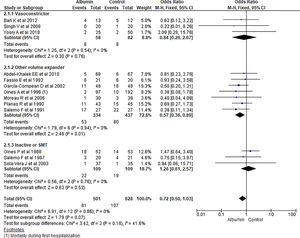
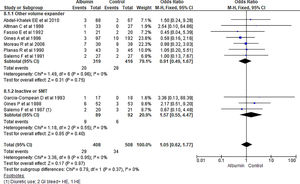
3.1.1Paracentesis induced circulatory dysfunction (PICD)Nine RCTs reported PICD as an outcome in their study. Overall use of albumin has reduced the odds of PICD by 60% (OR 0.40, 95% CI 0.27–0.58; n = 729; I2 = 0%). Further analysis showed albumin was superior to other volume expanders in terms of reducing PICD (OR 0.34, 95% CI 0.22–0.52; n = 606; I2 =0%), while comparing albumin with vasoconstrictor therapy did not reach statistical significance (OR 0.93, 95% CI 0.35–2.45; n = 123; I2 =0%) (Fig. 3.).
Further pooling of data on PICD based on time frame using fixed effect model showed significant reduction in odds of PICD in albumin group among studies published before 2000 (OR 0.32, 95% CI 0.19–0.54; n = 364; I2 =43%), and after 2000 (OR 0.50, 95% CI 0.29–0.87; n = 365; I2 =0%) (Supplementary file 3, Fig. 1).
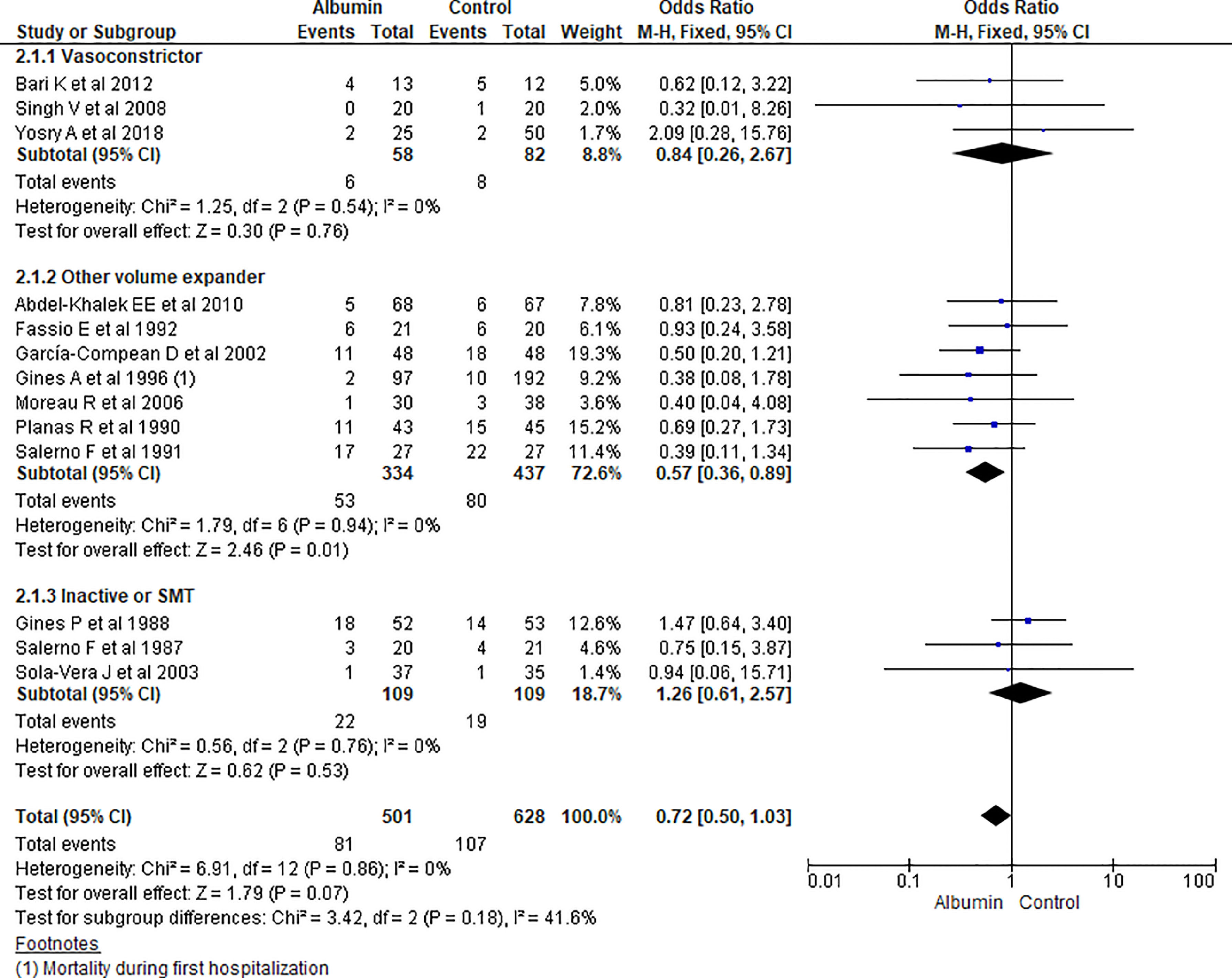
3.1.2Mortality outcomeThirteen studies reported the mortality as an outcome during the study period and follow up. Analysis showed some reduction in overall odds of mortality using albumin therapy but did not reach statistical significance (OR 0.72, 95% CI 0.50–1.03; n = 1129; I2 =0%). Further analysis comparing albumin therapy with vasoconstrictor agents (OR 0.84, 95% CI 0.26–2.67; n = 140; I2 =0%) and placebo or standard medical therapy (SMT) (OR 1.26, 95% CI 0.61–2.57; n = 218; I2 =0%) showed no significant differences of using albumin therapy over others. But, use of albumin showed a 43% lower odds of mortality compared to other volume expanders (OR 0.57, 95% CI 0.36–0.89; n = 771; I2 =0%) (Fig. 4).
