Special issue on hepatocellular carcinoma (HCC) and hepatitis B and C as its main causes worldwide
More infoIn 1999, a population-based survey showed a 5.6 % (102/1832) prevalence of HCV infection in O'Brien, a small rural town of Argentina. The aim of this study was to assess the impact of screening, clinical evaluation and antiviral therapy on elimination of HCV after 20 years of follow-up.
Patients and methodsHCV+ subjects (n=102) underwent clinical, biochemical and histological evaluation to assess the presence and severity of liver disease. Antiviral therapy included pegylated interferon + ribavirin in 2005 and direct antiviral agents from 2017.
ResultsAll viremic subjects (n=84) had genotype 1b with 90%-97.5% sequence homology scores, suggesting the existence of a common source of infection (use of unsafe injections administered by the same health professional). Liver biopsy (n=55) showed chronic hepatitis in all patients. The prevalence of cirrhosis was 28% overall (29/102) and 34.5% among viremic patients. Sustained virological response (SVR) was obtained in 20/34 (59%) patients treated with interferon. From 2005 to 2017, when oral antivirals became available 37/50 untreated patients died. Median age of this group in 2005 was 67 years. Six interferon non-responders and five naive subjects received direct antiviral agents and all developed SVR. Only 1/31 patient (3.2%) with SVR died and none developed decompensated cirrhosis or HCC. In 2019, a new population-based study showed that the prevalence of HCV in O'Brien decreased 20-fold, from 5.6% to 0.28% (3/1070).
ConclusionsDespite the high mortality rate precluding timely access to direct antiviral agents, the O'Brien Project is a good example of HCV micro-elimination studies
The prevalence of HCV infection worldwide is variable and largely depends on risk factors operating in each population or geographic area [1,2]. O'Brien is a small rural town of around 2300 inhabitants located within the County of Bragado in Buenos Aires Province, Argentina. In the mid 1990’s, an increasing number of asymptomatic individuals from O'Brien were found to have elevated aminotransferases in routine laboratory examinations. At that time, jaundice, cirrhosis, liver failure and liver cancer were frequent diagnoses mentioned in death certificates from the region and four patients from this small community required liver transplantation for HCV-cirrhosis. These findings prompted a large population-based study in July 1999 that revealed a high prevalence of HCV infection (5.7%) in the town of O´Brien [3]. Subsequently, HCV-positive individuals underwent clinical studies to assess disease severity and received antiviral therapy with the combination of pegylated interferon (PEG-IFN) and ribavirin (RBV), and more recently direct antiviral agents (DAAs). In 2019, 20 years after the initial study, we repeated the population-based survey to explore the possibility of HCV eradication from O´Brien.
2Patients and methods2.1PatientsThe total number of individuals studied in 1999 was 1832, 1637 included in the previous publication [3] and 195 enrolled shortly thereafter. All participants completed a questionnaire including demographics, risk factors and history of alcohol consumption. Adults provided written consent at the time of enrolment in both epidemiological studies and before liver biopsy, whereas parents consented the participation of children under 18 years of age.
2.2Serologic and virological methodsSerological tests used in the 1999 study for determining the presence of anti-HCV, HCV RNA, HBsAg, anti-HBc and anti-HBs are described elsewhere [3]. Rapid anti-HCV testing (Montebio-HCV, Montebio SRL) was utilized for the 2019 population-based study. Sequencing of HCV isolates was investigated on a 158 base pair fragment from the NS5B region utilizing a prototype software module (Visible Genetics Inc). Comparative sequence alignments and phylogenetic trees were generated using Clustral Wallis Multiple Sequence Alignment Program, v1.7 [4] HCV viral load was investigated by quantitative PCR: Amplicor HCV Monitor 2.0, Roche in 2004 for the 5-year reevaluation of the 1999 HCV-positive cohort and in 2005, before PEG-IFN/RBV therapy and Cobas HCV for use on the Cobas 4800 System, Roche, in 2019
2.3Antiviral therapyAntiviral therapy included PEG-IFN α-2A (Pegasys), 180 μg/week combined with RBV (Copegus) adjusted by body weight (1000 mg/day in <75 Kg and 1200/day in ≥ 75Kg). Starting in 2017, patients with contraindications for PEG-IFN/RBV and non-responders were offered treatment with DAAs, either with the combination of sofosbuvir (Sovaldi or Probirase) 400 mg/day and daclatasvir (Daklinza) 60 mg/day or ledipasvir (Harvoni) 90 mg for 12 weeks. Sofosbuvir was approved in Argentina in October 2015, and daclatasvir and ledipasvir in August 2016. Patients with cirrhosis received in addition RBV adjusted by body weight. Sustained virological response (SVR) was defined as undetectable HCV RNA in serum 24 weeks after completing treatment with PEG-IFN/RBV or 12 weeks for DAAs.
