The emergence of SARS-CoV-2, which causes the coronavirus disease (COVID-19) has caused a great impact on healthcare systems worldwide, including hepatitis B and C viruses screening and elimination programs. The high number of COVID-19 hospitalizations represent a great opportunity to screen patients for hepatitis B virus (HBV) and hepatitis C virus (HCV), which was the aim of this study.
Material and MethodsCross-sectional, retrospective study performed between April 2020 and 20201 at a referral center in Mexico dedicated to the care of adults with severe/critical COVID-19. We retrieved clinical, demographic, and laboratory results from each patient´s medical records, including antibodies against HCV (anti-HCV), HBV surface antigen (HBsAg), antibodies against the HBV core antigen (anti-HBcAg), and antibodies against HBsAg (anti-HBsAg).
ResultsOut of 3620 patients that were admitted to the hospital, 24 (0.66%), 4 (0.11%), and 72 (1.99%) tested positive for anti-HCV, HBsAg, and anti-HBcAg, respectively. Of all seronegative patients, 954 (27%) had undetectable anti-HBsAg and 401 (12%) had anti-HBsAg at protective levels. Blood transfusion was the most relevant risk factor. Only 9.7% of the anti-HBc positive, 25% of the HBsAg positive, and 52% of the anti-HCV positive were aware of their serological status.
ConclusionsIn this study we found a prevalence of anti-HCV of 0.66%, HBsAg in 0.11%, and isolated anti-HBcAg in 1.99%. We also found that HBV vaccination coverage has been suboptimal and needs to be reinforced. This study gave us a trustworthy insight of the actual seroprevalence in Mexico, which can help provide feedback to the Hepatitis National Elimination Plan.
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are still a very important public health threat, due to their high prevalence and morbimortality.
The epidemiology of the HBV varies according to the region. The worldwide prevalence of the hepatitis B surface antigen (HBsAg) is 3.61%, with high-endemic zones such as Africa and the Western Pacific harboring prevalence as high as 8.83% and 5.26%, respectively. In 2010 there were 248 million HBsAg-positive individuals [1]. Mexico is considered a low-stable endemic zone [2], with a HBsAg prevalence of 0.15-0.23 % [1,3]. The 2020 Mexican Annual Report of Epidemiological Surveillance reported a declining incidence rate of 0.28% [4]. The prevalence of positive anticore antibodies (Anti-HBc) in Mexico has been reported to be 3.3% [5], equivalent roughly to 3 million people [6].
On the other hand, there are more than 70 million people worldwide infected by the HCV, the prevalence depends on the region [7], being highest in zones such as Central Asia and Central Africa (e.g. 6.0%) [7]. Regarding Mexico, there is heterogeneity on the results of HCV prevalence, which may reflect the differential distribution of risk factors, but the most commonly reported is 1.4% [8]. In terms of incidence there are 2108 new cases every year [4].
The outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS CoV-2), which causes the Coronavirus disease (COVID-19), represents an opportunity for HBV/HCV screening due to the high number of COVID-19 inpatients, which can help mitigate the obstacles put by the lockdown. In Spain, this strategy has been done to evaluate the results of an HBV/HVC screening program in COVID-19 inpatients. A study of 4662 patients reported a seroprevalence of antibodies against the HBV core antigen (anti-HBcAg) of 8.75%, HBV surface antigen (HBsAg) of 0.38%, and antibodies against the HCV (anti-HCV) in 0.83% [9]. Therefore, the aim of this study was to estimate the seroprevalence of HBV, HCV, and of protective levels of antibodies against HBsAg (anti-HBsAg) in patients were moderate or severe COVID-19 admitted to our institution.
Material and MethodsStudy designThis was a cross-sectional retrospective study performed at a referral center in Mexico City dedicated to the care of adults with severe and critical COVID-19. We included all consecutive subjects with SARS-CoV-2 infection confirmed by real-time reverse transcription-polymerase chain reaction (RT-PCR) that were admitted between April 2020 and April 2021. The study protocol was reviewed and approved by the Institutional Review Board (Ref. 3779) with a waiver for informed consent because of its retrospective nature.
Data collection and hepatitis serologyWe extracted clinical, demographic, and laboratory results from the electronic medical record of each patient, we intentionally retrieved information regarding risk factors for hepatitis infection. Routine laboratory work-up for all patients with COVID-19 on admission at our institution includes anti-HCV, HBsAg, anti-HBsAg, anti-HBcAg, and antibodies against HIV. Protective levels of anti-HBsAg were defined as having ≥ 10 UI/L. Because the HBV vaccine was introduced into the national immunization program of the newborn in 1999 in Mexico, we analyzed the frequency of protective anti-HBsAg levels by age group.
