Special issue on hepatocellular carcinoma (HCC) and hepatitis B and C as its main causes worldwide
Más datosIntroduction and Objectives: Espírito Santo state is considered a region with a higher frequency of hepatitis B virus infection. This study characterized demographic, epidemiological, laboratory, virological and clinical aspects of 587 chronic HBV carriers followed up at the University of Espírito Santo Hospital. Materials and Methods: Demographic, epidemiological, laboratory and clinical data were extracted from medical records during the entire follow-up period. Classification of the evolutionary phases of chronic hepatitis B was defined as immunotolerant; inactive carrier; chronic active hepatitis HBeAg (+) and HBeAg (-). Characterization of HBV genotypes/subgenotypes was performed by sequencing of overlapping surface antigens and HBV DNA polymerase genes. Phylogenetic relationships were determined using BEAST 1.8.3 software. Results: and Conclusions: Genotypes found were A (132/65.3%) [A1 = 129 (63.9%) and A2 = 3 (1.5%)], D (66/32.7%) [D3 = 56 (27.7%), D4 = 8 (4.0%) and D2 = 2 (1.0%)] and F (4/2.0%) - all F2a. Subgenotypes A1 or D3 were not associated with age, sex, HIV/HCV co-infection, viral load, antiviral usage, HBeAg status or clinical stages of chronic hepatitis B. Mother –to-child-transmission (MTCT) was associated with the subgenotype A1 and intrafamilial transmission with subgenotype D3. Subgenotype A1 was more frequent than D3 among individuals born outside ES compared to those born in ES. Conclusions: The most predominant clinical phases were HBeAg (-), inactive carrier and chronic active hepatitis HBeAg (-). Subgenotypes A1 and D3 were most frequent and were associated were MTCT and intrafamilial transmission of HBV, respectively.
Hepatitis B virus (HBV) affects more than 270 million people worldwide, with about 78,000 deaths annually from complications of the disease. In Brazil, 7.4% of the population have been exposed to the virus and about 0.37% have chronic infection. The prognosis of this infection depends on several factors such as geographic location, age of infection, viral genotype and association with some coinfections, such as hepatitis C virus and HIV [1].
Globally, the predominant route of HBV transmission is vertical or mother-to-child transmission (MTCT), especially in regions with high rates of virus carriers such as the Asia-Pacific region. Other routes include blood and sexual transmission, as well as intrafamilial spread of the virus that can occur through prolonged contact between children or household contacts likely through exposure to fluids from infected individuals [2]
Chronic HBV infection has a non-linear progression through 4 stages. The natural history and clinical outcomes vary widely between individuals infected with HBV. Factors related to the virus (viral load, mutations and genotype) as well as to the host (age of adquisition, gender, immunosuppression, alcohol and genomic factors), seem to affect the outcomes. The classification of the clinical phases of chronic hepatitis B is based on clinical parameters, such as signs of advanced liver disease or extrahepatic manifestations, alanine transaminase (ALT) levels, viral markers (HBV DNA, HBeAg) and degree of inflammation and/or fibrosis in the liver. Several viral factors may influence the course of HBV infection including viral genotype, viral load levels over time and specific HBV mutations [3,4].
Espírito Santo State (ES) is located in the Southeast region of Brazil, with a current population of 4,108,508 million people (https://cidades.ibge.gov.br/brasil/es/panorama, accessed 08/09/2021), initially colonized by black people from Africa in the 16th century and later by European immigrants, mainly Italians in the 19th/20th century (Fig. 1).
Espírito Santo (ES) State divided in its 4 geographical regions. In the World Map, it is also showed the localization of this Brazilian state in Brazil. The distribution by regional health of the Health Department of ES shows the four macro-regions: north, central, metropolitan and south.
The aim of this study is to characterize the demographic, epidemiological, laboratory, virological (HBe/anti-HBe status, viral load, genotypes, subgenotypes) and clinical aspects of chronic HBV carriers followed up at the Hepatitis B Outpatient Clinic of the Infectious Diseases Service of the Cassiano Antônio de Moraes University Hospital, Federal University of Espirito Santo (HUCAM/UFES).
2Material and methodsThis cross-sectional cohort included 587 individuals, adults, of non-Asian origin, HBsAg+ > 6 months, followed at the viral hepatitis outpatient clinic of the Infectious Diseases Service of HUCAM/UFES between July 2005 and July 2017. Inclusion criteria were presence of HBsAg in serum for more than six months; availability of results from HBV serological markers HBeAg and anti-HBe in the medical records; at least one quantitative HBV DNA determination; age ≥ to 18 years old. Exclusion criteria were incomplete demographic epidemiological data in the medical record and follow-up for less than 6 months.