2.4Alcohol consumption and liver biopsiesSignificant alcohol consumption was defined as a daily intake >80 grams in males and >50 grams in females for at least ten years. Indications for liver biopsy were age <65 years and no other contraindications for PEG-IFN therapy. The Knodell-Ishak score was used for histologic grading and staging of hepatitis C [5].
2.5Statistical analysisStudent, chi square, Fisher, Kolmogorov-Smirnov and Mann-Whitney tests and logistic regression were utilized in different phases of the project.
The Secretary of Health of Bragado County approved all studies performed in O'Brien during the 20-year follow-up. Funding and support for the entire project was provided by Fundación para la Docencia e Investigación de las Enfermedades del Hígado (FUNDIEH), a non-profit foundation supporting research and education in liver diseases (www.fundieh.org.ar) and by Bragado County. Hoffmann-La Roche provided PEG-IFN α-2A and RBV at no cost for the patients or for Bragado County
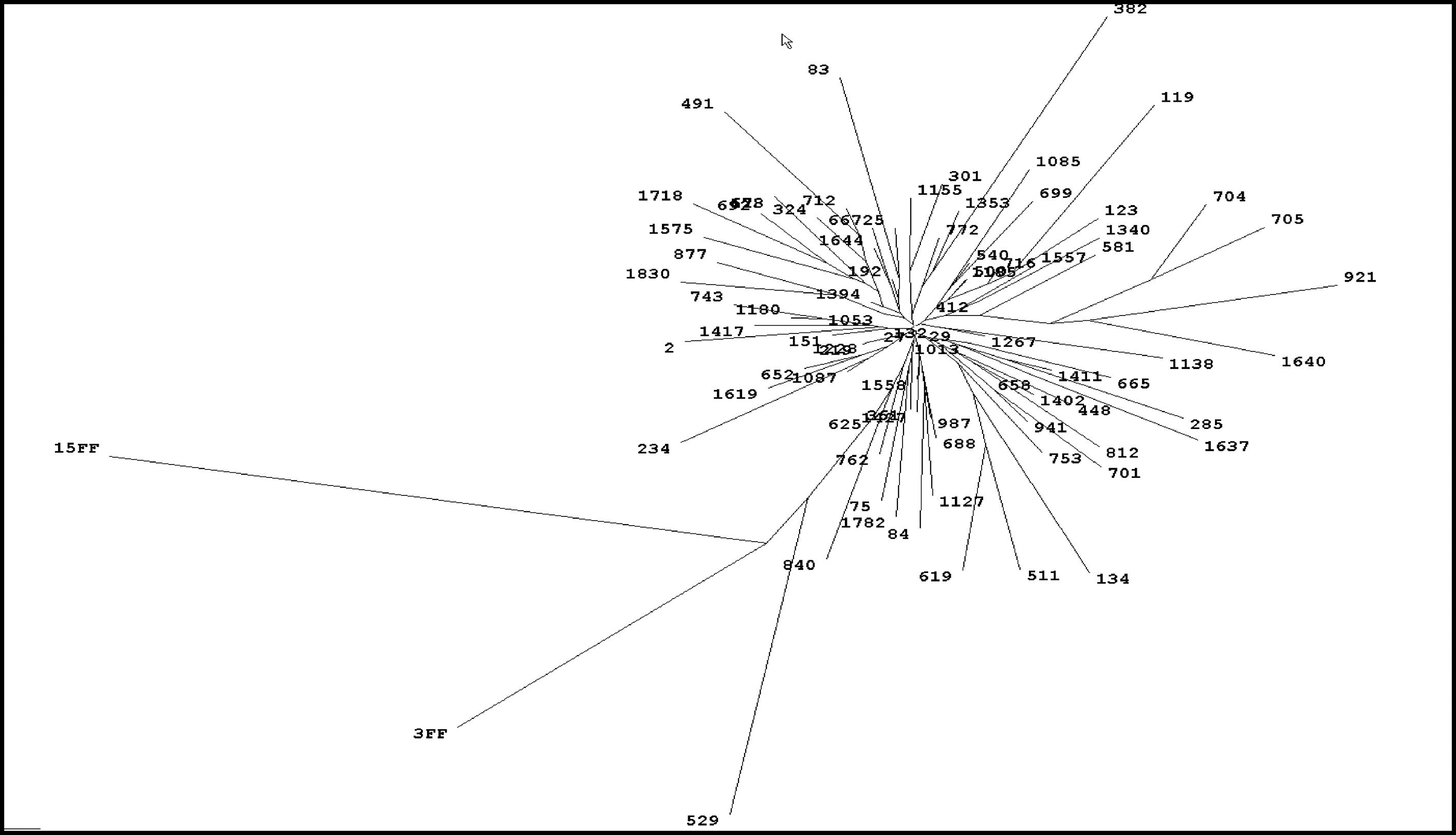
3ResultsThe initial study (1999) included 1832 of the 2300 (79.6%) inhabitants from O´Brien, both adults and children. HCV seroprevalence by enzyme immunoassay (EIA) was 5.6% (102/1832), 0.56% in people aged <40 years (6/1068) and 12.6% in those >40 years (97/764) with a peak of 23.4% between 60 and 70 years of age. Median age of seropositive individuals was 60 (7-81) years with 51% females and 49% males. There were no false-positive results of EIA with 89% of samples (91/102) being recombinant immunoblot assay (RIBA)-positive and the remaining 11 RIBA-indeterminate (c22 single band in 10 and c33 single band in 1). Serum HCV RNA was detectable in 82% (84/102) seropositive subjects. All patients with detectable viremia had genotype 1b infection with a single restriction pattern. Sequencing and phylogenetic analysis of 77 samples showed