Statistical analysisCategorical variables were reported as frequencies and proportions. Continuous variables were described using median and interquartile range (IQR). Comparisons between groups (i.e., patients with negative serologies, anti-HCV positive patients, HBsAg positive patients, and anti-HBcAg positive-HBsAg negative patients) were performed with Kruskal-Wallis and chi-squared tests. Mann-Whitney test and chi-square were used for between-group comparisons, a Bonferroni correction was conducted to adjust the level of significance, considering a p-value ≤ 0.017, based on the number of planned comparisons (i.e., control vs anti-HCV positive; control vs HBsAg positive; control vs anti-HBcAg positive-HBsAg negative).
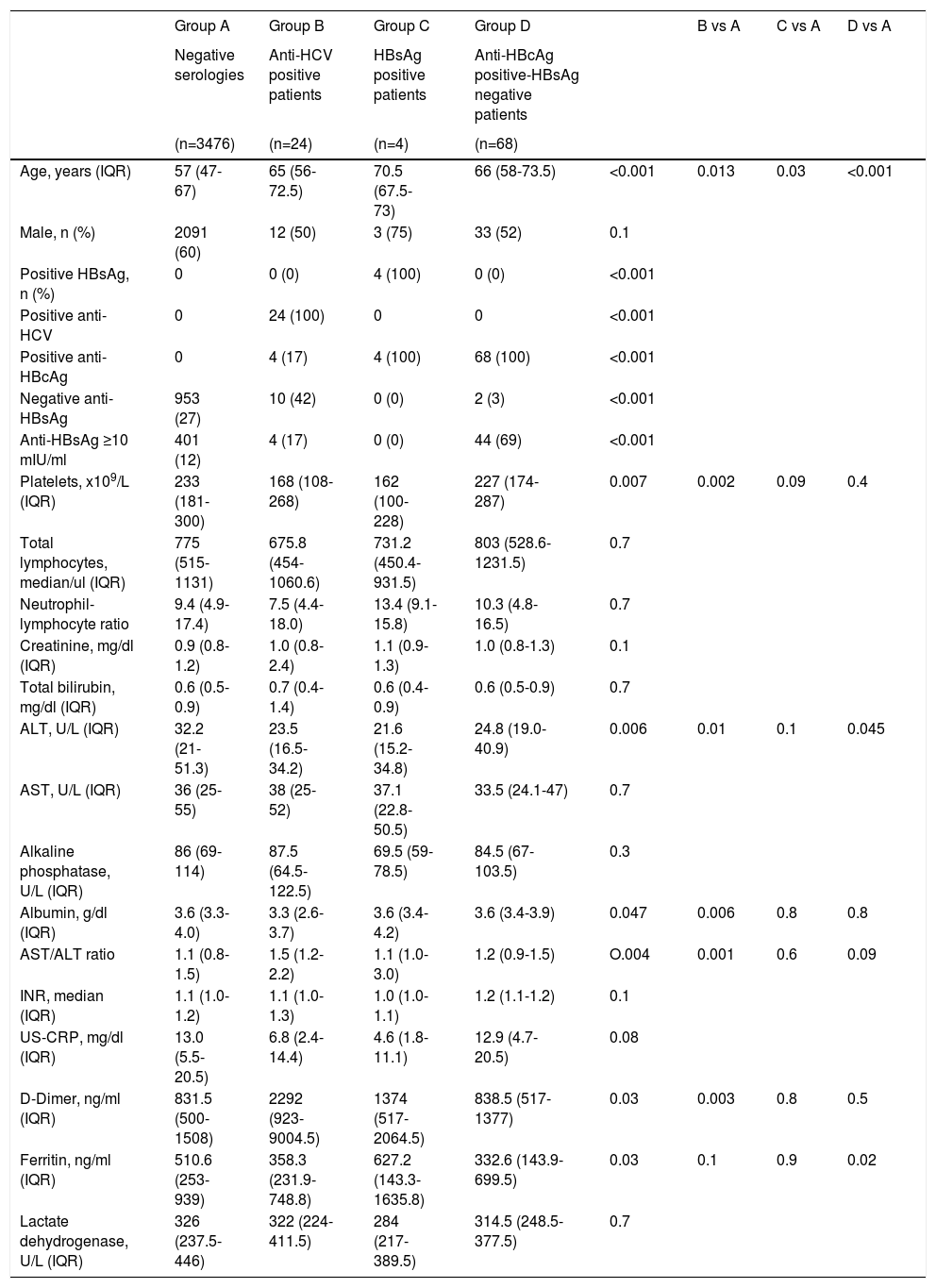
ResultsOut of 3620 patients that were admitted to the hospital due to moderate or severe COVID-19, 24 (0.66%), 4 (0.11%), and 72 (1.99%) tested positive for anti-HCV, HBsAg, and anti-HBcAg, respectively. In addition, there were 32(%), 3 (%), and 19(%) indeterminate results for anti-HCV, HBsAg, and anti-HBcAg, respectively. There were no HBV-HCV co-infected patients. One patient had HCV-HIV coinfection, one had HBV-HIV coinfection, and two anti-HBcAg positive-HBsAg negative patients had documented HIV infection Table 1. shows patients' characteristics by serological status.
