Progression of liver disease in chronic hepatitis C depends on several factors related to the host, virus and the environment which deserves further investigations. 120 candidates for blood donation with hepatitis C virus were divided into three groups according to alcohol intake: abstainers41, light drinkers36 and heavy drinkers43. Liver histopathology alterations, namely architectural staging, periportal and lobular inflammation as well as portal inflammatory infiltrate were graded from 0 to 4 and afterwards divided into light (0 to 2) and severe (3 to 4). There were more drinkers among men (83.5%) than among women (41.5%). Regarding the three groups, mild periportal inflammation was significantly related with abstainers and light drinkers groups whereas severe periportal inflammation was more predominant in heavy drinkers (p = 0.033). When we compared mild with severe histopathological alterations older age was significantly (p = 0.004) associated with severe fibrosis, periportal inflammation and portal inflammatory infiltrate. In relation to enzyme levels a significant difference in fibrosis and lobular activity was found for ALT, AST and GGT. Only AST was a marker of greater portal inflammatory infiltrate. Additionally, platelets were significantly lower in severe fibrosis and in periportal inflammation. Logistic regression analysis identified AST and platelets as independent predictors for severe fibrosis. In conclusion, a correlation was found between alcohol consumption and periportal inflammation. Fibrosis correlated with age, high enzymes levels and low platelets. AST and platelets were the best predictors for severe fibrosis.
Abbreviation used: HCV: hepatitis C virus; CHC: chronic hepatitis C; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma glutamyl transferase; BMI: body mass index; HBsAg: Hepatitis B surface antigen; IgG: immunoglobulin G; IgM: immunoglobulin M.
IntroductionHepatitis C virus (HCV) is recognized as the major cause of chronic hepatitis worldwide.1 Once HCV infection develops, it progresses silently to chronic hepatitis in about 80% of the cases. Cirrhosis and hepatocellular carcinoma will develop in respectively 20% and 4%.2 The natural history of HCV infection remains speculative, mainly due to the absence of symptoms that would require medical attention. Predictive factors for the progression of the disease continue to be a major challenge, mainly in asymptomatic patients, as candidates for blood donation. Many studies have been developed in order to try to identify which are the potential prognostic markers that could lead to the development of cirrhosis.3 Various factors of the host, the virus and the environment have been investigated, including older age, gender, age at contamination, viral load, genotype, alcohol intake and disease transmission patterns.4-6
The interaction between HCV infection and alcohol intake contributes to the severity of liver disease. According to many studies, excessive use of alcohol influences the progression of HCV infection.7-10 However, alcohol consumption alone may cause damage, but only 20% develop cirrhosis,11 suggesting that other factors may contribute to hepatic disorders. Some investigators, examining HCV carriers with different levels of alcohol consumption, found fibrosis progression especially in obese and diabetes patients.12
Controversies exist concerning histological findings in chronic hepatitis C13 and in general, hepatic enzyme values are considered to correlate poorly with histopathological changes.14-16 Although the architectural alterations, namely degree of fibrosis, are clinically relevant for staging chronic hepatitis C, the degree of the inflammatory process should also be considered in the natural history or the progression of the disease.
The aim of our study was to evaluate the relationship between different alcohol consumption and grading of histopathological variables.
Patients and methodsDuring a period of two years, 120 consecutive outpatients at the Hemocentro of Sao Paulo (Clinic Hospital of FM-USP) were studied. Inclusion of these candidates for blood donation was made according to the following criteria: a) Anti-HCV positive, confirmed by supplementary recombinant immunoblot assay (RIBA or LIA and HCV RNA by PCR); b) alanine aminotransferase (ALT) levels above normal; c) age ranging from 18 to 65 years for both genders; d) a written informed consent of the patients, as approved by our hospital ethics committee; e) acceptance of liver biopsy performance. In all patients the following clinical and demographic information were obtained: gender, age, Body Mass Index (BMI) (calculated as weight in kilograms divided by height in meters squared), source of HCV infection (transfusion, intravenous drug use, surgery, tattoos, acupuncture, sexual contacts), estimated duration of HCV infection (defined as the number of years since the time of the earliest exposure to an identified risk factor), laboratory data at the time of liver biopsy: serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), serum gamma glutamyl transferase (GGT), platelet number (mm3), serum Immunoglobulin M (IgM), Immunoglobulin G (IgG). Exclusion criteria were: presence of anti-nuclear antibodies, anti-smooth muscle antibodies, anti-liver-kidney microsomes antibodies, anti-mitochondrial antibodies in titles > 1/80; Anti-HIV, HBsAg and hepatocellular carcinoma.