a very high homology score (90%-97.5%) with very little divergence between isolates, strongly suggesting the existence of a common source of infection [4]. (Fig. 1). There was a high prevalence of anti-HBc positivity among patients with HCV infection (55/102, 54%) of which 35 (64%) were also anti-HBs positive. However, none tested positive for HBsAg or had HIV co-infection. Serological and virological HCV and HBV markers are summarized in Fig. 2. Sixty patients (59%) had elevated aminotransferases, associated with detectable serum HCV RNA in all cases.
From mid-40´s to mid-80´s, 95% of HCV-positive subjects reported receiving from the same health professional intramuscular or intravenous injections with non-disposable materials and poorly sterilized syringes and needles. Based on the study questionnaire and individual evaluations, the estimated time-interval from injections to HCV diagnosis was 35 ± 6 years. A history of alcohol consumption was recorded in 39 HCV-positive individuals (38%) being significant in 17 (17%).
In 2000, liver biopsies were performed in 55 of 84 (65%) viremic patients, all of which had chronic hepatitis [6]. Tissue specimens were inadequate for histological examination in three cases. Fibrosis stage was F0-F1 in 12 (23%), F2 in 13 (25%), F3 in 14 (27%) and F4-6 (cirrhosis) in 13 (25%). Among the 32 viremic patients who were not biopsied, 16 (50%) had clinical, biochemical, radiologic or endoscopic abnormalities highly suggestive of cirrhosis. Therefore, the overall, the prevalence of cirrhosis in O'Brien was 28% (29/102) and 34.5% (29/84) in those with detectable viremia. Cirrhosis was present in 22% of females and 23.5% of males with no history of alcohol consumption and in 33% and 39% respectively in those who reported alcohol intake. None of the 18 HCV RNA-negative individuals had clinical evidence of chronic liver disease [6,7].