Demographic, epidemiological (age, sex and city of birth), epidemiological, laboratory and clinical data were extracted from medical records during the entire follow-up period. For the distribution of individuals by place of birth in Espírito Santo (ES), the four macro-regions by which the Health Department of ES surveys regional health were used: north, central, metropolitan and south [5] (Fig. 1).
The following parameters were evaluated: demographics (gender, age, region of birth, probable mode of transmission); serological (Anti-HBc, HBeAg, Anti-HBe, Anti-HCV, Anti-HIV); biochemical (two alanine transaminases - ALT - measurements with a minimum interval of 4 weeks), virologic (HCV RNA, HIV RNA, HBV DNA, HBV genotype and subgenotype) in the study subjects.
Hepatitis B transmission route was categorized as:
Mother-to-childtransmission (MTCT) or vertical, defined when the mother was HBsAg positive;
intrafamilial, when the father was HBsAg positive or had chronic hepatitis, with a susceptible seronegative mother, or serological evidence of a chronic hepatitis B patient among relatives living in the household of the index case;
parenteral, from the use of injectable or inhaled drugs or by blood transfusion;
sexual, in individuals with unsafe sex, multiple partners (more than two sexual partners in the 6 months prior to diagnosis), absence of family history and negative HBV infection markers in parents,
unknown, when the individual did not fill any criteria above or when there was no possibility of family investigation.
When more than one criterion was found, the one with the greatest potential for infectivity was considered.
The classification of the evolutionary phases of chronic hepatitis B was defined in 4 phases: immunotolerant; inactive carrier; chronic active hepatitis HBeAg (+); and chronic active hepatitis HBeAg (-) [4].
The characterization of HBV genotypes/subgenotypes was performed by amplification and sequencing of overlapping surface antigens and HBV DNA polymerase genes (S/POL) as previously described [6]. The generated sequences were aligned using the Clustal W program integrated with the BioEdit program [7], together with the sequences of the different HBV genotypes and subgenotypes obtained from the GenBank. To identify the HBV genotypes and subgenotypes of each case and to study the phylogenetic relationships between the characterized sequences, the alignment was submitted to phylogenetic analysis using the BEAST 1.8.3 program [8] with 10,000,000 steps of the Markov Monte Carlo chain (MCMC) and sampling every 1,000 steps, and rejecting the first 1,000,000 steps as burn-in.
3Statistical analysisDescriptive analysis of demographic, epidemiological, laboratory and clinical aspects of the entire study population was performed for: mean and median age, female to male sex ratio, probable route of transmission, region of birth, HBeAg +/- status, presence of co-infections with HIV and/or HCV, viral load level expressed in IU/mL and logarithm in base 10, clinical stage of the disease and antiviral use. The mean viral load was also described in patients naïve to treatment and using antiviral drugs at the time of classification of the clinical phase of chronic hepatitis B.
Patients were divided into subgroups according to the evolutionary phase of chronic HBV infection and demographic, epidemiological and clinical data between subgroups were compared.
The frequency of the different HBV genotypes and subgenotypes was analyzed in the whole sample and then evaluated according to the place of birth of the individuals, separated between individuals: born in ES or in other regions of Brazil. The significance level used in all statistical tests was p < 0.05.
The results were analyzed using the statistical programs GraphPadPrism (Software Inc. San Diego, CA, version 5.01), Minitab (Minitab Inc., version 17.3.1) and the SPSS program (IBM SPSS ® Statistics, version 23.0). When indicated, the following analyzes of the results obtained were carried out: mean, median, standard deviation; Fisher's exact test, Chi-square test, Mann Whitney non-parametric test and Kruskal-Wallis non-parametric test [9,10].
4Results4.1Study populationBetween July 2005 and July 20175, 617 individuals with chronic hepatitis caused by HBV were enrolled in the Infectious Diseases Service of HUCAM/UFES. However, 30 were excluded, 20 due to loss of follow-up, 9 for being in follow-up for less than 6 months, and 1 HBsAg negative patient with occult hepatitis B. The follow-up time of most individuals included in the study (81.4%) was greater than one year. Demographic, epidemiological, laboratory and clinical and demographic characteristics are summarized in Table 1.