Patients´ characteristics by serological status
Kruskal-Wallis and chi-squared tests. Mann-Whitney test and chi-square were used for between-group comparisons, a Bonferroni correction was conducted to adjust the level of significance, considering a p-value ≤ 0.017.
ALT: Alanine aminotransferase; Anti-HBcAg: Antibodies against the hepatitis B virus core antigen; Anti-HBsAg: Antibodies against the hepatitis B virus surface antigen; Anti-HCV: Antibodies against the hepatitis C virus; AST: Aspartate aminotransferase; HBsAg: Hepatitis B virus surface antigen; INR: International normalized ratio; IQR: Interquartile range; US-CRP: Ultra-sensitive C reactive protein.
A total of 24 patients were positive for anti-HCV; 12 (50%) were male. Compared to patients with negative serologies, patients with anti-HCV were older [65 (IQR 56-72.5) vs 57 (IQR 47-67) years, p=0.013], had lower platelet counts [168 (IQR 108-268) vs 233 (IQR 181-300) x109/L, p=0.002], lower albumin levels [3.3 (IQR 2.6-3.7) vs 3.6 (IQR 3.3-4.0) g/dl, p=0.006], and a higher AST/ALT ratio [1.5 (1.2-2.2) vs 1.1 (0.8-5.0), p=001].
Thirteen 13 (52%) patients were aware of their HCV status; two patients were currently undergoing treatment for HCV. The following risk factors for HCV infection were identified: 8 patients (32%) had been transfused, 7 of them before 1995, two patients (8%) had a tattoo, and one patient (4%) was a regular user of cocaine.
HBV Positive PatientsThere were only 4 patients that were positive for the HBsAg, median age was 70.5 years (IQR 67.5-73), and 3 (75%) were male. Only one patient was aware of the diagnosis and was under treatment for HIV-HBV coinfection.
Patients that were anti-core positive-HBsAg negative were older compared to patients with negative serologies [66 (IQR 58-73.5) vs 57 (IQR 47-67) years, p<0.001]; and 33 (52%) were male. Two patients had isolated anti-HBcAg, with negative anti-HBsAg. Of anti-HBcAg positive-HBsAg negative patients, 7 (9.7%) patients were aware of their serological status. Regarding risk factors for HBV infection, 5 patients (6.9%) had been transfused in the past, 2 of them in the year 2018, and the others in 1962, 1980, and 1981, and one patient had a tattoo.
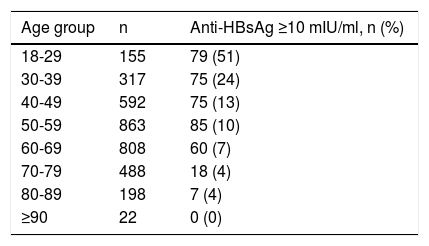
Of patients with negative serologies, 953 (27%) had undetectable anti-HBsAg, and 401 (12%) had anti-HBsAg levels ≥10 mIU/ml Table 2. shows the frequency of protective levels of anti-HBsAg according to the age group in patients with negative serologies. Protective levels of anti-HBsAg were found in 14(40%) of patients that were under 23 years old. In the case of anti-HBcAg positive-HBsAg negative patients, 44 (69%) had anti-HBsAg levels ≥10 mIU/ml.