Alcohol quantification. All patients and family members were interviewed to obtain informations about the type of alcoholic drink, the amount of alcohol consumed per day, duration and complete stopping of alcoholic intake. The quantification of the daily alcohol intake was submitted to the following formula: volume in ml multiplied by 0.8 (constant) and by the alcoholic degree of the beverage, divided by 100 and express in g/day. In this study the patients were divided into three groups: Group A: abstainers; Group B: light alcohol intake as < 40 g/day for men and < 20 g/day for women and Group C: heavy alcohol intake, defined as > 40 g/day for men and > 20 g/ day for women. Complete stopping was considered only after six months of abstinence. At the time of liver biopsy, complete stopping was observed in 24.3% of patients in group B and in 29.5% of patients in group C.
Liver Histology. Liver biopsy samples were obtained by percutaneous punction ultrasound oriented. Mean length of biopsy samples was 1.8 ± 0.5 cm and they were formalin-fixed, paraffin-embedded and stained with Hematoxylin-Eosin, Perls, Masson’s trichrome and reticulin impregnation by silver salts. The mean number of portal tracts was 10 ± 4. The histological inflammatory activity (grade) and degree of architectural derangement or fibrosis (stage) of the viral hepatitis were assessed according to the Brazilian classification of the chronic hepatitis17 as follows: the stage of fibrosis (F) or architectural alterations was assessed on a four-point scale: F0 = normal lobular architecture; F1 = fibrous portal expansion; F2 = fibrous portal expansion with portal-portal septa; F3 = partial preservation of the lobular architecture with portal-portal and portal-central septa, with occasional sketch of nodules; F4 = cirrhosis. Periportal activity (PA) was graded according to the intensity of necroinflammatory lesions: PA0 = absence of inflammation; PA1 = spill-over of inflammatory cells without interface hepatitis; PA2 = mild interface hepatitis; PA3 = moderate interface hepatitis; PA4 = severe interface hepatitis. Lobular activity (LA) was graded as following: LA0 =normal hepatocytes; LA1 = mild alterations in hepatocytes, including ballooning degeneration or acidophil body, associated with lymphocytes/histiocytes infiltrate and rare foci of necrosis; LA2 = foci necrosis of hepatocytes, numerous areas of mononuclear aggregates; LA3 = foci necrosis of hepatocytes surrounded by mononuclear aggregates in several areas, limited areas of confluent necrosis; LA4 = foci necrosis of hepatocytes surrounded by mononuclear aggregates in numerous areas, with extensive/multiple confluent necrosis. Portal Infiltrate (PI) was graded: PI0 = rare portal lymphocytes; PI1 = mild increase of the number of portal lymphocytes; PI2 = moderate increase of the number of portal lymphocytes; PI3 = marked increase of the number of portal lymphocytes; PI4 = very marked increase of the number of portal lymphocytes. Steatosis was scored as present or absent.
For statistical comparisons between groups or variables, two levels of histopathological alterations were considered: grades 0, 1 and 2 were grouped as light (absent or mild) and grades 3 and 4 as severe.
Statistical Analysis. Chi-square test was used for analysis of qualitative variables, whereas quantitative variables as age, BMI, duration of infection and platelet were assessed using analysis of variance (ANOVA). When significant, the Tukey or the Student’s t-test was used to discriminate the differences. Kruskal-Wallis test, Dunn test or Wilcoxon rank-sum test were also used to analyze variables as ethanol (g/d), duration of alcohol intake, ALT, AST, GGT, IgG, IgM, and abstinence. Multiple logistic regression analysis with stepwise of selected variables was performed to correlate the degrees and stages of the histopathological alterations with other clinical and biochemical data. Odds ratios and 95% confidence intervals were calculated. Values of p < 0.05 were considered to be significant.