Demographic, epidemiological, laboratory and clinical characteristics of the 587 individuals with chronic HBV under follow-up at HUCAM/UFES from January 2005 to July 2017
Of the 587 study participants, 279 had a sample collected available and with detectable HBV DNA, which were submitted to the amplification and sequencing procedures of the S/Polymerase region of the HBV genome. In 202 of these samples, it was possible to amplify and sequence the HBV genome to characterize the genotypes and subgenotypes.
The most frequent genotype was A (132/65.3%) [A1 = 129 (63.9%) and A2 = 3 (1.5%)], followed by genotypes D (66/32.7%) [D3 = 56 (27.7%), D4 = 8 (4.0%) and D2 = 2 (1.0%)] and F (4/2.0%) - all subgenotype F2a.
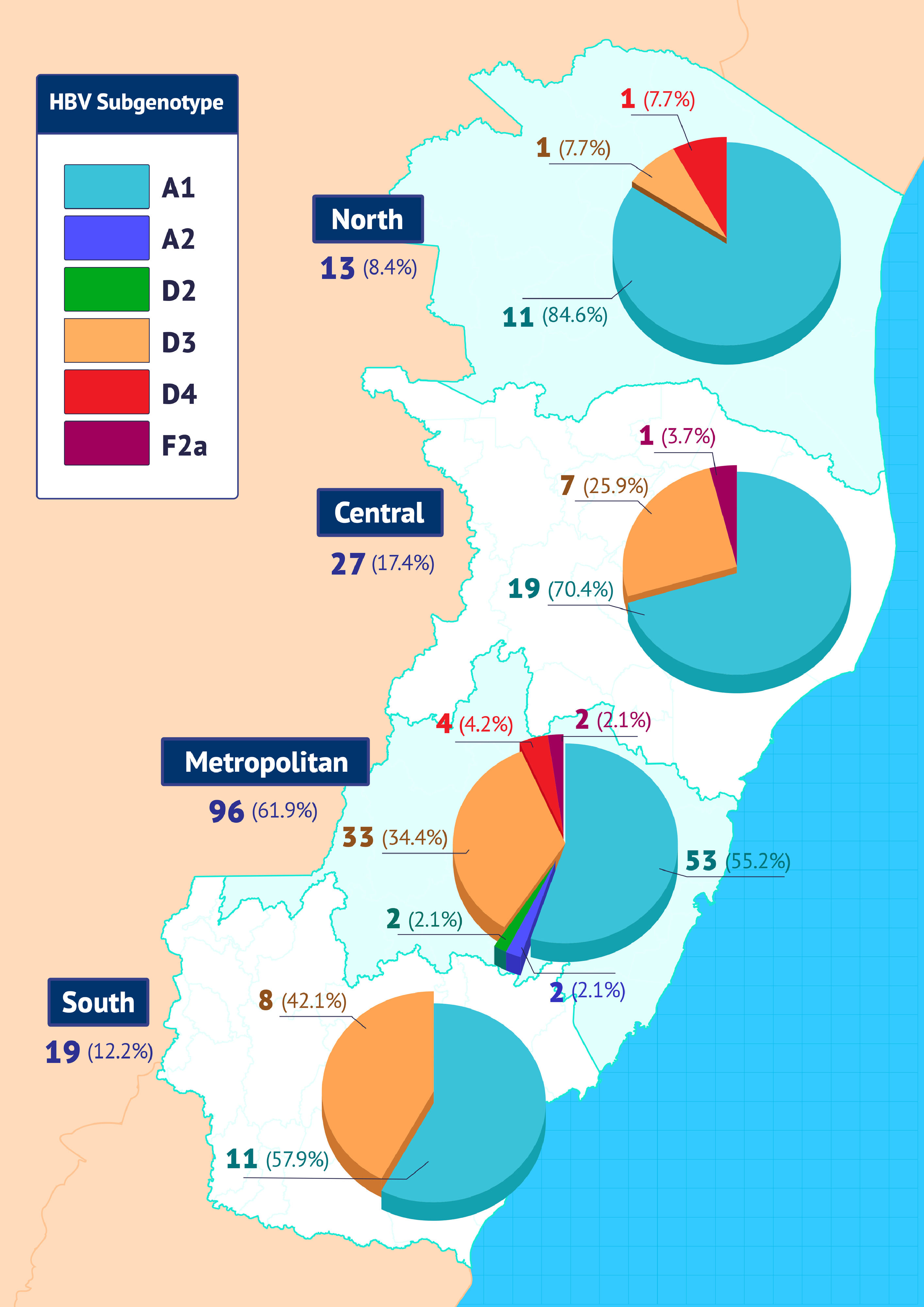
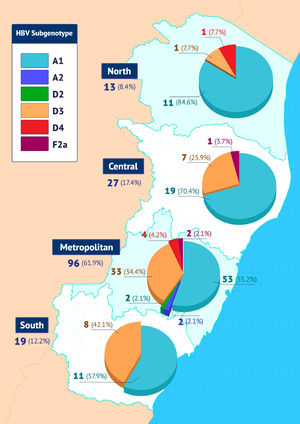
The distribution of subgenotypes in the four ES macro-regions is shown in Fig. 2.