Five years after the initial survey, in 2004, 85 of the 102 (83%) individuals with HCV infection were reevaluated. All remained anti-HCV positive. Serum HCV RNA was undetectable in 1/67 viremic patients (1.5%) and in all 18 of the non-viremic group.
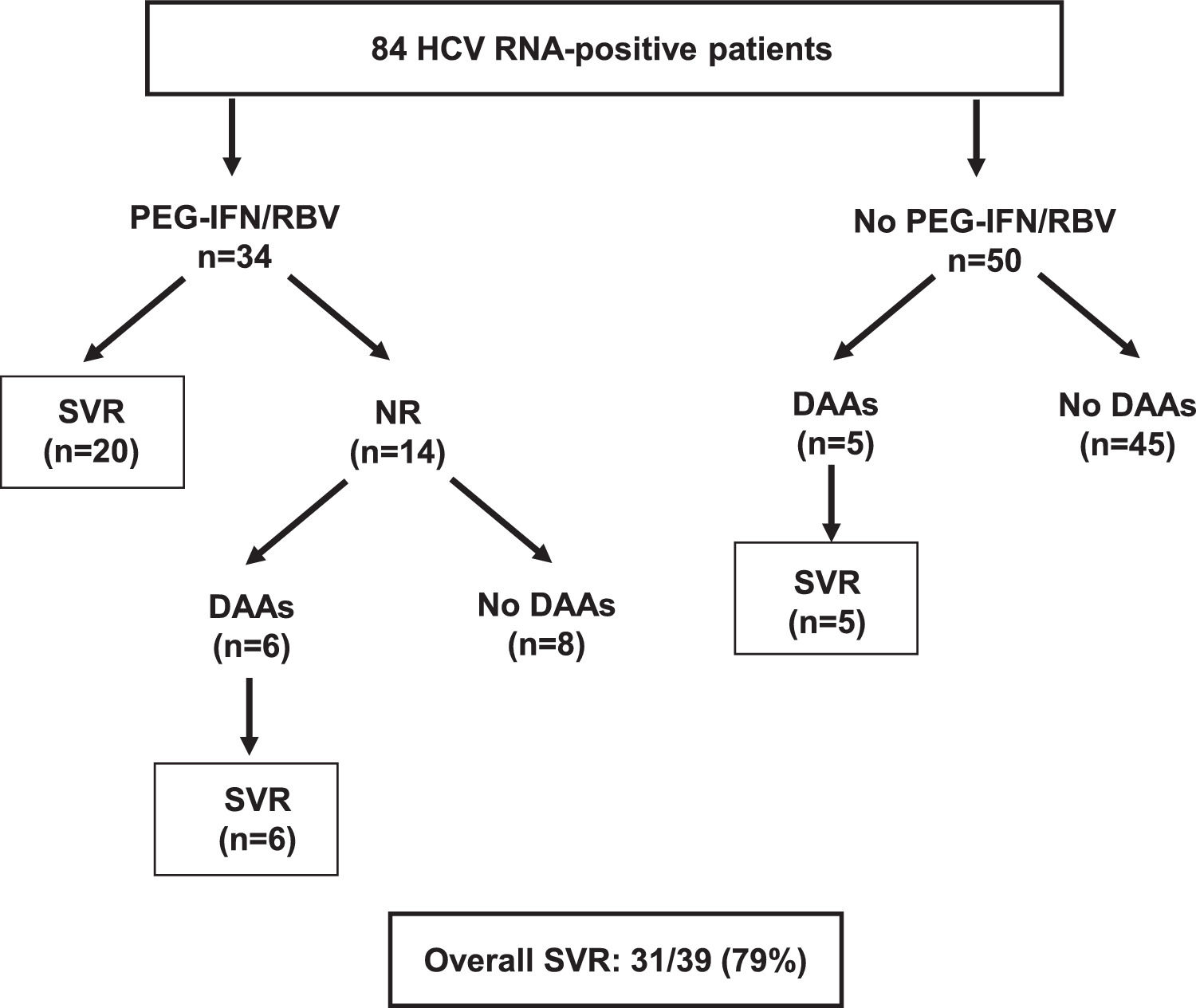
The next step of the study, starting in 2005, was antiviral therapy with the administration of PEG-IFN/RBV for 48 weeks to 34 of the 84 (40%) viremic patients. The remaining 50 viremic patients had contraindications for PEG-IFN therapy, mostly due to advanced age and comorbidities. Mean age of treated patients was 56 ± 10 years and serum HCV RNA 6.4 ± 0.8 log. At baseline, eleven (33%) had advanced fibrosis or cirrhosis (F3 in six patients and F4-6 in five). Rapid virological response, end of treatment response and SVR were 91%, 91% and 59% (20/34) respectively. Fibrosis stage was the only independent (negative) predictor of SVR [8]. All treated patients fulfilled the “80% adherence rule” at all time-points of treatment [9].
