Introduction and objectives It has been suggested that albumin administration could alter the natural history of cirrhosis, and also, that long‐term treatment with albumin might be associated with improvement in survival, control of ascites, reduction in the incidence bacterial infections, renal dysfunction, hepatic encephalopathy (HE) and hyponatremia, as well as reduction in length of hospitalization in patients with cirrhosis and ascites. The objective of the present study is to evaluate the role of albumin in the management of HE. Materiales and methods:: This is a systematic review of randomized controlled trials that evaluated the use of albumin in adult patients with cirrhosis and HE. The search for eligible studies was performed in MEDLINE, EMBASE, and Cochrane CENTRAL databases until June 2020. The outcomes of interest were the complete reversal of HE and mortality. Meta‐analysis was performed using the random effects model, through the Mantel–Haenszel method.
Results: This systematic review was registered at the PROSPERO platform (CRD42020194181). The search strategy retrieved 1,118 articles. After reviewing titles and abstracts, 24 studies were considered potentially eligible, but 22 were excluded after full-text analysis. Finally, 2 studies were included. In the meta-analysis, albumin was associated to significant lower risks of persistent HE (risk ratio – RR = 0.60; 95% confidence interval – CI = 0.38–0.95, p = 0.03) and mortality (RR = 0.54; 95% CI = 0.33–0.90, p = 0.02).
Conclusion: Albumin administration improves HE and reduces mortality in patients with cirrhosis and HE.
Until a few years ago, the pathophysiology of decompensated cirrhosis was supported by the hypothesis of peripheral vasodilation [1]. However, currently the hypothesis to be considered is that of systemic inflammation [2], in which the importance of the presence of a sustained pro-inflammatory and pro-oxidant state is also recognized. Within this perspective, we should consider not only the role of albumin in plasma expansion, correcting effective arterial hypovolemia, but also its non-oncotic properties. Thus, albumin should be considered as a drug with antioxidant, detoxifying, endothelial stabilizing and immunomodulating properties [3].
Another important factor to be considered is that, in cirrhosis, there is not only a quantitative deficit of albumin, but also a qualitative one, which leads to the concept of effective concentration of albumin [4,5]. In this context, it has been suggested that albumin administration could alter the natural history of this disease. Moreover, it has been demonstrated that long‐term treatment with albumin might be associated with improvement in survival, control of ascites, reduction in the incidence bacterial infections, renal dysfunction, grade III-IV hepatic encephalopathy (HE) and hyponatremia, as well as reduction in length of hospitalization in patients with cirrhosis and ascites [6,7]. Nevertheless, it seems likely that high doses of albumin should be used in order to achieve such benefits [8].
Considering the pathophysiology of decompensated cirrhosis and the potential effects of albumin, aside from the traditional albumin indications (spontaneous bacterial peritonitis [9,10], hepatorenal syndrome [11,12] and prevention of large volume paracentesis-induced circulatory dysfunction [13,14] other indications are under study, such as cirrhosis with ascites [15,16,17,18,19,20] and extraperitoneal infections [21,22,23,24,25]. In this context, one could hypothesize a role for albumin administration in the treatment of HE [2]. The pathophysiology of HE still is not completely understood, but cerebral edema related to an imbalance of neuroactive chemicals (primarily ammonia) which are not properly metabolized by the liver, as well as other possible inflammatory neurochemicals produced by the human microbiome, are thought to play a central role in this process [26,27]. An excessive formation of reactive oxygen and nitrogen species, which triggers multiple RNA and protein modifications, has also been proposed to play a role in HE [28]. Albumin infusion could be beneficial in patients with HE due to its anti-inflammatory and antioxidant properties, as well as due to its capacity of binding and clearing many toxic substances which accumulate in patients with liver dysfunction. Indeed, one non-randomized study has shown improved mental status and decreased oxidative stress markers in patients with diuretic-induced HE when treated with albumin [5]. It has also been suggested that albumin leads to a more substantial improvement than other colloids [5,29].
The objective of the present systematic review and meta-analysis is to evaluate the role of albumin in the management of HE.
MethodsProtocol and registrationThis systematic review was registered at the PROSPERO International Prospective Register of Systematic Reviews platform (CRD42020194181). This study followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement [30].
