Special issue on hepatocellular carcinoma (HCC) and hepatitis B and C as its main causes worldwide
Más datosViral hepatitis is a global health problem with unequal distribution of disease burden in which low-income people are at higher risk for acquisition and underlying liver diseases. This study aimed to seek the prevalence of hepatitis B and C viruses, HIV, and liver damage among low-income patients attending a public tertiary care hospital in West Mexico.
MethodsA retrospective/cross-sectional study at the Department of Genomic Medicine in Hepatology was conducted between March 1, 2016 to March 30, 2017. A total of 10,352 patients tested for anti-HCV, HBsAg, or anti-HIV (n=23,074) were included. Age, gender, and hospital service were registered. Liver fibrosis was assessed using APRI and FIB-4 scores.
ResultsOverall, 3.9% were anti-HCV+ (305/7848), 1.0% were HBsAg+ (80/7894), and 2.9% were anti-HIV+ (210/7332). A 43.8% (750/1959) of patients negative for all viruses had either abnormal AST, ALT, or GGT (≥40 UI/L). Also, significant liver fibrosis (APRI ≥ 0.7) was prevalent in 10.6% (191/1804). In patients who tested positive for viral infections, liver fibrosis was detected in 20.4% (11/54) of HBsAg+, 34.2% (53/155) in anti-HCV+ and 15.5% (16/103) in anti-HIV+. Anti-HCV+ was highest in Geriatrics (11.1%), HBsAg+ in HIV patients (3.0%) and anti-HIV+ in Emergency room attendees (33.3%).
ConclusionHigh seroprevalence of HCV, HBV, and HIV infections was found among the studied population. Significant liver fibrosis was detected in negative and positive patients for viral infections. Medical services need to continuously test for viral infections, promote early detection of chronic liver damage and identify target patients for elimination strategies to decrease disease burden.
Viral hepatitis is a global health problem where the relevant infectious agents are viral types A, B, C, D, and E. These hepatotropic viruses caused 1.45 million deaths in 2013, of which chronic hepatitis B and C virus infections accounted for 91% of the mortalities [1,2]. Currently, 1.4% of the worldwide prevalence of chronic hepatitis B (0.7%) and hepatitis C viruses (0.7%) occurs in the region of Latin America and the Caribbean (LAC) [1,3]. However, such percentages may be discrepant compared to studies reporting increased rates of hepatitis B and hepatitis C infections among high-risk groups [4]. For example, a 7.1% of hepatitis B infection was reported among hemodialysis patients [5] whereas up to 35.89% of injection drug users can test positive for hepatitis C [6]. Also, higher rates of viral infection are related to early initiation of sexual activity, predisposing people to sexually transmitted diseases such as hepatitis B alone or coinfection with human immunodeficiency virus (HIV) [7]. On the other hand, low vaccination coverage, lack of screening, and less harm reduction strategies also contribute to this situation [6,8]. However, low income is a definite social determinant placing individuals at risk for viral infections due to the inaccessibility of timely diagnosis and treatment [9,10].
In 2015, the World Health Organization (WHO) proposed reducing the incidence of viral hepatitis by 90% and mortality by 65% in 2030. However, this global goal will require regional actions to provide updated prevalence/incidence rates [11]. In Mexico, as in other LAC countries, epidemiological data on viral hepatitis are scarce or focused on middle or high-class urban inhabitants (i.e., blood donors) that may underestimate the current situation [12–14]. Furthermore, 43.9% of the Mexicans live in poverty (55.7 million), of which nearly half lack access to public health care services [15]. This social vulnerability places them at risk of acquiring viral hepatitis and being unaware of liver disease [16]. Hence, early diagnosis to avoid the progression of liver damage is warranted [17], and it can be accessed by minimally-invasive methods such as AST to Platelet Ratio Index (APRI) or the Fibrosis-4 (FIB-4) [18,19].
Therefore, to achieve the WHO's viral hepatitis elimination target, evidence at the regional level regarding the rate of viral hepatitis infections and liver disease may prove valuable [14]. This study aimed to seek the prevalence of hepatitis B and C viruses, HIV, and the degree of liver damage among low-income patients attending a public tertiary care hospital in West Mexico.