Antiviral therapy with DAAs became available in 2017, mostly through the Argentina National Program of Viral Hepatitis. Six non-responders to PEG-IFN and five naive subjects were treated with sofosbuvir-based regimens and all achieved SVR. The overall SVR rate in O'Brien was 79% (31/39) (Fig. 3).
Of note, in 2005 median age of the 50 subjects that did not receive PEG-IFN therapy was 67 (45-91) years. From 2005 to 2017, when DAAs became available, 37/50 (74%) patients of this group died.
At the time of last follow-up, in 2019, 52 of the 102 (50.9%) HCV+ individuals have died, 7/18 (38.8%) of the HCV RNA-negative group and 45/84 (53.6%) of the viremic cohort. Among HCV RNA–positive patients (n=84), mortality was significantly higher in those who were not treated (38/45, 84.4%) compared to the treatment group (7/39, 17.9%) (p<0.0001) and in cirrhotics (23/29, 79.3%) compared to non-cirrhotics (24/55 (43.6%) (p=0.002). Liver-related deaths were significantly higher in untreated (14/38, 36.8%) compared to treated patients (1/39, 2.6%) (p=0.0013). During long-term follow-up, only 1/31 patients (3.2%) with SVR died due to cardiovascular disease and none developed decompensated cirrhosis or HCC. Causes of death among HCV-positive patients other than cirrhosis or HCC were not available
Finally, after 20 years of follow-up, we repeated the population-based survey to assess the impact of medical interventions on the prevalence of HCV infection in O'Brien. At the same time, we restudied 39 of 50 HCV-positive patients of the original cohort who were alive in 2019. All remained HCV-seropositive. HCV RNA was undetectable in 8 non-viremic/untreated subjects and in 29 patients who achieved SVR and was detectable in only two who refused antiviral therapy.
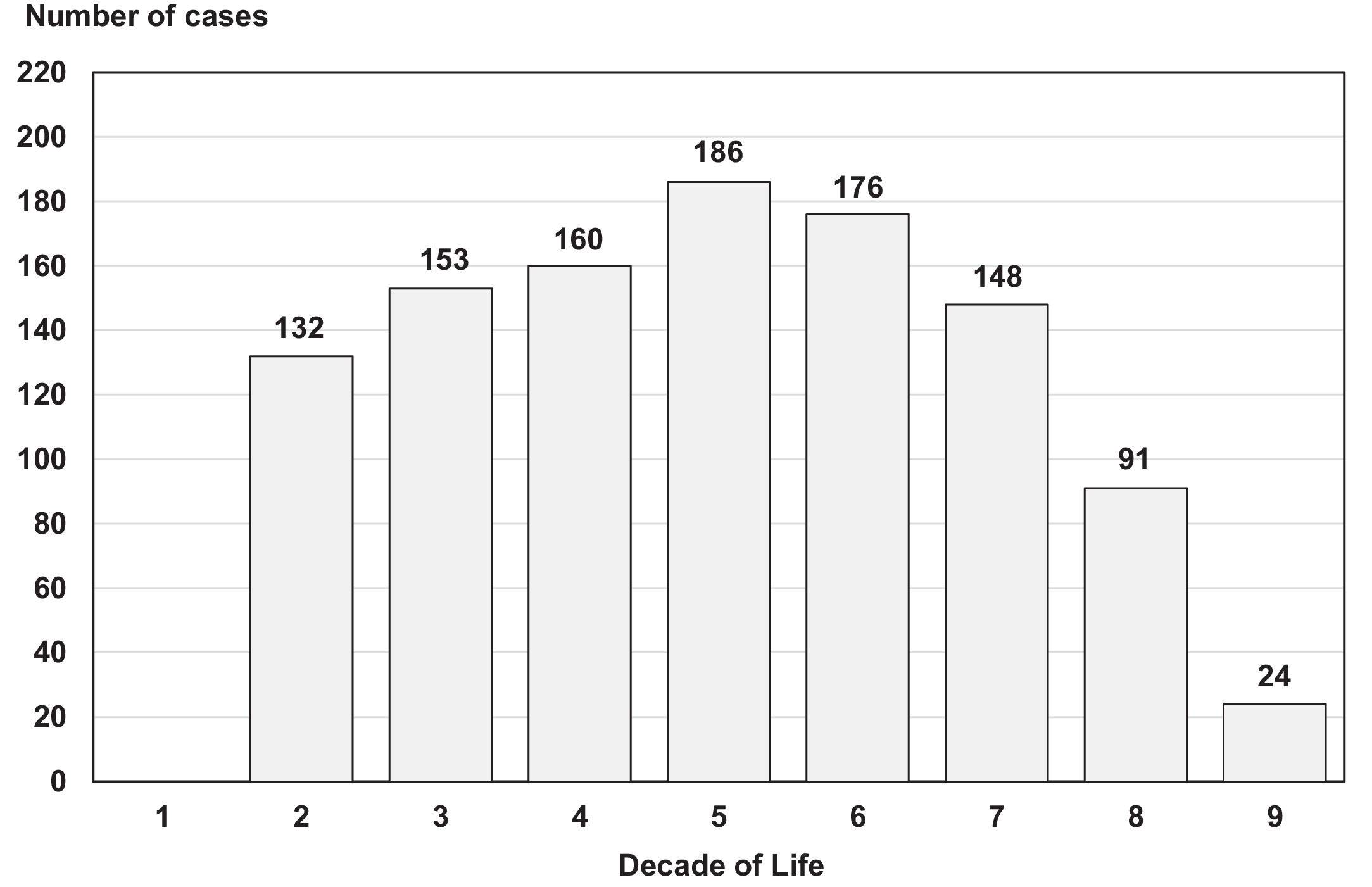