ResultsThis study included 120 patients, 83 (69.2%) men and 37 (30.8%) women with chronic hepatitis C divided according to alcohol intake as follow: the group A, abstainers: n = 41; group B, light drinkers: n = 36 and group C, heavy drinkers: n = 43. Male patients were common in groups B and C (69.4% and 95.3% respectively), whereas female patients more common in group A (58.5%) (p < 0.001). The epidemiological, clinical and demographic data of the 120 patients at the time of the liver biopsy are shown in table I. The three groups were not different in terms of age, BMI, duration of infection or surgery as a contamination factor. Groups B and C were not different in terms of duration of alcohol intake and abstinence. Transfusion as a risk factor was more frequent in the abstainers (p = 0.056) whereas drug addiction predominated in heavy drinkers (p < 0.001). The three groups were not different in terms of ALT, AST, GGT and platelet count (Table I). AST/ALT index was also analyzed in the three groups and no differences were found (p = 0.106).
Comparative analysis of the demographic data, epidemiological and biochemical characterization of the patients at the time of liver biopsy
| Parameters | Group A | Group B | Group C | “p” |
|---|---|---|---|---|
| Number of patients | 41 | 36 | 43 | |
| Gender n (M/F)**** | 17/24 | 25/11 | 41/2 | < 0.001 |
| Age (year)* | 40.8 ± 11.8 | 36.7 ± 10.4 | 38.8 ± 9.9 | 0.248 |
| Alcohol Intake (mean) | ||||
| Amount (g/d)*** | 0 | 18.1 ± 11.1 | 149.9 ± 119.2 | < 0.001 |
| Duration (yr)*** | 0 | 12.9 ± 9.7 | 15.2 ± 8.4 | 0.137 |
| Abstinence (days)*** | 0 | 375.8 ± 918.6 | 413.3 ± 853.1 | 0.911 |
| BMI (kg/m2)* | 25.3 ± 3.7 | 25.8 ± 3.9 | 26.1 ± 4.1 | 0.672 |
| Contamination factors**** | ||||
| Transfusion n (%) | 17 (41.40) | 6 (16.70) | 12 (27.90) | 0.056 |
| Drug addicted n (%) | 1 (2.50) | 6 (16.70) | 17 (39.50) | < 0.001 |
| Surgery n (%) | 24 (58.50) | 17 (47.20) | 29 (67.40) | 0.192 |
| Others n (%) | 8 (19.50) | 6 (16.70) | 10 (23.30) | 0.763 |
| Duration of contamination (yr)* | ||||
| Laboratory data | 19.8 ± 7.9 | 15.9 ± 7.4 | 16.5 ± 7.7 | 0.108 |
| ALT (xULN)** | 2.1 ± 1.5 | 2.3 ± 1.4 | 2.4 ± 1.4 | 0.751 |
| AST (xULN)** | 1.8 ± 1.0 | 1.7 ± 0.8 | 2.2 ± 1.4 | 0.365 |
| GGT (xULN)** | 1.3 ± 1.0 | 1.5 ± 0.9 | 1.7 ± 0.1 | 0.194 |
| Platelet (mm3)* | 204.541 ± 57.281 | 197.583 ± 56.052 | 190.813 ± 71.173 | 0.333 |
| AST/ALT (mean)** | 0.8 ± 0.3 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.106 |
Abbreviations: Group A: abstainers; Group B: light drinkers; Group C: heavy drinkers.
M, male; F, female; xULN, x upper limit of normal; BMI: body mass index Values in mean and SD
Histopathological Data. There was no difference between findings of liver histology concerning the degree of fibrosis, periportal inflammatory activity, lobular inflammation, portal inflammatory infiltrate and steatosis, among the three groups.
Grouping the results of these variables as light (grades 0, 1 and 2) or severe (grades 3 and 4), no differences were found in architectural alterations, lobular inflammatory activity or portal inflammatory infiltrate. Nevertheless, heavy drinkers have shown higher percentages of grades 3 and 4 of interface hepatitis when compared to abstainers and light drinkers (p = 0.033), as shown in table II.