Subgenotype A1 was more frequent in all regions, with higher prevalence in the northern region of the state. Conversely, the second most frequent subgenotype, D3, was more prevalent in the southern region of ES.
Considering only individuals from ES, 61.9% (96/155) of them were from the metropolitan macro-region, 8.4% (13/155) were from the northern macro-region, 17.4% (27/155) from the central macro-region and 12.2 % (19/155) from the southern macro-region of the state. The frequencies of HBV subgenotypes in each of these macroregions were similar to those of the general study population, with a predominance of A1 and D3, but with some particularities. Progressive increase in the frequencies of the subgenotype D3 in the north-south direction of the state (7.7%; 25.9%; 34.4%; 42.1%), and the opposite in relation to the subgenotype A1 in the south-north direction of the state (57.9%; 55 .2%; 70.4% 84.6%). As expected, the greatest diversity of subgenotypes (A1, A2, D2, D3, D4 and F2a) was observed in individuals born in the metropolitan macro-region.
An association analysis was performed only between the most frequent subgenotypes, A1 and D3, with demographic, epidemiological, laboratory, clinical, epidemiological, and virological characteristics is showed in Table 2. An association was observed between the probable HBV transmission routes and the viral subgenotype: MTCT was associated with the subgenotype A1 and intrafamilial transmission with subgenotype D3 (p=0.001). There was also a significant association between subgenotype and birthplace: subgenotype A1 was more frequent than subgenotype D3 among individuals born outside ES compared to individuals born in ES (p=0.029).
Demographic, epidemiological, laboratory and clinical characteristics of the 185 patients with chronic HBV carriers of subgenotypes A1 or D3under follow-up at HUCAM/UFES from January 2005 to July 2017.
On the other hand, we did not observe an association between infections by subgenotypes A1 or D3 with age, sex, co-infection with HIV or HCV, viral load, antiviral usage, HBeAg status or clinical stages of chronic hepatitis B.
5DiscussionHBV infection is a major public health problem in Brazil. In some areas or countries, MTCT and intrafamilial transmission are the main mode of spread of HBV. Even after more than two decades of HBV vaccine implementation, epidemiological studies such as ours, carried out in some regions of Brazil, still show a strong familial component in HBV transmission [11–14].
Chronic hepatitis B is slowly progressive and large clinical cohorts characterizing these populations are scarce, but in general, HBeAg negative clinical forms predominate in endemic areas both in East Asia (81%) [15] and in the West (62.1%) [16] where intrafamilial transmission predominates. In fact, our study detected an even greater predominance (94%) of the HBeAg negative forms of chronic hepatitis B. In addition, our study identified the MTCT and intrafamilial routes as a likely route of HBV transmission in 56.2% of cases. In Brazil, previous cohorts showed a lower predominance of negative HBeAg forms: 70.2% [17] and 74.1% [18].The proportion of active forms of the disease in our series differed from studies in areas of medium/high prevalence of HBV worldwide. While our study showed an eleven times higher proportion (37.6% versus 3.4%) of active forms HBeAg(-) compared to HBeAg(+), another Chinese study with 8,439 individuals showed that, despite the predominance of active forms, the relationship between HBeAg(-) and HBeAg(+) cases presented a proportion three times lower than in our study. In our study, the MTCT and intrafamilial route was similar, but as genotype C was more frequent in this Chinese study, the HBeAg+/- seroconversion rate was characteristically lower [15].