Outcomes of interestPrimary outcome was the complete reversal of HE. Secondary outcome was mortality.
Eligibility criteriaRandomized controlled trials that evaluated the use of albumin in adult patients with cirrhosis and HE were included as long as they provided information on at least one of the outcomes of interest. Articles published exclusively as abstracts, studies evaluating children and those which were not characterized as randomized controlled trials were excluded. No language or date of publication restrictions were applied.
Search strategy and study selectionThe search for eligible studies was performed in MEDLINE, EMBASE and Cochrane CENTRAL databases until June 2020, without language or date restriction. The search strategy for MEDLINE was the following:
((((Serum Albumin, Human) OR Albumin)) AND Hepatic Encephalopathy) AND (((randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized controlled trials[mh] OR random allocation[mh] OR double-blind method[mh] OR single-blind method[mh] OR clinical trial[pt] OR clinical trials[mh] OR (“clinical trial”[tw]) OR ((singl*[tw] OR doubl*[tw] OR trebl*[tw] OR tripl*[tw]) AND (mask*[tw] OR blind*[tw])) OR (“latin square”[tw]) OR placebos[mh] OR placebo*[tw] OR random*[tw] OR research design[mh:noexp] OR follow-up studies[mh] OR prospective studies[mh] OR cross-over studies[mh] OR control*[tw] OR prospective*[tw] OR volunteer*[tw]) NOT (animal[mh] NOT human[mh]))).
Similar search strategies were used for the other databasesAll studies retrieved by the search had titles and abstracts evaluated by independent reviewers (CVT, MA, IB, TC and AAM). Full-text of studies considered potentially eligible were then analyzed. Data were finally extracted from studies which fulfilled all of the inclusion criteria and none of the exclusion criteria. In case of divergence between reviewers another researcher was consulted (AZM).
Data collection processData was collected in a predefined electronic spreadsheet by independent investigators for the following variables: first author of the study, year of publication, country, interventions, number of patients per treatment group, mean age of subjects, evaluated outcomes and main results. If necessary, the first author of each study would be contacted for clarifications.
Risk of biasThe reviewers independently assessed the included studies for quality and risk of bias using the Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB 2.0) [31]. The risk of bias referred to the primary outcome of the present study (HE).
Statistical analysisMeta-analysis was performed using Review Manager 5.3 software (The Nordic Cochrane Center, Copenhagen, Denmark). The Mantel-Haenszel method was used for the meta-analyses. Risk ratio (RR) with 95% confidence interval (CI) was the chosen effect measure. Statistical significance was set at p < 0.05. The fixed-effects or the random-effects models were planned to be used according, respectively, to the absence or presence of substantial heterogeneity among studies. Statistical heterogeneity among studies was evaluated using the Cochran Q-test (p < 0.10) and the Higgins I2 statistic (I2 ≥ 50% indicated substantial level of heterogeneity).
Sensitivity analyses by repeating the meta-analyses after the exclusion of each of the studies at a time were planned. Publication bias assessment using funnel plots was planned if more than 10 studies were included in the meta-analysis.