The population study of 2019 included 1070 volunteers aged ≥12 years of which 629 (59%) were screened for the first time and 441 (41%) who were seronegative in 1999. There were 601 females (56%) and 469 males with a median age of 44 (11-89) years. The age distribution by decade is shown on Fig. 4. Only three of the 1070 participants (0.28%) tested positive for HCV antibodies, 2/629 (0.32%) studied for the first time and 1/441 (0.22%) who seroconverted after the 1999 survey. This patient was the son of an 85-year/old female with active HCV infection diagnosed in 1999 and who never received antiviral therapy. The two other seropositive individuals, aged 68 and 78 years, most likely belonged to the historical cohort of infected subjects but remained untested until 2019. All three newly diagnosed patients had detectable serum HCV RNA and genotype 1b.
4DiscussionThe O'Brien Project represents well important aspects of HCV infection such as transmission mode, natural history and the possibility of disease eradication. Wasley and Alter described three different epidemiological patterns of HCV infection [1] The one described in Italy [10,11] and Japan [12,13] showed low prevalence of infection in children and young adults followed thereafter by a progressive increase with age. In this pattern, transmission occurred during the previous 30-50 years and resulted from the indiscriminate use of injections or other medical/cultural practices with non-disposable materials and poor sterilization techniques. The O´Brien epidemiology followed exactly this pattern with an estimated interval between exposure and diagnosis of around 35 years, history of unsafe injections practices in 95% of cases, low prevalence in people aged <40 years (0.56%) and peak infection rate of 23.4% at age 60-70 years. The unique feature of the O'Brien study is that 100% of patients had genotype 1b infection with a high degree of sequence homology, something not previously reported in other HCV population-based surveys. This virological finding, in addition to epidemiological data, strongly suggest the existence of a common source of infection.