Further pooling of data on the mortality outcome during the study period and follow up based on time frame using fixed effect model showed some reduction in mortality in the albumin group but it could not reach the level of significance among studies published before 2000 (OR 0.78, 95% CI 0.50–1.23; n = 618; I2 =0%), and after 2000 (OR 0.64, 95% CI 0.36–1.13; n = 511; I2 =0%) (Supplementary file 3, Fig. 2).
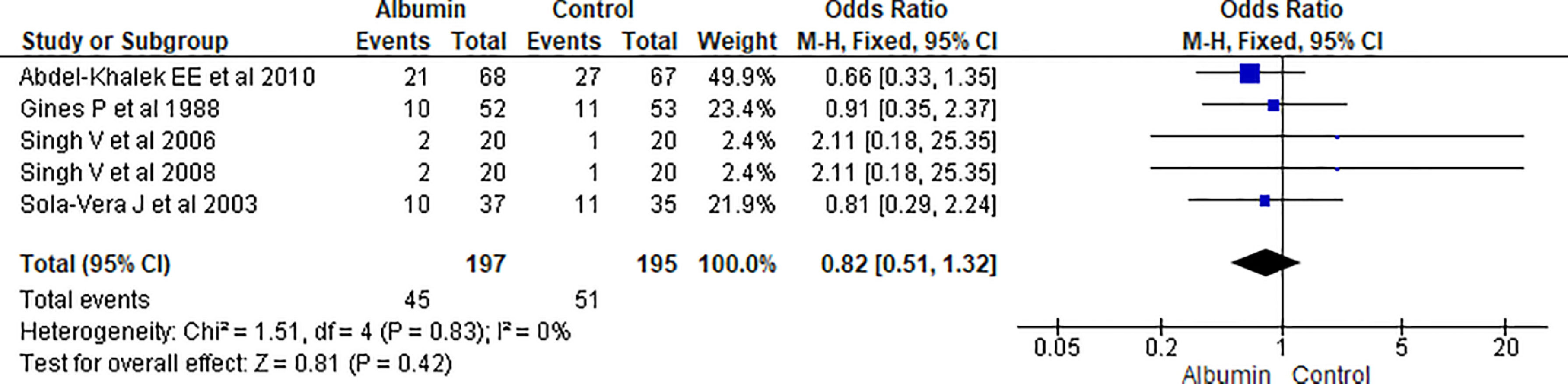
3.1.3Re-appearance of ascitesAnalysis from pooled data from 5 studies showed no significant reduction in the reappearance of ascites in albumin-treated patients compared to patients receiving other interventions (OR 0.82, 95% CI 0.51–1.32; n = 392; I2 =0%) (Fig. 5)
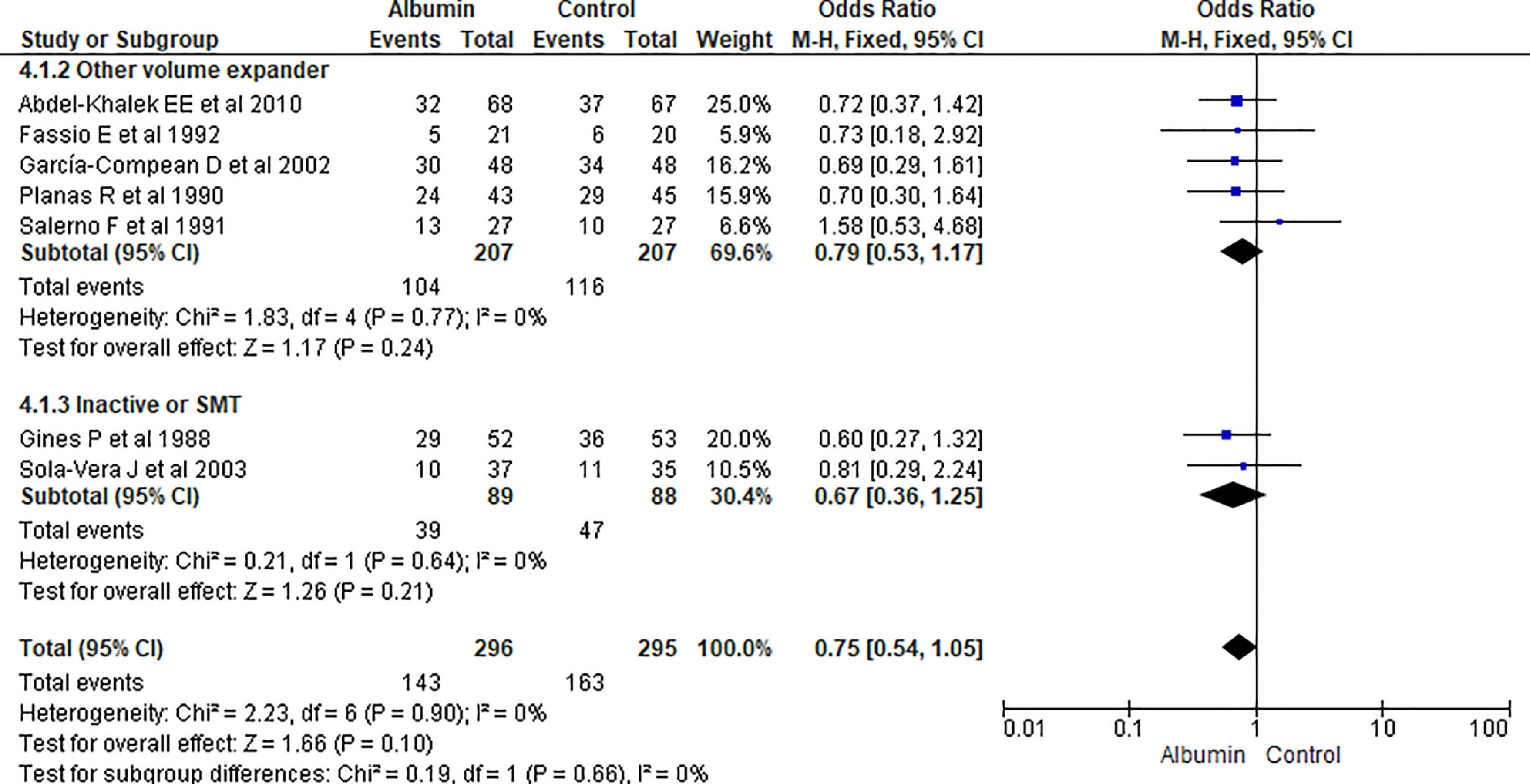
3.1.4Hospital readmissionPooling data from seven studies showed that the use of albumn therapy did not reach statistical significance for hospital readmission while comparing with other therapies (OR 0.75, 95% CI 0.54–1.05; n = 591; I2 =0%). Comparing albumin therapy with other volume expanders (OR 0.79, 95% CI 0.53–1.17; n = 414; I2 =0%), and SMT (OR 0.67, 95% CI 0.36–1.25; n = 177; I2 =0%) did not show a significant reduction in readmission either (Fig. 6).