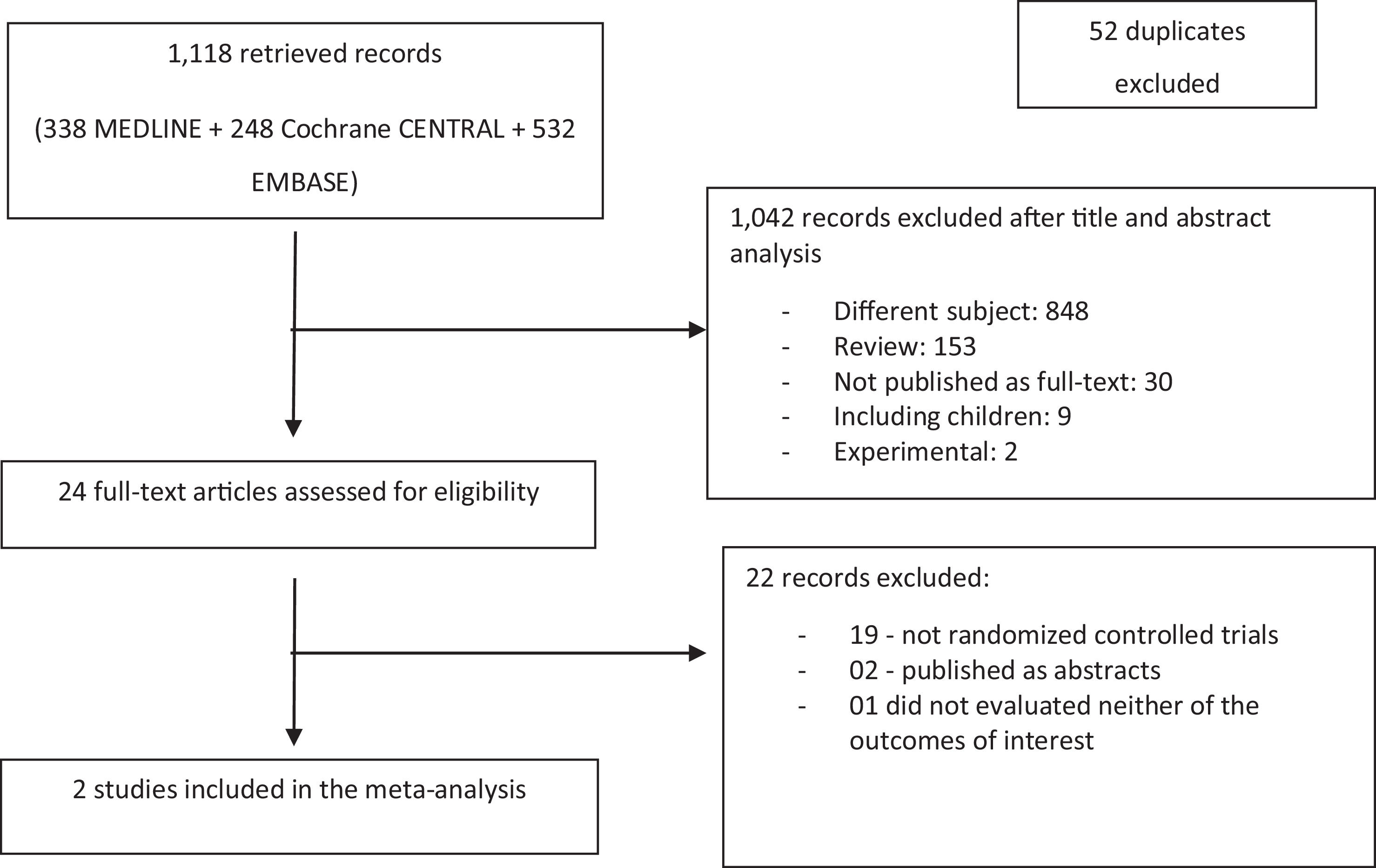
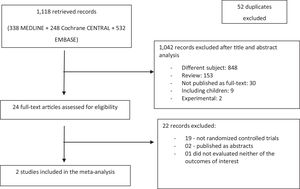
ResultsInitially, 1118 articles were retrieved. After excluding 52 duplicates, the remaining 1066 references had their titles and abstracts reviewed. From these, 1042 articles were excluded (848 for not meeting the subject of the systematic review; 153 for being review papers; 30 for not being published as full-text; 9 for including children; and 2 for being experimental studies) and 24 articles were considered to be potentially eligible. After full-text analysis, 22 studies were excluded (19 for not being randomized controlled studies; two for being published just as abstracts; one not evaluating neither of the outcomes of interest for this systematic review).
Finally, 2 articles were included in the systematic review [32,33], evaluating 176 patients (86 in the albumin group and 90 in the control group). The flowchart for the selection of studies is depicted in Fig. 1.
The study by Sharma et al. [32]. is a study from India, in which 120 patients with cirrhosis and HE grades II-IV (West-Haven classification) were evaluated. A group of 60 patients received lactulose plus albumin and the remaining 60 patients were randomized to receive lactulose alone. The authors observed the reversal of HE in 45 subjects from the intervention group and in 32 patients from the control group (75.0 vs. 53.3%, p = 0.03). Eleven patients randomized to receive albumin and 19 controls died during the study (18.3 vs. 31.6%, p = 0.04).