Histopathological results in the three studied groups.
| Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Abstainers | Light drinkers | Heavy drinkers | Total | p* | |||||
| Fibrosis | 0.868 | ||||||||
| 0/1/2 | 30 | 73.20% | 27 | 75.00% | 30 | 69.80% | 87 | 72.50% | |
| 3/4 | 11 | 26.80% | 9 | 25.00% | 13 | 30.20% | 33 | 27.50% | |
| Periportal activity | 0.033 | ||||||||
| 0/1/2 | 26 | 63.40% | 26 | 72.20% | 18 | 43.90% | 70 | 59.30% | |
| 3/4 | 15 | 36.60% | 10 | 27.80% | 23 | 56.10% | 48 | 40.70% | |
| Portal inflammatory infiltrate | 0.675 | ||||||||
| 0/1/2 | 27 | 67.50% | 21 | 58.30% | 24 | 60.00% | 72 | 62.10% | |
| 3/4 | 13 | 32.50% | 15 | 41.70% | 16 | 40.00% | 44 | 37.90% | |
| Lobular activity | 0.481 | ||||||||
| 0/1/2 | 31 | 79.50% | 29 | 80.60% | 28 | 70.00% | 88 | 76.50% | |
| 3/4 | 8 | 20.50% | 7 | 19.40% | 12 | 30.00% | 27 | 23.50% | |
| Abstinence | 0.272 | ||||||||
| < 6 months | - | - | 29 | 80.60% | 30 | 69.80% | 59 | 74.70% | |
| > 6 months | - | - | 7 | 19.40% | 13 | 30.20% | 20 | 25.30% | |
Demographic, epidemiological and biochemical data analyzed according to the different degrees of fibrosis and periportal inflammatory activity are shown in table III. Among the demographic variables, older age (p = 0.004) and BMI (p = 0.013) were significantly associated with severe fibrosis. Otherwise, the gender, duration of infection and duration of alcohol intake were not associated with severe fibrosis. The serum ALT (p < 0.001), AST (p < 0.001), GGT (p = 0.012) and IgG (p = 0.040) values were significantly higher in severe fibrosis compared to no or mild fibrosis. Additionally, the platelet count was significantly lower (p < 0.001) in severe fibrosis compared to no or mild fibrosis. The periportal inflammatory activity was shown to be significantly more severe in older age (p = 0.001) and duration of alcohol intake (p = 0.001), compared to no or mild periportal activity. The ALT (p = 0.031), AST (p < 0.001) and GGT (p = 0.013) values were significantly higher in severe periportal inflammatory activity (grades 3 and 4), whereas the platelet count was significantly (p = 0.049) lower. On the other hand, gender, BMI, duration of infection, IgG, IgM, did not differentiate between mild and severe periportal inflammatory activity.
Demographic, epidemiological and biochemical data, analyzed according to different degrees of fibrosis and periportal inflammatory activity.