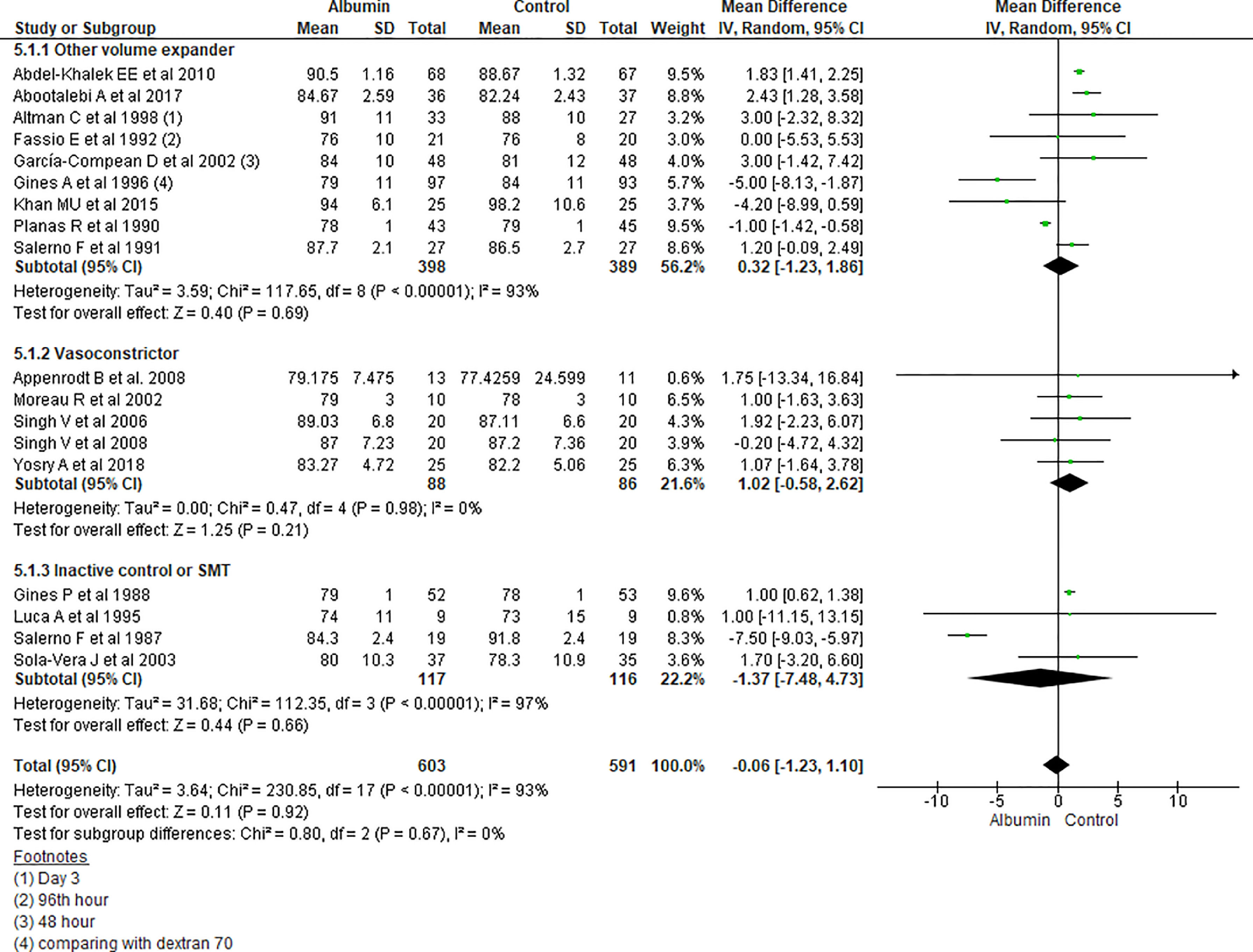
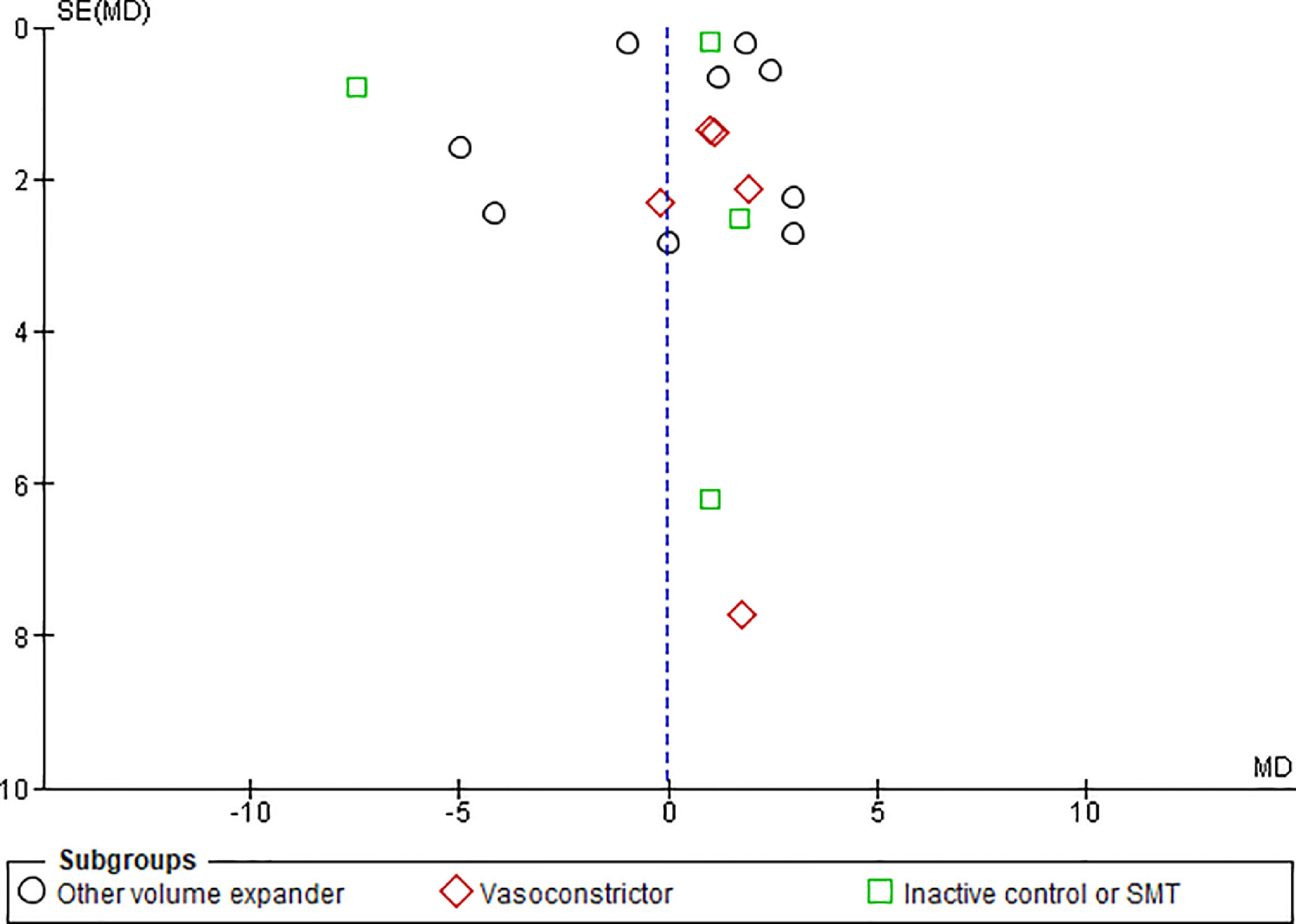
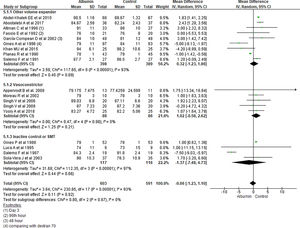
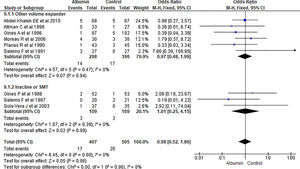
3.1.5Mean arterial pressure (MAP) after 4–6 days of therapyAnalysis taking data of 18 studies reporting MAP did not show significant differences in MAP between albumin and other treatment groups (MD -0.06, 95% CI -1.23 to 1.10; n = 1194; I2 =93%). Further analysis considering type of the control therapy also did not show significant differences in MAP among albumin group over other volume expanders (MD 0.32, 95% CI -1.23 to 1.86; n = 787; I2 =93%); vasoconstrictor agents use (MD 1.02, 95% CI -0.58 to 2.62; n = 174; I2 =0%); and SMT (MD -1.37, 95% CI -7.48 to 4.73; n = 233; I2 =97%) (Fig. 7).
Further pooling of data in MAP based on time frame using random-effect model among studies published before 2000 showed no significant differences in MAP between albumin and other treatment groups (MD -1.41, 