2Materials and methods2.1Study design, data recollection, clinical and laboratory measurementsThis cross-sectional hospital-based study was conducted from March 1, 2016 to March 30, 2017 at the Department of Genomic Medicine in Hepatology (GMH), Civil Hospital of Guadalajara, “Fray Antonio Alcalde.” The patient's sociodemographic information was collected from the laboratory´s database, such as age, gender, and hospital service. Serological diagnosis of viral infections was based on the detection of hepatitis B surface antigen (HBsAg) for hepatitis B virus (HBV) , antibody to hepatitis C virus (anti-HCV), and HIV-1/2 antibodies (anti-HIV). Laboratory parameter measurements were platelets, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT). The hospital's clinical laboratory conducted all tests using chemiluminescence microparticle immunoassay (CMIA) with the Abbott Architect i2000 Automated Analyzer (Abbott Diagnosis, Chicago, USA). At least 82.6% of the hospital patients earn low salaries, as previously reported [20].
Two non-invasive methods were used to assess liver fibrosis, AST to Platelet Ratio Index (APRI) and Fibrosis-4 (FIB-4). APRI was calculated using AST (IU/L)/AST (Upper Normal Limit) (UI/L)/ PLT (109/L) x 100, using the limit of 40 IU/L for AST, as indicated by some authors [18]. Significant liver fibrosis was considered with an APRI value ≥ 0.7. FIB-4 was calculated using Age x AST level (IU/L)/(Platelet Count (109/L) x √ALT (IU/L)) [19]. A criterion of advanced fibrosis was a score ≥3.25. In this study, values ≥ 40 IU/L of ALT, AST or GGT were considered to indicate liver damage.
2.2Statistical analysisThe complete database was revised to eliminate duplicated entries. Continuous variables were represented as mean and standard deviation, and categorical variables were expressed in percentages. Pearson's Chi-square or t-Student statistical test was performed to determine the differences between groups. The statistical analysis was performed with SPSS version 20.0 (IBM Inc. Armonk, NY) and graphed with Graphpad Prism, Ver 7.0. A p < 0.05 was considered significant.
2.3Ethics statementThe Institutional Review Board approved this study (#CI-07218), and patient's data were anonymized.
3Results3.1Sociodemographic data and viral infection's prevalenceA total of 10,352 patients were evaluated during the study period, of which half were male (51.3%, 5,310/10,352) with a mean age of 38.7 ± 15.40 years, as displayed in Table 1. The prevalence of HBsAg, anti-HCV, and anti-HIV positivity was 1.0% (80/7894), 3.9% (305/7848) and 2.9% (210/7332), respectively. Among the patients who were positive for HIV, 9.04% (n=19/210) presented positivity to anti-HCV (11/210, 5.23%) or HBsAg (8/210, 3.8%). All viral infections were more frequent in men than women at a ratio of 6:1 (HBsAg), 2:1 (anti-HCV), and 4:1 (anti-HIV).
General characteristics, prevalence of viral infections, abnormal liver enzymes and fibrosis scores among the study population (N=10,352).
| Variable | n (%) |
|---|---|
| Gender | |
| Male | 5310 (51.3) |
| Female | 5042 (48.7) |
| Age (Years) Mean (± SD) (Range) | 38.7 ±15.40 (18-99) |
| Anti-HCV | |
| Negative | 7543 (96.1) |
| Positive | 305 (3.9) |
| HBsAg | |
| Negative | 7814 (99.0) |
| Positive | 80 (1.0) |
| Anti-HIV | |
| Negative | 7122 (97.1) |
| Positive | 210 (2.9) |
| Liver enzymes | |
| ALT or AST or GGT < 40 IU/mL | 2668 (56.8) |
| ALT or AST or GGT ≥ 40 IU/mL | 2031 (43.2) |
| APRI* | |
| Without significant liver fibrosis | 3911 (89.4) |
| With significant liver fibrosis | 466 (10.6) |
| FIB-4* | |
| Without advanced fibrosis | 4138 (94.6) |
| With advanced fibrosis | 237 (5.4) |
HCV: hepatitis C virus; HBsAg: Hepatitis B surface antigen; HIV: human immunodeficiency virus; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; GGT: Gamma-glutamyltransferase; APRI: AST to Platelet Ratio Index; FIB-4: Fibrosis-4; APRI* and FIB-4* estimated in patients who had available data.