The study by Simón-Talero et al. [33]. was performed in Spain and included 56 patients with cirrhosis and HE grades II–IV (West-Haven classification). Patients were randomly assigned to receive albumin (n = 26) or saline solution (n = 30). The complete resolution of HE was achieved in 57.7% of patients from the albumin group and in 53.3% individuals from the saline group (p > 0.05). The 90-day survival was 69.2% in the albumin group and 40.0% in the saline group (p = 0.02).
Table 1 summarizes the baselines characteristics of the patients, and Table 2 the characteristics of the included trials.
Baseline characteristics of patients.
| Characteristic | Sharma BC, 2017 [32] | Simón-Talero M, 2013 [33] |
| Mean age ± SD (years) * | 42.5 ± 8.7 vs. 38.4 ± 9.6 | 63.7 ± 11.3 vs. 66.3 ± 9.7 |
| Male sex; n (%) | 100 (83.3) | 42 (75.0) |
| Etiology; n (%)Viral hepatitisAlcoholOther | 67 (55.8)36 (30.0)17 (14.2) | 19 (33.9)24 (42.9)13 (23.2) |
| MELD score; mean ± SD* | 26.4 ± 5.8 vs. 25.8 ± 5.1 | 16.8 ± 3.8 vs. 16.1 ± 5.1 |
| Severity of HE; n (%)Grade II-IIIGrade IV | 84 (70.0)36 (30.0) | 46 (82.1)10 (17.8) |
N = number; SD = standard deviation; MELD = model for end-stage liver disease; HE = hepatic encephalopathy; vs. = versus.
*Intervention group compared to the control group, non significant.
Characteristics of included studies.
| Author, year | Country | N | Treatment | Outcomes | Results |
| Sharma BC, 2017 [32] | India | 120 | Lactulose + albumin (n = 60) vs. lactulose (n = 60) | -Primary: reversal of HE;-Secondary: mortality (day 10) | -Reversal of HE: 45 (75.0%) vs. 32 (53.3%); p = 0.03-Mortality:11 (18.3%) vs. 19 (31.6%); p = 0.04 |
| Simón-Talero M, 2013 [33] | Spain | 56 | Albumin (n = 26) vs. isotonic saline (n = 30) | -Primary: reversal of HE;-Secondary: survival (day 90) | -Reversal of HE: 21 (80.8%) vs. 24 (80.0%); p > 0.05-Survival: 18 (69.2%) vs. 12 (40.0%); p = 0.02 |
N = number of patients; SD = standard deviation; HE = hepatic encephalopathy; vs. = versus.
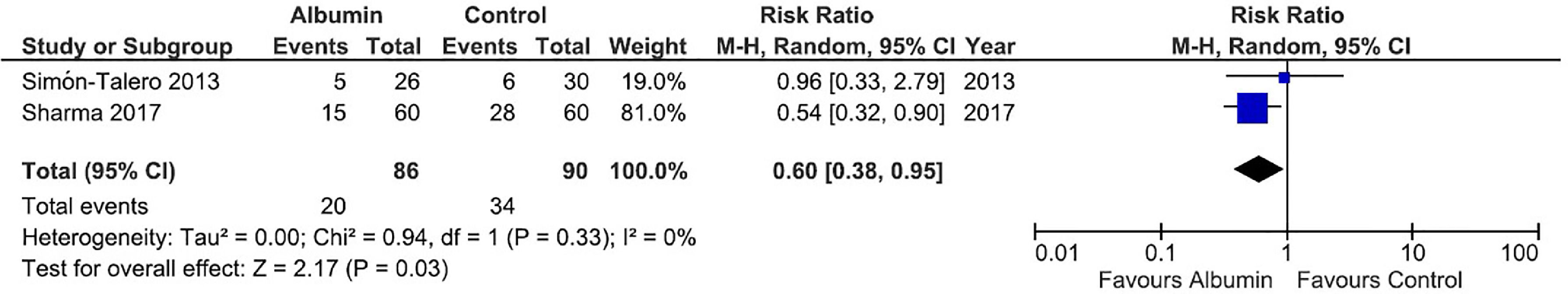
Regarding the meta-analysis on persistence of HE (evaluated by West-Haven classification), data were pooled at 10 days for the study by Sharma et al. [32]. and at 7 days for the study by Simón-Talero et al. [33].. Albumin was associated with a significant reduction in the risk of persistence of HE (RR = 0.60, 95% CI = 0.38–0.95, p = 0.03). There was no significant heterogeneity between studies (I² = 0%). The forest plot for this outcome is shown in Fig. 2.