| Parameter | Fibrosis | Periportal inflammatory activity | ||||
|---|---|---|---|---|---|---|
| Degrees | Mean | SD | p | Mean | SD | p |
| Gender n (M/F)*** | ||||||
| 0/1/2 | 60/27 | – | 0.938 | 51/19 | – | 0.234 |
| 3/4 | 23/10 | – | – | 30/18 | – | – |
| Age (year)* | ||||||
| 0/1/2 | 37.2 | 11.3 | 0.004 | 35.4 | 10.6 | 0.001 |
| 3/4 | 43.4 | 7.9 | 43.9 | 9.1 | ||
| BMI (kg/m2)* | ||||||
| 0/1/2 | 25.2 | 3.9 | 0.013 | 25.4 | 4.3 | 0.299 |
| 3/4 | 27.2 | 3.7 | 26.2 | 3.4 | ||
| Duration of infection (yr)* | ||||||
| 0/1/2 | 17.3 | 7.9 | 0.790 | 16.4 | 7.9 | 0.100 |
| 3/4 | 17.8 | 7.9 | 19.1 | 7.6 | ||
| Duration of alcohol intake (yr)** | ||||||
| 0/1/2 | 13.3 | 8.6 | 0.207 | 11.1 | 7.6 | 0.001 |
| 3/4 | 16.6 | 9.8 | 18.2 | 9.5 | ||
| ALT (xULN)** | ||||||
| 0/1/2 | 2.1 | 1.5 | < 0.001 | 2.1 | 1.5 | 0.031 |
| 3/4 | 2.7 | 1.1 | 2.4 | 1.1 | ||
| AST (xULN)** | ||||||
| 0/1/2 | 1.6 | 0.8 | < 0.001 | 1.6 | 0.9 | < 0.001 |
| 3/4 | 2.8 | 1.3 | 2.4 | 1.2 | ||
| Platelet (mm3)* | ||||||
| 0/1/2 | 209.850 | 49.965 | < 0.001 | 206.800 | 51.607 | 0.049 |
| 3/4 | 165.006 | 78.109 | 184.108 | 72.607 | ||
| GGT (xULN)** | ||||||
| 0/1/2 | 1.3 | 0.9 | 0.012 | 1.3 | 0.8 | 0.013 |
| 3/4 | 1.9 | 1.2 | 1.7 | 1.1 | ||
| IgG (mg/dL)** | ||||||
| 0/1/2 | 1.539 | 307 | 0.040 | 1.533 | 314 | 0.078 |
| 3/4 | 1.771 | 567 | 1.706 | 497 | ||
| IgM (mg/dL)** | ||||||
| 0/1/2 | 134 | 70 | 0.052 | 138 | 77 | 0.456 |
| 3/4 | 176 | 119 | 156 | 102 | ||
M, male, F, female; xULN, x upper limit of normal
Demographic, epidemiological and biochemical data analyzed according to the different degrees of lobular inflammatory activity and portal inflammatory infiltrate are shown in table IV. There was a significant association between BMI (P = 0.039), higher levels of ALT (p = 0.013), AST (p = 0.001) and GGT (p = 0.003) and severe lobular inflammation. The other studied variables did not show differences between the groups. There was a significant association between older age (p = 0.001) and AST (p = 0.018) with severe portal inflammatory infiltrate. For the other variables there were no significant correlations.