95% CI -3.28 to 0.45; n = 594; I2 =95%), however among studies published after 2000 there were some but significantly higher MAP after 4–6 days of therapy in albumin group (MD 1.82, 95% CI 1.44 to 2.20; n = 600; I2 =0%) (Supplementary file 3, Fig. 3).
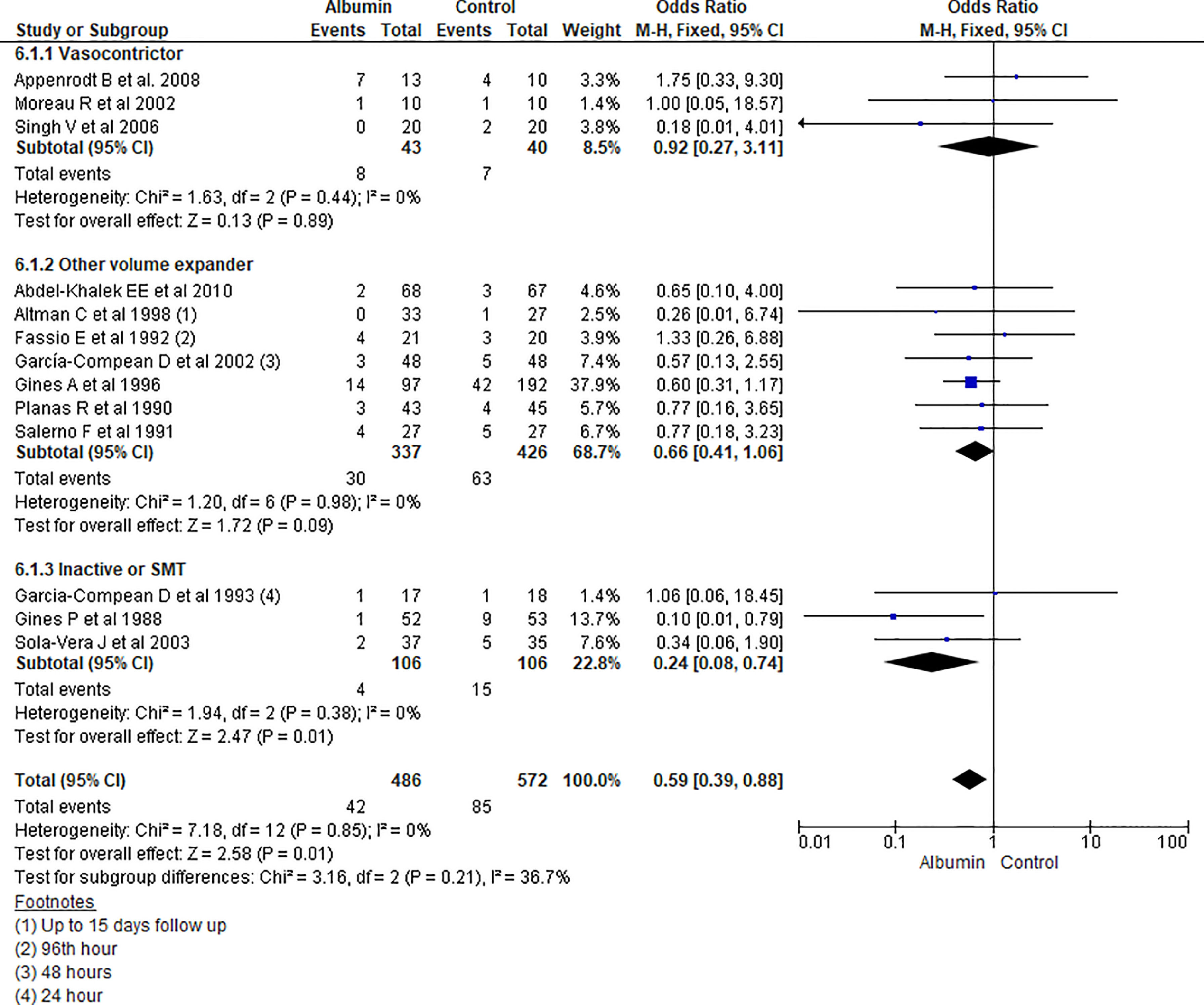
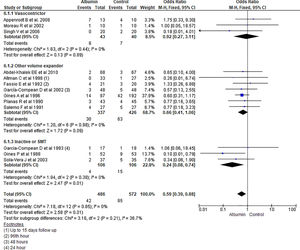
3.1.6HyponatremiaAmong 13 studies reporting hyponatremia outcome, pooling of the data showed overall significant low incidence of hyponatremia among albumin treated group than other treatment (OR 0.59, 95% CI 0.39–0.88; n = 1058; I2 =0%). On further analysis, there was significant reduction of incidence of hyponatremia in patients recieving albumin therapy as compared to SMT (OR 0.24, 95% CI 0.08–0.74; n = 212; I2 =0%), but was not statistically significant while comparing with volume expanders (OR 0.66, 95% CI 0.41–1.06; n = 763; I2 =0%) or vasopressor group (OR 0.92, 95% CI 0.27–3.11; n = 83; I2 =0%) (Fig. 8.).