Forest plot of the fixed-effects model meta-analysis for the comparison between the albumin group and the control group regarding persistence of hepatic encephalopathy. Captions: Each study is identified by the name of the first author. Squares indicate the RR, and their sizes are proportional to the weights of the trials. The horizontal bars refer to the 95% CI of the RR. The vertical line is the equivalence line, where the RR is 1. The diamond represents the 95% CI of the pooled RR. M-H, Mantel-Haenszel method.
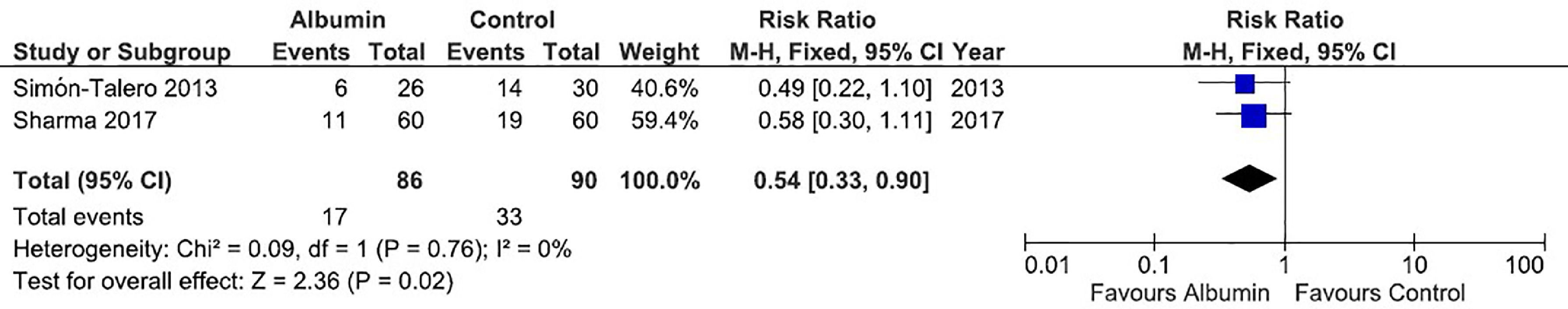
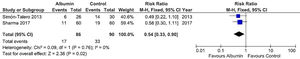
Concerning the meta-analysis on mortality, albumin significantly reduced the risk of death (RR = 0.54, 95% CI = 0.33–0.90, p = 0.02). There was no significant heterogeneity between studies (I² = 0%). The forest plot for this outcome is shown in Fig. 3.
Forest plot of the fixed-effects model meta-analysis for the comparison between the albumin group and the control group regarding mortality. Captions: Each study is identified by the name of the first author. Squares indicate the RR, and their sizes are proportional to the weights of the trials. The horizontal bars refer to the 95% CI of the RR. The vertical line is the equivalence line, where the RR is 1. The diamond represents the 95% CI of the pooled RR. M-H, Mantel-Haenszel method.