Demographic, epidemiological and biochemical data analyzed according to different degree of lobular inflammatory activity and portal inflammatory infiltrate.
| Parameter | Lobular inflammatory Activity | Portal inflammatory Infiltrate | ||||
|---|---|---|---|---|---|---|
| Degree | Mean | SD | p | Mean | SD | p |
| Gender n (M/F)*** | ||||||
| 0/1/2 | 61/27 | - | 0.795 | 47/25 | - | 0.404 |
| 3/4 | 18/9 | - | - | 32/12 | - | - |
| Age (year)* | ||||||
| 0/1/2 | 38.1 | 11.2 | 0.252 | 36.3 | 11.1 | 0.001 |
| 3/4 | 40.8 | 9.0 | 42.9 | 8.8 | ||
| BMI (kg/m2)* | ||||||
| 0/1/2 | 25.3 | 3.9 | 0.039 | 25.4 | 4.1 | 0.371 |
| 3/4 | 27.1 | 3.9 | 26.1 | 3.7 | ||
| Duration of infection (yr)* | ||||||
| 0/1/2 | 18.2 | 7.9 | 0.219 | 17.1 | 8.2 | 0.494 |
| 3/4 | 15.8 | 7.8 | 18.3 | 7.5 | ||
| Duration of alcohol intake (yr)* | ||||||
| 0/1/2 | 13.9 | 9.1 | 0.696 | 12.9 | 9.1 | 0.072 |
| 3/4 | 15.2 | 9.6 | 16.3 | 8.9 | ||
| ALT (xULN)** | ||||||
| 0/1/2 | 2.1 | 1.3 | 0.013 | 2.2 | 1.6 | 0.104 |
| 3/4 | 2.8 | 1.4 | 2.3 | 0.9 | ||
| AST (xULN)** | ||||||
| 0/1/2 | 1.6 | 0.8 | 0.001 | 1.7 | 1.1 | 0.018 |
| 3/4 | 2.6 | 1.4 | 2.1 | 1.0 | ||
| Platelet (mm3)* | ||||||
| 0/1/2 | 200.845 | 54.834 | 0.469 | 203.211 | 52.817 | 0.052 |
| 3/4 | 190.562 | 82.832 | 189.318 | 74.648 | ||
| GGT (xULN)** | ||||||
| 0/1/2 | 1.3 | 0.8 | 0.003 | 1.5 | 0.9 | 0.680 |
| 3/4 | 2.0 | 1.7 | 1.5 | 1.1 | ||
| IgG (mg/dL)** | ||||||
| 0/1/2 | 1.561 | 390 | 0.048 | 1.588 | 412 | 0.606 |
| 3/4 | 1.734 | 438 | 1.630 | 399 | ||
| IgM (mg/dL)** | ||||||
| 0/1/2 | 138 | 81 | 0.090 | 137 | 77 | 0.222 |
| 3/4 | 171 | 106 | 161 | 103 | ||
M, male; F, Female ULN, x upper limit of normal
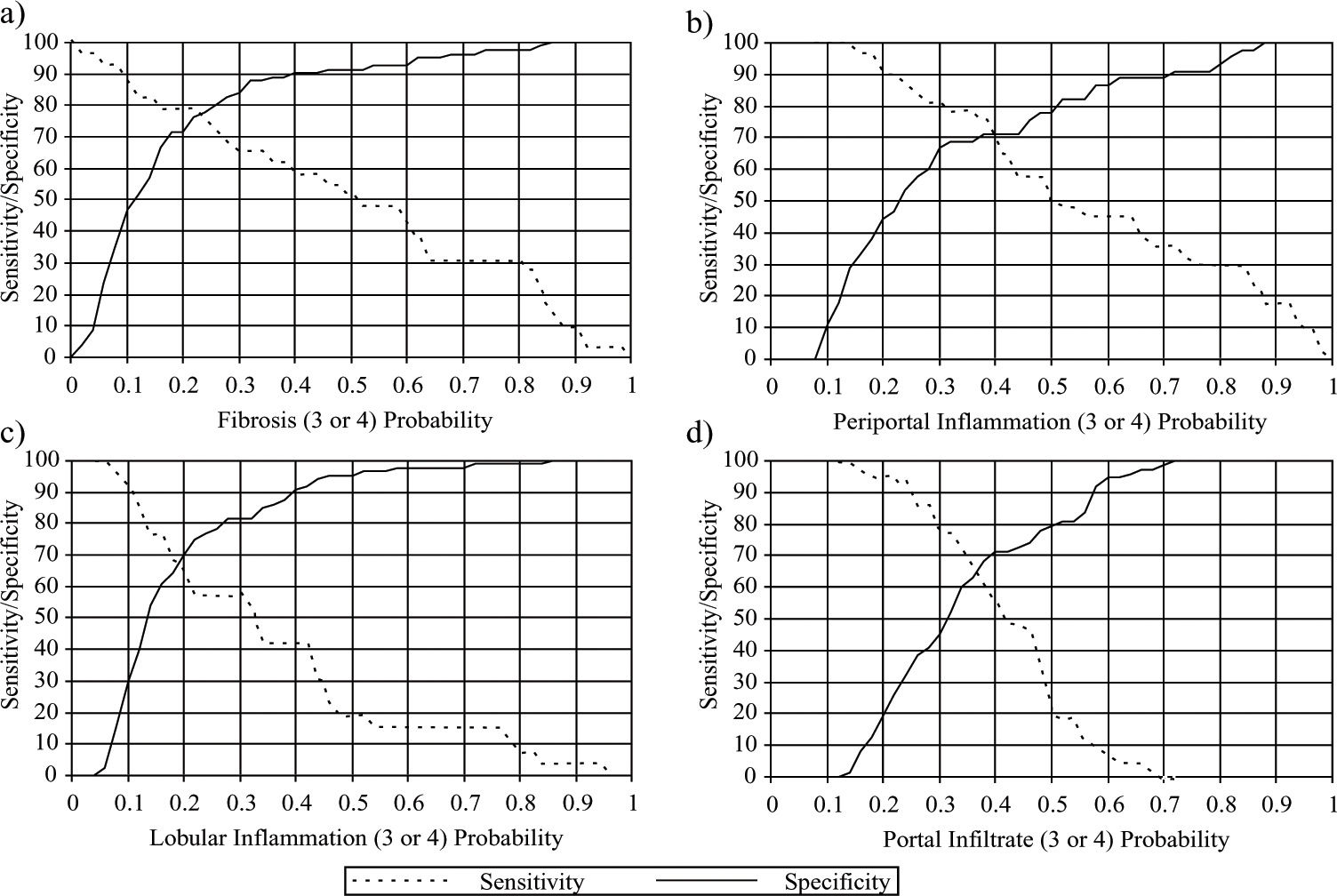
Logistic regression analyses: Considering the two levels of the architectural alterations in relation to the different clinical variables, it was shown that the only independent predictive factor of severe fibrosis were AST (odds ratio = 2.734 and CI 95% 1.687-4.431) and platelet count (odds ratio = 0.862 and CI 95% 0.7820.950). Considering