Further pooling of data for hyponatremia outcome based on time frame using fixed effect model among studies published before 2000 showed significantly lower incidence of hyponatremia among albumin treated group than other treatment (OR 0.57, 95% CI 0.35–0.93; n = 672; I2 =0%), however, among studies published after 2000, there was some lower odds of hyponatremia in albumin group but could not reach the level of significance (OR 0.63, 95% CI 0.30–1.32; n = 386; I2 =0%) (Supplementary file 3, Fig. 4).
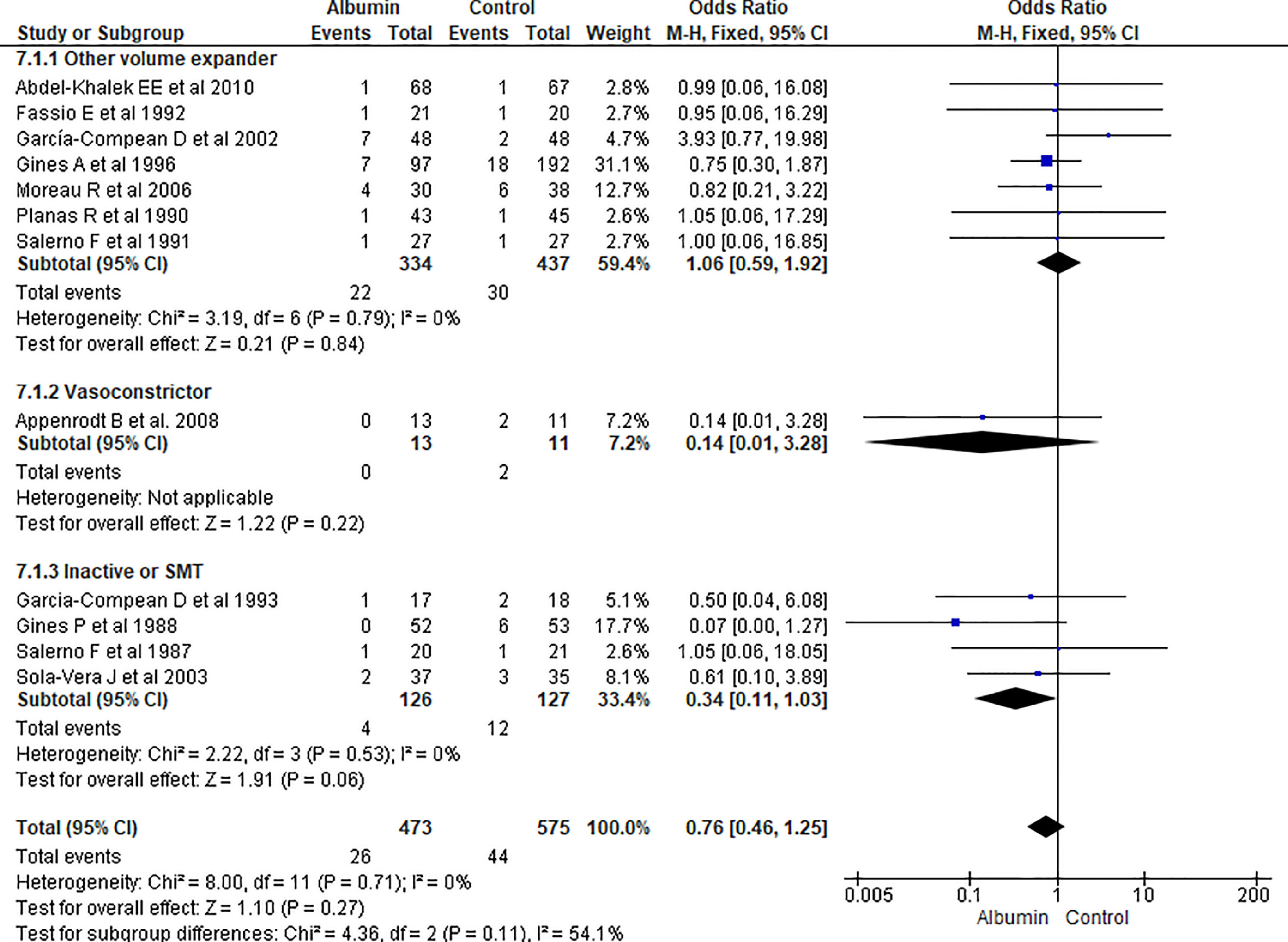
3.1.7Renal impairmentOverall incidence of renal impairment was not statistically significant between albumin and other therapy (OR 0.76, 95% CI 0.46–1.25; n = 1048; I2 =0%). On further analysis, there was no significant reduction in incidence of renal impairment in patients treated with albumin as compared with other volume expanders (OR 1.06, 95% CI 0.59–1.92; n = 771; I2 =0%), and SMT (OR 1.57, 95% CI 0.55–4.47; n = 181; I2 =0%) (Fig. 9.).