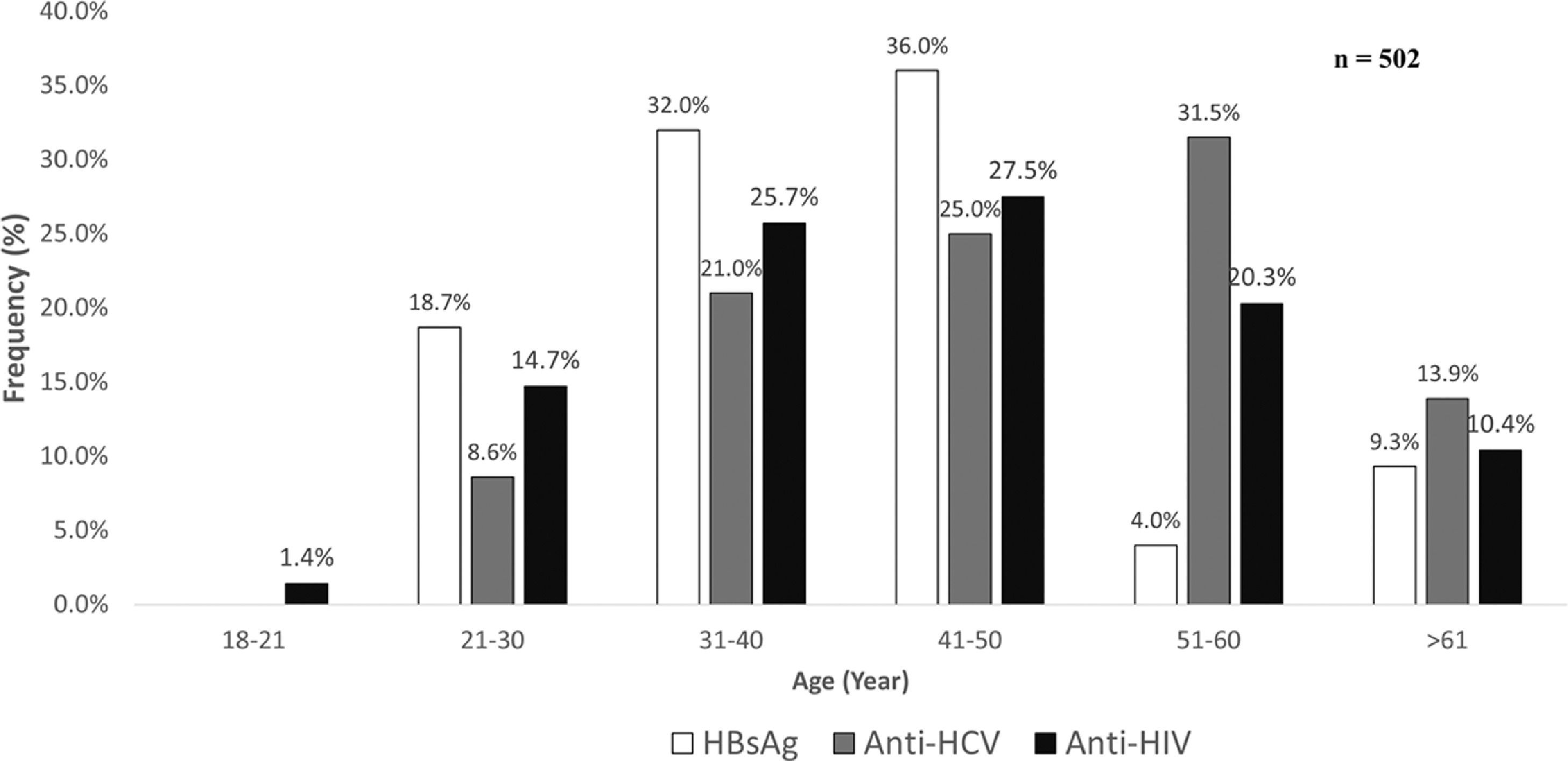
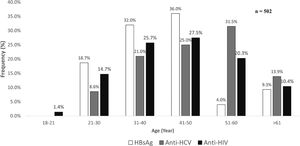
As shown in Fig 1, the prevalence of anti-HCV increased by age, starting at the age group between 21-30 years it reached the highest number of cases between 51-60 years. For HBsAg, a polynomial trend was observed in which the leading age group was 41-50 years, whereas the highest number of cases anti-HIV positive were found between 41-50 years of age.