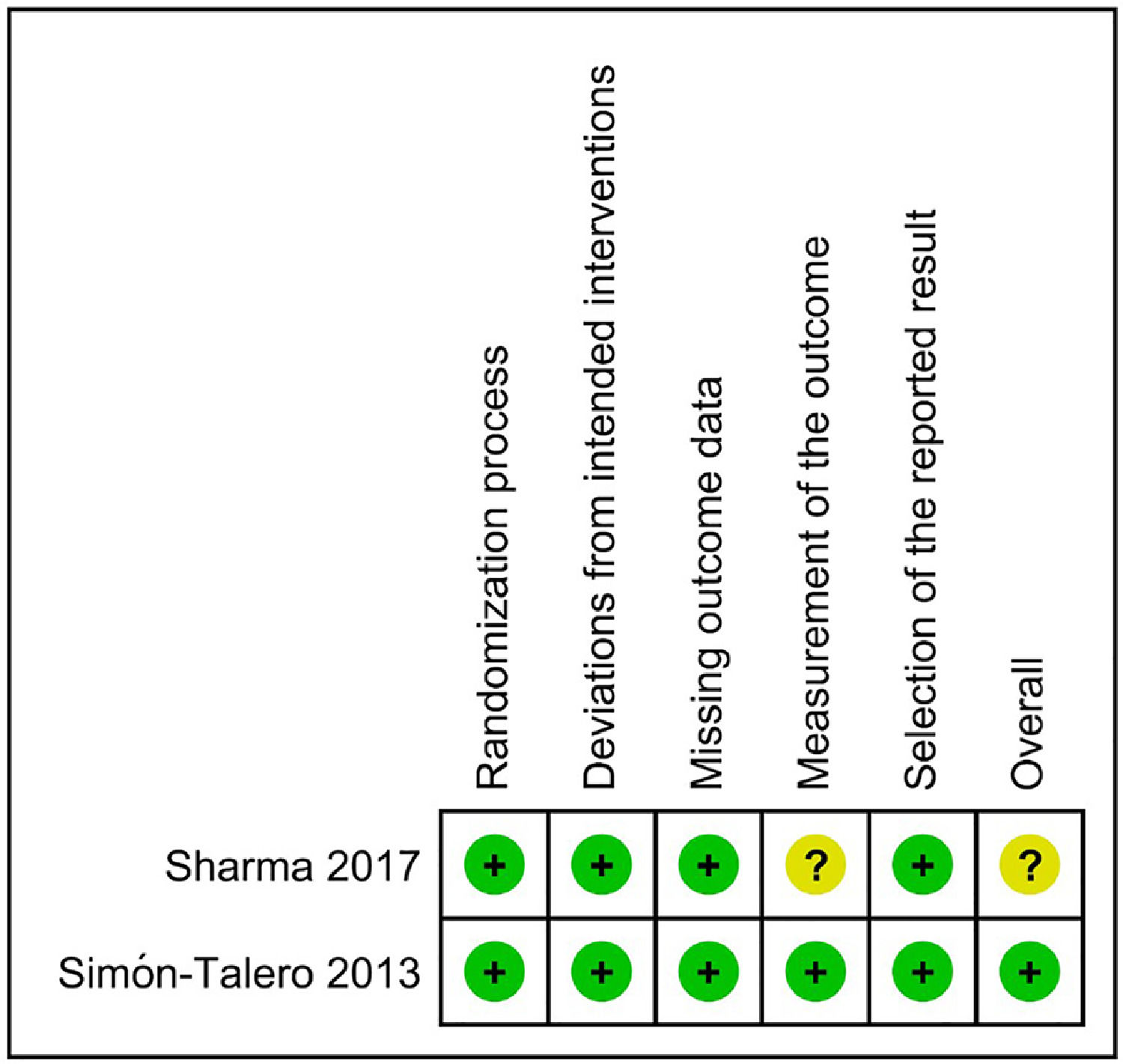
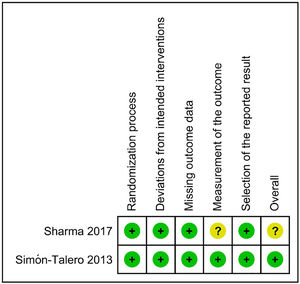
Regarding the risk of bias assessment, the study by Simón-Talero et al. [33] was considered to have low risk of bias. On the other hand, the trial by Sharma et al. [32] raised some concerns because of the lack of blinding, which could have influenced the measurement of an outcome such as HE. Fig. 4 shows the risk of bias assessment.
Due to the limited number of studies included in this systematic review, it was not possible to perform sensitivity analyses or a publication bias assessment.
DiscussionThe effect of albumin administration in patients with HE has been investigated, but its role remains unclear until now. It has been suggested that albumin could decrease the incidence of HE or improve overt HE in patients with cirrhosis, but most of the studies did not have an adequate design or a sufficiently large sample of patients to allow for a strong conclusion [5,32,–34]. In this systematic review of randomized controlled trials with meta-analysis, including 176 patients, it was demonstrated that the administration of albumin significantly improves HE and decreases mortality.