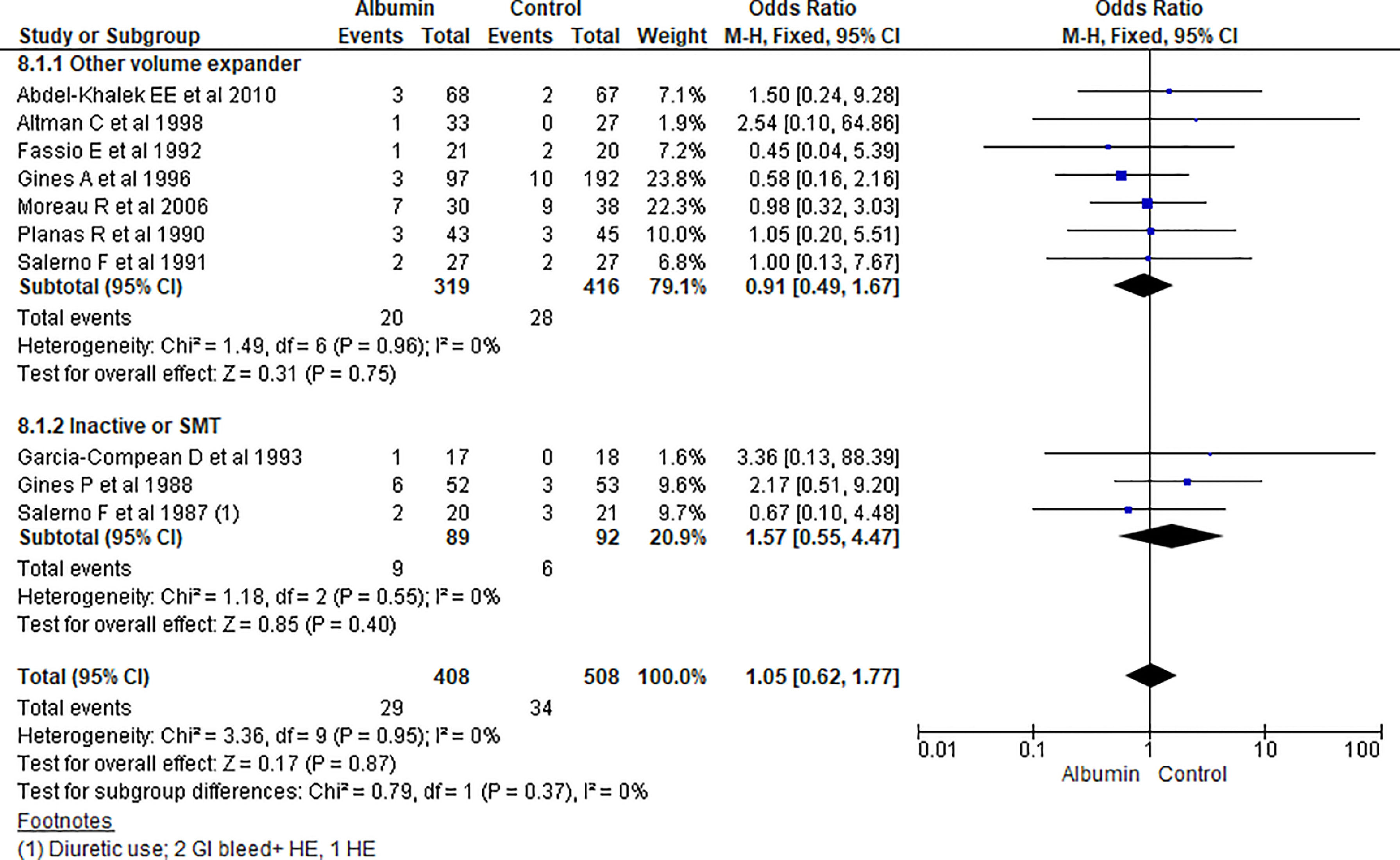
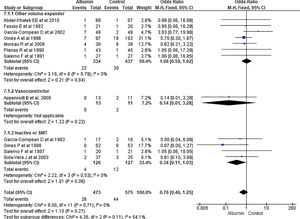
3.1.8Hepatic encephalopathy (HE)Overall hepatic encephalopathy incidence among albumin and other treatment group was reported in ten studies. Pooling of the data showed no significant differences among albumin and other treatment for incidence of HE (OR 1.05, 95% CI 0.62–1.77; n = 916; I2 =0%). Further contrasting albumin with other volume expanders (OR 0.91, 95% CI 0.49–1.67; n = 735; I2 =0%), and SMT (OR 1.57, 95% CI 0.55–4.47; n = 181; I2 =0%) was not statistically significant (Fig. 10).