A total of 53 medical services within the hospital requested 23,074 tests to detect anti-HCV (n=7848), HBsAg (n=7894), or anti-HIV (7332) serological markers during the study period. As shown in Table 2. the highest positivity for anti-HCV was seen in Geriatrics (11.1%), Gastroenterology (11.0%), Thorax & Cardiovascular (10.6%), and Prison Inmates tested at the hospital (10.0%). Although the HIV Unit, Outpatients, and Internal Medicine requested the most significant number of tests, these services reported nearly two-thirds less anti-HCV positive tests.
Medical service rank by the number of tests requested and frequency of positive cases for viral infections.
| ANTI-HCV | HBsAg | ANTI-HIV | ||||||
|---|---|---|---|---|---|---|---|---|
| SERVICE | RANK/ #TESTS | % CASES | SERVICE | RANK # TESTS | % CASES | SERVICE | RANK # TESTS | % CASES |
| GMH | 11 | 21.6 | GMH | 11 | 3.73 | HIVU | 10 | 56.32 |
| GERIA | 26 | 11.1 | PROCT | 30 | 3.00 | EMERG | 46 | 33.33 |
| GASTRO | 5 | 11.0 | HIVU | 1 | 3.00 | PROCT | 29 | 14.63 |
| T&C | 21 | 10.6 | RHEUMA | 16 | 2.08 | PRISON | 39 | 12.50 |
| PRISON | 34 | 10.0 | URO | 23 | 1.69 | PED | 33 | 11.54 |
| OTORHIN | 20 | 5.8 | GAST | 5 | 1.44 | NICU | 37 | 10.00 |
| LMS | 18 | 5.6 | NEUR | 15 | 0.98 | GMH | 12 | 9.15 |
| PROC | 30 | 5.4 | OUTPAT | 3 | 0.87 | UROLO | 23 | 4.55 |
| GSUR | 13 | 5.4 | OBS | 14 | 0.84 | TER | 32 | 3.70 |
| OP | 35 | 5.3 | GSUR | 13 | 0.68 | EMERG/ADUL | 8 | 3.49 |
| ANEST | 29 | 5.0 | INFEC | 7 | 0.42 | PSY | 31 | 3.33 |
| INFEC | 7 | 4.6 | NEPHRO | 6 | 0.18 | NEUSUR | 24 | 3.28 |
| HIVU | 1 | 3.8 | INTMED | 4 | 0.16 | NEUROL | 15 | 2.61 |
| ITER | 32 | 3.7 | PRENAT | 2 | 0.00 | T&C | 21 | 2.60 |
| ORTH | 19 | 3.5 | HEMA | 8 | 0.00 | RHE | 19 | 2.44 |
| OUTPAT | 2 | 3.4 | EMERG | 9 | 0.00 | LMS | 18 | 2.27 |
| URO | 24 | 3.2 | TRANS | 10 | 0.00 | ORTH | 17 | 2.20 |
| HEMA | 8 | 3.0 | GYN | 12 | 0.00 | ANES | 26 | 1.85 |
| ONCO | 22 | 3.0 | CARD | 17 | 0.00 | OUTPAT | 2 | 1.83 |
| NEPHRO | 6 | 2.6 | ORTH | 18 | 0.00 | OTO | 22 | 1.47 |
| INTMED | 4 | 2.3 | LMS | 19 | 0.00 | INFEC | 6 | 1.39 |
| RHEUMA | 17 | 2.1 | T&C | 20 | 0.00 | GSUR | 13 | 1.30 |
| NEUROL | 16 | 2.0 | OTO | 21 | 0.00 | ONCO | 20 | 1.27 |
| ICU | 25 | 1.9 | ONCO | 22 | 0.00 | HEMA | 7 | 1.16 |
| EMERG | 9 | 1.9 | ICU | 24 | 0.00 | GYN | 9 | 1.05 |
| GYN | 12 | 1.9 | NEURSUR | 25 | 0.00 | CARD | 16 | 0.92 |
| NEUSUR | 23 | 1.6 | DERM | 26 | 0.00 | INTMED | 3 | 0.59 |
| TRANS | 10 | 1.1 | ENDO | 27 | 0.00 | GASTRO | 5 | 0.36 |
| CARD | 15 | 1.0 | GERIA | 28 | 0.00 | NEPHRO | 4 | 0.34 |
| PNATCU | 3 | 0.0 | PNATCU | 1 | 0.25 | |||
GMH: Genomic Medicine in Hepatology
Serological HBsAg was the next highest hepatitis virus marker detected among the attending patients. In this case, the HIV Unit requested the most tests (position #1) and presented a similar prevalence of 3.0% of HBsAg marker as Proctology that ranked in the 30th position followed by Rheumatology with 2.08% (position #16).