When analyzing controversies about the natural history of HCV infection, Alter et al [14] and Seeff [15,16] suggested that the ideal study should provide information on the date of infection, clinical status of all infected individuals, existence of co-factors that may modify disease severity, long-term follow-up and analysis of outcome in the absence of therapy. The O'Brien Project fulfills most of these requirements. Based on the questionnaire and clinical histories, the estimated duration of HCV infection before diagnosis was 3-4 decades. Epidemiologically, the study was very representative because it included 80% of the entire population of O'Brien with the vast majority of participants being asymptomatic and unaware of having HCV infection. All seropositive individuals underwent clinical, biochemical, radiological or histological studies to assess the presence and severity of hepatitis C. The study cohort covered the whole spectrum of HCV-related disease, from resolved infections to advanced cirrhosis or HCC. Regarding cofactors, alcohol consumption increased the severity of hepatitis C, as has been previously reported [17–19]. The prevalence of cirrhosis increased by 11% in females and by 15.5% in males when HCV infection was associated with a history of alcohol intake. Despite the high rate of anti-HBc positivity, there were no HCV-HBV or HCV-HIV co-infections. During the 30-40 years of HCV infection preceding the 1999 study, and up to 2005, no patient from O'Brien received antiviral therapy. Lastly, we were able to evaluate HCV-positive patients up to 20 years after the initial diagnosis, an important issue when assessing the natural history of the disease.
The O´Brien Project should be included in the retrospective-prospective category of studies assessing the natural history of HCV infection, where a large number of individuals acquired the infection from a common source [15,16].
The best examples reported of retrospective-prospective studies are past exposure to anti-Rho contaminated immunoglobulins [20,21]. or to blood transfusions [22–24] After two decades of follow-up, the risk of developing cirrhosis was negligible in recipients of immunoglobulins and 8%-24% in those with post-transfusion hepatitis. The estimated duration of HCV infection in O´Brien was much longer, something that could explain the 28% rate of cirrhosis, although in patients with hepatitis C and no alcohol consumption it was around 23%.
Despite being a difficult-to-treat cohort with high viral loads and significant fibrosis at baseline, the 59% SVR obtained with PEG-IFN α-2A in combination with RBV was higher than the 42%-52% reported for genotype 1 in phase III clinical trials [25–27]. This suggests that the HCV strain circulating in O'Brien may share common determinants of PEG-RBV susceptibility. Studies reported in 2009 showed that genetic polymorphism near the IL28B gene is an excellent determinant of response to treatment with PEG-RBV [28,29] and also enhances spontaneous resolution of HCV infection [30]. Unfortunately, this test was not available at the time of the initial survey in O'Brien and in 2005 when interferon therapy was started. In addition, serum samples were not stored for retrospective analysis. All 11 patients who received DAAs, 6 non-responders to PEG-RBV and 5 naïve, achieved SVR. Overall, treatment with PEG-RBV followed by DAAs was significantly associated with improved outcome and reduced mortality. No patient with SVR developed decompensated cirrhosis or HCC, complications that occurred in around one third of the untreated group.
Previously reported community-based epidemiological studies concentrated on describing the prevalence of HCV, risk factors and viral characteristics but did not include strategies to link diagnosis to clinical care, antiviral therapy and long-term follow-up [10-13,31-34]. The O´Brien Project is unique in this respect. The Global Viral Hepatitis Strategy of the World Health organization aim to eliminate viral hepatitis by 2030, reducing new infections by 90% and mortality by 65% [35]. This proposal requires identification of the main mode of transmission and risk factors, study of vulnerable at risk and affected populations, assessment of the heath burden in terms of cirrhosis and the coverage of hepatitis services. Most of these goals were achieved in the O'Brien Project. The elevated age of the untreated cohort in 2005 and the 12-year interval between the availability of PEG-IFN and DAAs resulted in significant mortality precluding timely access to antiviral therapy. The repeated population-based study in 2019 showed a 20-fold decrease in HCV prevalence, from 5.6% to 0.28% and only one new HCV infection during a 20-year follow-up. Therefore, the O'Brien Project is an example of the effectiveness of micro-elimination strategies allowing eradication of HCV infection from a small geographic area of Argentina [36–38]