these variables, the best sensitivity and specificity cut-off was reached as respectively 78.8 and 71.3 (see figure 1a). Considering the two levels of periportal inflammation activity in relation to the different clinical parameters of alcoholic groups B and C it was shown that the only independent predictive factors of severe periportal inflammation were AST (odds ratio = 2.684 and CI 95% 1.437-5.013) and duration of alcohol intake (odds ratio 1.112 CI 95% 1.041-1.188). Considering the studied parameters, the best cut-off for the sensitivity was 75.8% and for specificity 70.5% (see figure 1b).
The cut-off values near the crossing between the sensitivity and specificity are shown in the figure above.
They correspond to the probability of grades 3 or 4 of each histopathological variable be detected, by clinic and biochemical results, according to the logistic regression analysis.
Concerning fibrosis, the sensitivity was 79.30 and specificity 76.50 for AST and platelets. Concerning periportal inflammation, the sensitivity was 69.70 and specificity 71.10 for AST and duration of alcohol intake. Concerning lobular inflammation, the sensitivity was 69.20 and specificity 64.40 for AST and GGT.
Concerning portal inflammatory infiltrate, the sensitivity was 65.10 and specificity 63.00 for age.
Considering the two levels of lobular inflammation activity in relation to the different clinical parameters, it was shown that the only independent predictive factors of severe lobular inflammation were AST (odds ratio 2.049 CI 95% 1.304-3.221) and GGT (odds ratio 1.866 CI 95% 1.134-3.069). Considering these variables, the best cutoff for sensitivity was 66.7 and specificity 70.5 as shown in figure 1c.
Considering the two levels of portal inflammatory infiltrate in relation to the different clinical parameters, it was shown that the only independent predictive factor of severe portal inflammatory infiltrate was age (odds ratio 1.071 CI 95% 1.028-1.115). Considering this variable, the best cut-off for sensitivity was 63.6 and specificity 67.6 as shown in figure 1d.