The study by Sharma et al. [32]. evaluated 120 patients with cirrhosis and HE and showed that treatment with lactulose and albumin is more effective than lactulose alone regarding reversal of overt HE, reduction of mortality, shortening of hospital stay and reduction of levels of circulating cytokines and endotoxins. The authors observed that the complete recovery of HE was lower than previously observed in other studies [35,36], which could probably be explained by the inclusion of a high number of patients with grades III and IV HE, as well as by the requirement of complete reversal of HE as the primary endpoint of the study, instead of any improvement in the baseline degree of HE. The study also demonstrated that the albumin plus lactulose group had lower mortality than the control group, mainly due to a decrease in sepsis-related deaths, which could be associated with a decrease in cytokines and endotoxin levels in blood. One of the strengths of this study was its large sample size. On the other hand, a limitation of the study was the lack of blinding, that could have influenced the evaluation of HE, which might be subject to some degree of subjectivity.
However, the beneficial effects of albumin on HE resolution were not verified in the other randomized controlled trial on the matter. In the multicenter study by Simón-Talero et al. [33]., in which 56 patients with cirrhosis and HE grades II–IV were randomly assigned to receive albumin or saline, there was no significant difference between groups regarding reversal of HE. In addition, there were no differences in the evaluated oxidative stress markers and pro-inflammatory cytokines. This study also assessed other outcomes in order to investigate potential benefits of albumin in patients with HE, and one major finding was the improved 90-day survival among subjects who received albumin. Despite being a secondary endpoint of this study, survival is a solid outcome, not as susceptible to bias as others. The results of this trial were reinforced by the blinded assessment of HE, the inclusion of an independent assessor for all centers and the use of several scales of HE. On the other hand, the study had some limitations, as the short follow-up and the small sample.
Regarding the risk of bias assessment, the study by Simón-Talero et al. [33]. was considered to have a low risk of bias. Due to the lack of blinding and to the subjectivity possibly involved in the evaluation of HE, the study by Sharma et al. [32]. was considered to raise some concerns. Regarding mortality, the study by Sharma et al. [32]. would be considered to have a low risk of bias, since this outcome is not susceptible to bias by an unblinded assessment.
Some non-randomized studies aimed to evaluate the role of albumin in the management of HE. The cohort study by Jalan et al. [5] evaluated patients with cirrhosis and HE receiving albumin or not. The authors observed that the severity of HE was significantly improved in the albumin group both at 24 and at 72 h (p < 0.01), which was not observed in the control group (p = 0.21). In another retrospective cohort study of 182 patients [34], albumin infusion significantly improved overt HE (84.6 vs. 68.1%, p = 0.009) and decreased in-hospital mortality (7.7 vs. 19.8%, p = 0.018).
Two studies that evaluated the role of long-term albumin administration in decompensated cirrhosis showed a lower incidence of HE during the evolution of these patients [6,7]. On the other hand, two other trials and a meta-analysis published by our group failed to verify a significant benefit of long-term albumin in preventing the development of HE [17,18,20].
Considering the still limited body of evidence (only two studies) there is some concern regarding the fact that in the article by Sharma et al. [32]. the included patients presented more severe disease and mortality was evaluated on day 10, and in the article by Simón-Talero et al. [33]. mortality was assessed on day 90. Also, the fact that one of the included studies raised some concerns in the risk of bias assessment, may be a limiting data.
In conclusion, this meta-analysis demonstrates that albumin improves HE and reduces mortality in patients with cirrhosis and HE, but further randomized controlled trials with larger samples of patients would be useful in order to increase the confidence of recommending albumin administration in the treatment of HE.
CRediT authorship contribution statementIsadora Z. Bombassaro: Conceptualization, Visualization, Funding acquisition, Formal analysis, Data curation, Writing – original draft, Supervision, Writing – review & editing. Cristiane V. Tovo: Conceptualization, Visualization, Funding acquisition, Formal analysis, Data curation, Writing – original draft, Supervision, Writing – review & editing. Ângelo Z. de Mattos: Conceptualization, Visualization, Funding acquisition, Formal analysis, Data curation, Writing – original draft, Supervision, Writing – review & editing. Marcelo Ahlert: Conceptualization, Visualization, Funding acquisition, Formal analysis, Data curation, Writing – original draft, Supervision, Writing – review & editing. Talita Chiesa: Conceptualization, Visualization, Funding acquisition, Formal analysis, Data curation, Writing – original draft, Supervision, Writing – review & editing. Angelo A. de Mattos: Conceptualization, Visualization, Funding acquisition, Formal analysis, Data curation, Writing – original draft, Supervision, Writing – review & editing.
Albumin in the management of hepatic encephalopathy