Positive testing for anti-HIV was highest as expected from the HIV Unit with a prevalence of 56.32%, although it ranked in the 10th position on the list of services soliciting anti-HIV tests. Emergency services and Proctology were the next highest with an anti-HIV positivity prevalence of 33.33% and 14.63%, respectively. Furthermore, testing among prison inmates revealed a 12.50% of positivity for anti-HIV. Notably, the GMH Department reported 21.6% (anti-HCV), 3.73% (HBsAg), and 9.15% (anti-HIV) prevalence rates while ranking between 11th and 12th position of the testing list.
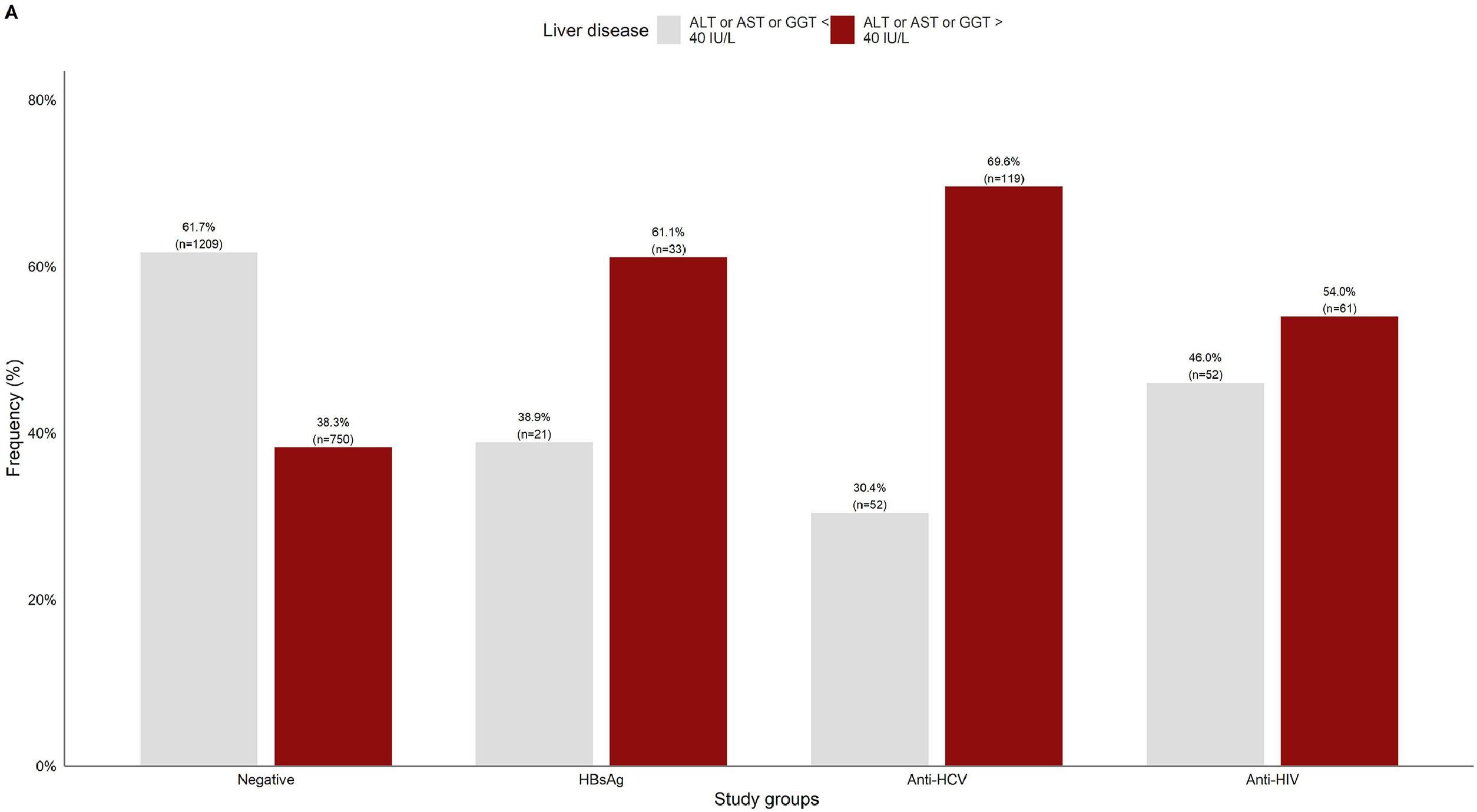
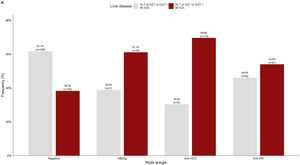
3.2Liver damage assessmentFig 2 shows that overall, 38.3% (750/1959) of the patients who were negative for any viral infection (HBV, HCV, or HIV) had at least one altered liver enzyme (ALT, AST, or GGT ≥40 IU/L). Among those who were positive to any of the infections, over 60% of them had abnormal liver enzymes, especially in anti-HCV positive patients (69.6%).