The characterization of HBV genotypes contributes to a better understanding of the natural history of hepatitis B, since the genetic heterogeneity of the virus seems to imply biological properties that influence clinical outcomes. Our study did not find an association between the two most frequent subgenotypes (A1 and D3) with the different clinical stages of chronic hepatitis B, epidemiological (age, sex) or virological variables (HBeAg +/-, HBV DNA, status) of HBV.
This study showed that genotype A was the most prevalent in ES, followed by D and F. The predominance of HBV/A and HBV/D may be the result of the African population brought in during the colonial period of slavery (16th century) and the European immigrant population, mainly Italians (in the 19th century and early 20th century). The small frequency of the genotype F (1.3%) in our study may be related to the intense internal migration of native Indians to Bahia and Pernambuco in the second half of the 16th century [19].
MTCT is frequent when mothers are HBeAg (+), whereas it occurs less frequently when mothers are HBeAg (‐): 85 to 90% vs. 32%, respectively. It occurs particularly in resource-limited countries with high endemicity, but it is lower in sub-Saharan than in Asian countries, associated to the lower frequency of HBeAg (+) mothers. HBV genotypes are involved in the different transmission rates: B and C are more frequent in Asia while A, D, and E are more frequent in sub-Saharan Africa. The frequency of virus expressing HBeAg might vary according its genotype or subgenotype, and it may explain the differences found in the MTCT rates. Differences can also be observed inside a single place and may depend on ethnicity, socio- economic reasons or HBV genotypes [20,21].
Subgenotypes A1 and D3 are not usually associated with an increase in MTCT as they generally evolve rapidly to HBeAg (-). Indeed, in our population, no difference in HBeAg frequencies were observed comparing subgenotypes A1 and D3, the two most common in ES. Our study showed an association between MTCT with subgenotype A1 and intrafamilial transmission with subgenotype D3. Strong evidence of intrafamilial HBV transmission has been described predominantly in Asia where genotypes B and C predominate [22–24]. Regarding genotype A, a study evaluating HBV/A transmission in a rural African community demonstrated that in addition to perinatal transmission, horizontal transmission during childhood was a common mechanism of HBV transmission. One study carried out in a rural community in the Republic of Cameroon showed evidence of family transmission with a predominance of HBV/A in 7 of the 11 families with more than one HBsAg+ individual identified [25].
In fact, persistent HBV infection was the risk factor most frequently associated with HCC with and without cirrhosis associated in ES, demonstrating the epidemiological importance of HBV in the context of advanced liver disease [26]. Likewise, a study in India reported that genotype D is associated with more severe liver disease and HCC than individuals with HBV/A [27].
We have not addressed genetic factors or social economical level from our population to evaluate other factors that might be involved in the differences found by our group, e.g., other genetic factors such as HLA polymorphisms might influence the MTCT that have not been addressed in this study [28].
Subgenotype A1 is particularly found in Africa from where most of the Brazilian population have been originated while subgenotype D3 is frequent in Italy, and Brazil has a very important Italian migration history, including ES. The distribution of subgenotypes among the four macroregions of ES showed increasing rates of the subgenotype A1 in the South-North direction of the state, closer to Bahia, where African descendants are more frequent. Conversely, subgenotype D3 showed increasing rates in the North-South direction of the state. In fact, Italian and German European immigrants settled in the south-central region of the state during the coffee cycle and others migrated to and from the southern region of the country where this subgenotype predominates [6,13,14,29–31].
We also found a significant association between subgenotype and birthplace: A1 was more frequent than subgenotype D3 among individuals born outside ES compared to individuals born in ES. This is related to the large population movement found in this Brazilian state, located between Rio de Janeiro, Minas Gerais and Bahia, all Brazilian states where subgenotype A1 is more frequent, while D3 is more frequent also in other Brazilian regions with a relevant Italian immigration. The role of family transmission of HBV/D was also identified in southern (Rio Grande do Sul and Paraná States) and southeastern (Ribeirão Preto, São Paulo State) Brazil [6,13,14,29–31]. In Espírito Santo, HBV MTCT was associated with the HBV/A1 subgenotype and the intrafamilial transmission with HBV/D3. It is important to emphasize that these genotypes were introduced several centuries ago in a highly mixed population, which contributed to the non-formation of specific clusters both in samples from different regions of ES and in different regions of Brazil.
In conclusion, in this study, the clinical phases HBeAg negative, inactive carrier and HBeAg negative chronic active hepatitis B were predominant. The most frequent subgenotypes are A1 and D3 and are associated with MTCT and intrafamilial transmission of HBV.