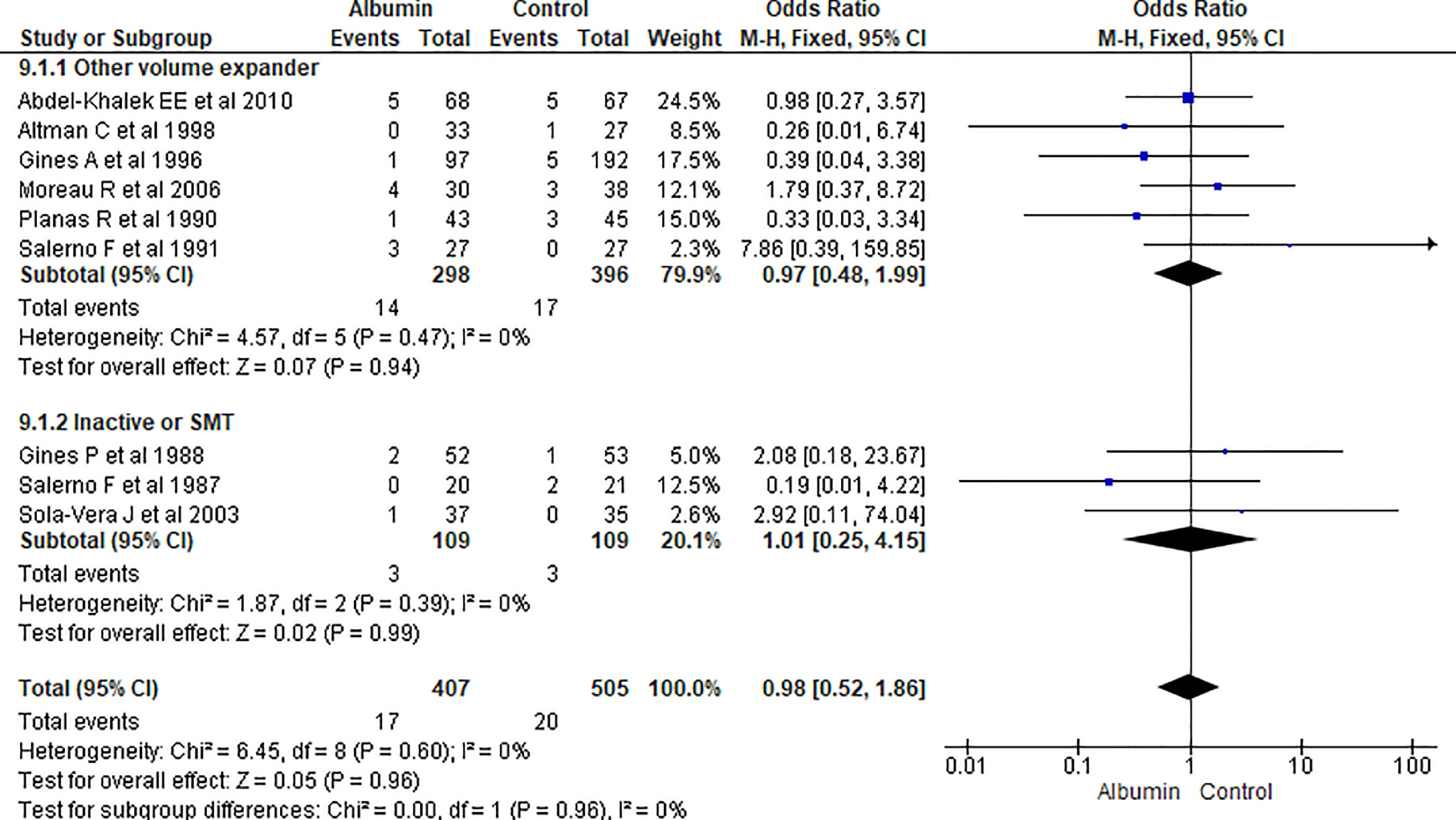
3.1.9Gastrointestinal (GI) bleedingOverall nine papers reported GI bleeding. Pooling of the data showed no significant differences between using albumin or other therapies (OR 0.98, 95% CI 0.52–1.86; n = 912; I2 =0%). Further analysis comparing albumin with other volume expanders (OR 0.97, 95% CI 0.48–1.99; n = 694; I2 =0%), and SMT (OR 1.01, 95% CI 0.25–4.15; n = 218; I2 =0%) also did not showed significant differences in GI bleeding (Fig. 11.).
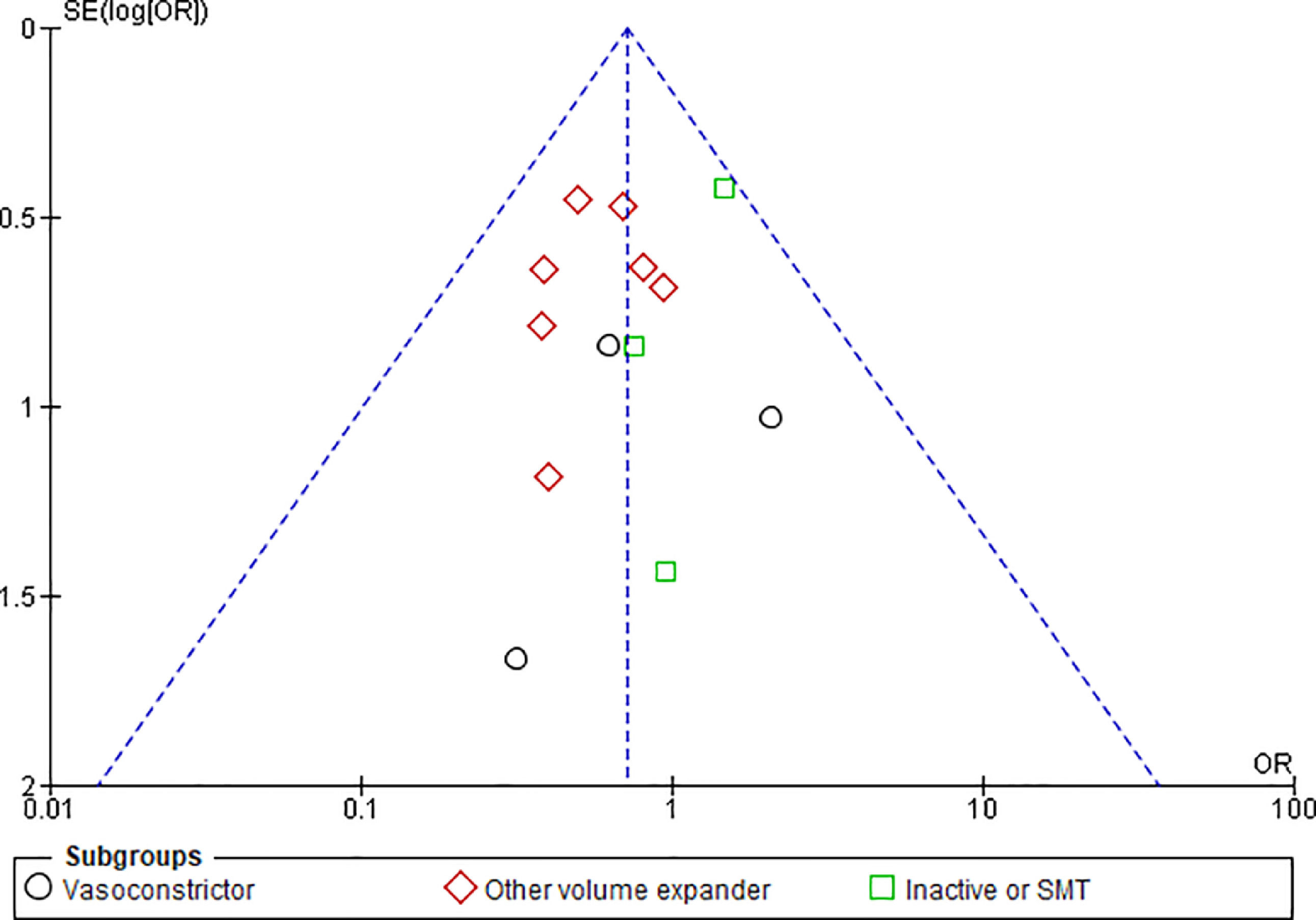
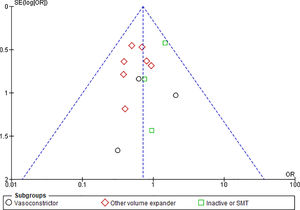
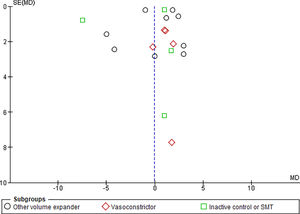
3.1.10Publication biasFunnel plots were used to evaluate the publication bias across the studied outcomes. Among the studied outcomes, there was no significant publication bias except the MAP outcome. Publication bias was not significant in outcomes like overall mortality outcome suggested by symmetry of plot (Fig. 12), however significant publication bias was observed for MAP outcome as suggested by the asymmetry of the plot (Fig. 13).
In this systematic review and meta-analysis, we have compared albumin to alternative treatment modalities including sodium restriction with diuretics, vasoconstrictors (midodrine and octreotide), and other volume expanders in patients with cirrhotic ascites. The major findings of our study were reduction in paracentesis induced circulatory dysfunction and decrease in hyponatremia with albumin use. Our finding of a reduction in paracentesis induced circulatory dysfunction with albumin by 60% was consistent with the finding of Bernardi et al [36]. In a subgroup analysis, we found that albumin was superior to other volume expanders in the prevention of PICD. However, no significant difference was observed between albumin and vasoconstrictors. PICD is regarded as the most significant predictor of mortality in patients with tense ascites that are treated by large-volume paracentesis [25]. Thus, PICD prevention with albumin is a significant finding of our study. Hyponatremia is an important risk factor for hepatic encephalopathy and a predictor of mortality in patients with cirrhosis [37,38]. Thus, the benefit of albumin treatment in the reduction of hyponatremia in patients with cirrhosis and ascites can have implications in reducing hospitalization and long-term clinical outcomes. We found no significant benefits with albumin in preventing recurrence of ascites, renal impairment, hepatic encephalopathy, and GI bleeding, although the combination of antibiotics and albumin was found to decrease renal function in the study done by Sort et al [39].