Proportion of patients with protective levels of anti-HBsAg by age group
| Age group | n | Anti-HBsAg ≥10 mIU/ml, n (%) |
|---|---|---|
| 18-29 | 155 | 79 (51) |
| 30-39 | 317 | 75 (24) |
| 40-49 | 592 | 75 (13) |
| 50-59 | 863 | 85 (10) |
| 60-69 | 808 | 60 (7) |
| 70-79 | 488 | 18 (4) |
| 80-89 | 198 | 7 (4) |
| ≥90 | 22 | 0 (0) |
Anti-HBsAg: Antibodies against the surface antigen of the hepatitis B virus
With the limited availability of specific antiviral therapy against SARS CoV-2, immunosuppressors such as steroids and IL-6 pathway inhibitors have been the pilar drugs against COVID-19, which are considered to be of moderate risk for HBV reactivation [12, 13]. Hence, all inpatients with COVID-19 were tested for HCV and HBV on admission at our institution, which represented a good opportunity for screening. We found a prevalence of anti-HCV, HBsAg, and isolated anti-HBcAg of 0.66%, 0.11%, and 1.99%, respectively. The 0.66% HCV prevalence resembles that reported by Sedeno-Monge V et al. [8] in blood donors and is lower than the 1.4% reported in the general population [8], and most probably represents the fact that high-risk groups such as drug users and prison inmates were underrepresented in our sample. Nonetheless we do think it would be important to screen all patients under the assumption that immunosuppressive treatments in patients with active hepatitis may have adverse consequences.
Our results may help provide feedback to the Hepatitis National Elimination Plan, and we would like to underscore some key findings. First of all, we need to work on simplifying the cascade of diagnosis and treatment of HCV, as we have not been able to contact the majority of patients that were positive for anti-HCV to perform a viral load [10]. Secondly, screening strategies need to be individualized according to local/national epidemiology, with some countries performing universal screening, but the low prevalence of HCV found in our study suggests Mexico should continue with a risk-based screening strategy [11]. Thirdly, the low prevalence of protective levels of anti-HBsAg are indicative of an ineffective HBV vaccination campaign. Only 50% of the youngest patients of our study, who supposedly already received HBV vaccination as newborns, were protected against HBV. This rate is similar to that reported in the 2012 National Health and Nutrition Survey (ENSANUT) [3], which was 40.8% in 20-25-year-olds, and suggests patients are not being fully vaccinated, either because of lack of health promotion or because of shortage of doses. Finally, based on the lower platelet count in patients that were positive for HCV or HBV it is most probable that some of these patients already have compensated advanced chronic liver disease which reflects a late diagnosis considering the natural history of these diseases.
An alarming finding was the fact that only 9.7% of the anti-HBc positive population, 25% of the HBsAg positive patients, and 52% of the anti-HCV positive were aware of their serological status, making it clear that more health promotion campaigns about HBV and HCV infection are needed, with special emphasis in risk factors and screening.
Regarding risk factors, we identified the usual ones, but it is worth noting that essentially there were no drug users in our population, most probably because of barriers in having access to the health system and because it is more in the Northern part of the country where HCV Is transmitted by this means.
Our study does have some limitations, being the most important its retrospective design; prospective studies allow to interrogate for specific risk factors of hepatitis transmission once the physician and/or the patient is aware of a positive result. Also, there were indeterminate serologies and no follow-up was given at that time to those patients to determine if they had an active infection. Likewise, we were not able to determine if positive anti-HCV were viremic. Finally, we did not collect information regarding the outcomes of these patients from the COVID-19 perspective to assess if viral hepatitis can modify their prognosis, as suggested by others [12].
In conclusion, the COVID-19 pandemic has harmed viral hepatitis elimination strategic plans. However, the high number of hospitalized patients supposed an excellent opportunity to screen all inpatients. In this study, we found a prevalence of anti-HCV of 0.66%, of HBsAg of 0.11%, and of isolated anti-HBcAg of 1.99%, which are lower than those previously reported. We also demonstrated that HBV vaccination coverage has been suboptimal and needs to be reinforced. Studies like ours may provide feedback to help tailor the strategies of Mexico´s Hepatitis National Elimination Plan.
AuthorshipJimenez-Mendoza JC: conceptualization, data curation, methodology, formal analysis, writing – original draft, writing – review and editing
Rivera-López FE: data curation, writing – original draft, writing – review and editing, translation.
González-Lara MF: data curation, proofreading, writing – review and editing.
Valdez-Echeverría RD: conceptualization, data curation, methodology, proof reading
Castro-Narro GE: proofreading, writing – review and editing
Torre A: proofreading, writing – review and editing
Uscanga-Domínguez LF: proofreading, writing – review and editing
Moctezuma-Velazquez C: conceptualization, data curation, methodology, formal analysis, writing – original draft, writing – review and editing
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Appendices