DiscussionThis study shows that histopathological parameters like fibrosis, lobular inflammation and portal infiltrate in candidates for blood donation with hepatitis C did not differ when alcohol abstainers, light drinkers and heavy drinkers were compared. Only periportal inflammation was more severe in heavy drinkers. The most commonly used parameter to evaluate the progression of liver disease is fibrosis,18 and many studies have indicated an increased risk of progression of chronic hepatitis C with increasing levels of alcohol consumption.14,19 As some of these studies have included higher percentages of cirrhotic patients than ours, our results may reflect an earlier phase of disease progression.20 As the inflammatory process precedes fibrosis development, it is understandable that there was a statistically significant difference for inflammation but not for fibrosis. The histopathological finding of periportal inflammation cannot be associated with isolated alcohol abuse. In fact, the most common histopathological alcoholic alterations predominate in zone 3 of the Rappaport acinus.21 On the other hand, we are dealing with an association of hepatitis C and alcohol, and the histopathological parameters, suggestive of alcoholic damage, are different from those of viral hepatitis.22 Another possibility which explains no differences in fibrosis, when abstainers were compared to light or heavy drinkers, is a beta error. In fact, the greater degree of histological architectural alterations was found in only 13 patients, the majority of them (61.5%) being heavy drinkers. One can speculate that, in a larger population, a significant difference would appear, as shown by other authors.6,14
The comparison of demographic and clinical parameters between groups have shown interesting differences, namely, more female are abstainers (58.5%).23 In relation to contamination factors, the two major risk factors for HCV infection were prevalent in different groups: blood transfusion was more frequently found in abstainers whereas drug addiction was significantly higher in heavy drinkers. It is uncertain why heavy drinkers are more likely to be infected with HCV, although alcoholics are more prone to have used injection drugs or have had other exposures to the virus.24,25 Interaction of alcohol and drugs are considered to enhance susceptibility to infections,26 probably due to alterations in the immune system.27
ALT is commonly used as a marker of hepatic inflammation and damage, especially in HCV infection,28 although it can be more pronounced in patients with both HCV infection and alcoholic liver disease.29 In contrast, some studies have found persistently normal ALT levels in alcoholic patients HCV infected with a slow progression rate of fibrosis.30
Differently from other studies,31 we have analyzed histopathological variables separately and in relation to epidemiological and laboratory parameters. An independent risk factor associated with fibrosis is older age, as shown in several epidemiological studies.32,33 Like others,14 we have found older age related to fibrosis and periportal inflammation. The amount of portal inflammatory infiltrate is not usually taken into consideration in the various classifications of chronic hepatitis,31,34 but is considered in our classification.17 The only parameter that differentiated slight from severe portal infiltrate was age. Younger people more frequently had lower grades of portal inflammatory infiltrate. The strong relationship of age with severity of chronic hepatitis may reflect qualitative and quantitative changes in the immune response which occur with increasing age, or more likely indicate the longer disease duration.35 To our knowledge this is the first report of relation between older age and greater portal infiltrate in hepatitis C.
Duration of infection is an important parameter in the progression of the disease, but it has been described in community-based studies that HCV infection may persist for decades without causing clinically apparent liver disease.36,37 Lack of differences regarding duration of infection in our study may be related to the blood donor population, in which a mild liver disease was more frequently found.
Elevated ALT, AST and GGT levels are associated with fibrosis38,39 and were correlated also with periportal and lobular inflammation in our study although only AST was an independent predictor of severe fibrosis. One of the possible reasons to explain higher AST values could be HCV-induced liver injury in the mitochondrial fraction of hepatocytes where it is present, and/or due to the presence of alcohol.40 On the other hand, decreased platelet count has been used to non-invasively assess the severity of disease in patients with chronic liver disease.41 Platelet counts clearly separated early from late stages of fibrosis as well as the degrees of periportal inflammation. Although the hepatitis C virus by itself may be related to low platelet counts, many authors have had similar results.16,42,43 To amplify the opposite relationship between AST levels and platelets count in determining the stage of fibrosis, a novel index, called “AST to platelet ratio index” (APRI) has recently been proposed.44 Our results are in keeping with this study.
Obesity has also been associated with more advanced degrees of fibrosis in CHC28 and an association between BMI and fibrosis has been demonstrated.45 In our series, 39.3% of patients presented steatosis, similarly to other histopathological studies of CHC, which have reported microvesicular and macrovesicular fatty change in 31% to 72% of patients.46-48 Lately, even low alcohol intake has been shown to play a synergistic effect on HCV infection leading to steatosis.49
Using logistic regression analysis it was remarkable to see that AST was an independent predictor of severity not only for fibrosis and periportal inflammation but also for lobular inflammation. On the other hand, platelet was an independent predictor only for fibrosis. The levels of sensitivity varying from 65.1 up to 79.3, and specificity ranging from 63 up to 76.5, were unexpectedly high. There are no doubts about the clinical usefulness of fibrosis, recognized as the most important factor in the progression of liver disease. So, it was rewarding to find the best sensitivity and specificity levels for the detection of fibrosis, by the use of AST values and platelets counts.
In conclusion, epidemiological and biochemical data can predict both fibrosis and periportal inflammation with acceptable levels of sensitivity and specificity.