Human albumin is extracted from donated blood. Thus, there are concerns regarding supply, demand mismatch, and added cost of care with albumin use. Caraceni et al. suggested albumin as a more cost-effective alternative due to the reduction in readmission rate. However, we found no difference in readmission rates between albumin and standard medical treatment groups [40].
Our meta-analysis is the most comprehensive to date to evaluate the efficacy and safety of albumin in patients with cirrhotic ascites. The inclusion of 21 trials in the analysis adds to the power of the study. We compared albumin treatment to various other modalities separately in our analyses. However, our study has limitations. Most of the included trials are small in size and lack statistical power and carry inherent limitations. There was wide variation in inpatient population, treatment dose, and duration of albumin, and endpoints of various studies. Moreover, studies were conducted over a wide period over three decades. There has been substantial evolution in evidence and practice patterns over the past three decades which could have affected the results pertaining to clinical outcomes.
5ConclusionTreatment with albumin in cirrhotic ascites reduced paracentesis-induced circulatory dysfunction and hyponatremia compared to alternative treatment modalities. There was no benefit in mortality, hospital readmission, hepatic encephalopathy, and recurrence of refractory ascites with albumin treatment.
DeclarationsEthics approval and consent to participate Not applicable
Consent for publication Not applicable
Availability of data and materials the datasets analyzed during the current study are available from the corresponding author on reasonable request.
Funding This article did not receive any specific grant from funding agencies in the public, commercial, or any other sectors.
Authors' contributions: DBS, PB, YRS, RB and MGK contributed to the concept and design, analysis, and interpretation of data. DBS, PB, SA, JY, and LA contributed to the literature search, data extraction, review and initial manuscript drafting. YRS, RB, BD, MGK, and CAC interpretation of data, revising the manuscript for important intellectual content and approval of the final manuscript.
All authors were involved in drafting and revising the manuscript and approved the final version.
DBS Medical officer, Department of Emergency Medicine, Mangalbare Hospital, Morang, Nepal
Resident Physician, Department of Internal Medicine, Mount Sinai Hospital, Chicago, IL, USA
Resident Physician, Department of Internal Medicine, BronxCare Health System, Bronx, NY, USA
RB Hospitalist, Our Lady of the Lake Hospital, Baton Rouge LA, USA
SA Intern doctor, Nepalese Army Institute of Health Sciences, Kathmandu, Nepal