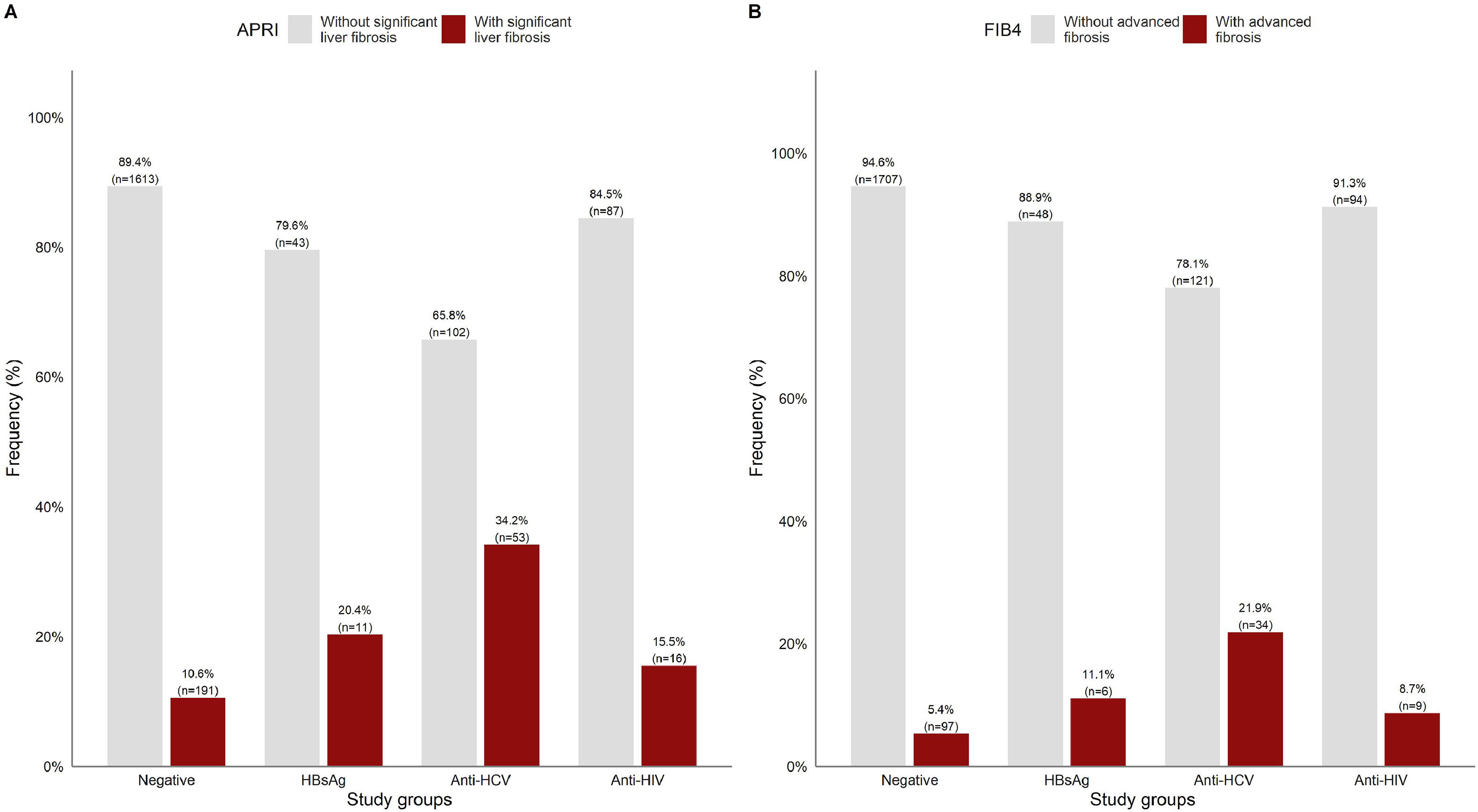
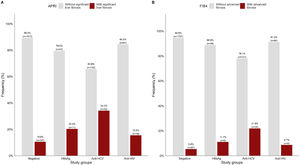
APRI and FIB-4 scores were analyzed in a subset of 2116 matched tests for the same patient. Overall, the patients with significant liver fibrosis were significantly older than those without (44.87 ± 15.5 vs. 39.77 ± 14.3 years, p<0.05). As shown in Fig 3A, significant liver fibrosis estimated by APRI was found in 10.6% of the negative patients. Among the patients who were positive for viral infections, the highest number of cases with liver fibrosis were among anti-HCV+ (34.2%), followed by HBsAg+ (20.4%) and anti-HIV+ (15.5%).
In Fig. 3B, FIB-4 scores showed advanced fibrosis in comparatively half of the negative patients (5.4%), whereas a lower percentage of positive cases was detected in the other subgroups.
4DiscussionThis study aimed to establish the prevalence of viral infections and liver damage among low-income patients attending one of the oldest governmental tertiary-care hospitals in West Mexico. HIV and HCV were the most frequent infectious agents among the studied population, followed by HBV, whereas an abnormal APRI score was found mainly in HCV-positive patients. This study shows that viral infections due to HCV, HBV, HIV, and liver fibrosis are persistent in patients with low socioeconomic status who may be unaware of their health condition.
Acute and chronic viral hepatitis confer the highest burden of disease in low-income and middle-income populations worldwide due to the lack of strategies at the levels of awareness campaigns, diagnosis, and access to care and treatment [9,10]. Among the WHO's region of the Americas, the highest prevalence rates of viral hepatitis are seen in Central and South America, whereas Mexico is considered a region of low endemicity [12,14]. However. in this study, hepatitis B was at least three times higher compared to the general adult population [21] (1% vs. 0.3%), while HCV was nine times higher [6] (3.9% vs. 0.43%) and lastly, HIV was seven times higher than that reported by the federal government in the adult population aged 15 to 49 years [7]. These rates are equivalent to those found in other endemic Latin America (LA) regions [22]. Since the studied group was composed mainly of vulnerable adult people who have low socioeconomic resources, this may reflect that they are highly exposed to unhygienic conditions and risky behaviors.
Hepatitis A virus infections, as well as HBV, are preventable by vaccines [23]. However, vaccination is not mandatory in all LA countries. In Mexico, better sanitation conditions have contributed to decrease the risk of infection for hepatitis A since vaccination against this viral type is not mandatory in Mexico [24]. In contrast, the HBV vaccine is included in the universal vaccination scheme since the year 2000 at 0, 2, and 6 months of age which may explain the higher infection rates seen in, the older patients who may not have been immunized [25]. As for HCV, the risk of acquiring this infection by contaminated blood transfusion was more common in the ’70s and ’80s, however, it currently has shifted to the use of unsafe needles in people who inject drugs and by tattooing [6]. Patients with HIV are also at risk of acquiring viral hepatitis (HBV and HCV) because they share similar routes of transmission. In this study, 9.0% of the positive cases of HIV had coinfection with HBV or HCV, which imposes a higher burden of disease and management. Furthermore, in the case HIV/HBV confection, patients can present mixed infections with two or three HBV genotypes coursing with significant liver damage [26]. In this study, patients with HIV also showed a high rate of liver fibrosis in agreement with previous studies [26].
Furthermore, the underlying epidemiological conditions coincided with the range of ages in which each virus was mainly reported in this study. In this case, HBV was highest between the ages of 41 to 50 in susceptible males. HCV prevalence was higher among women in the 60-year decade age group who may have been exposed to contaminated blood during surgeries and transfusion at younger ages. An important finding to highlight was that HIV was reported in ages 18-20 years compared to what has been reported by the national HIV/AIDS control center (Centro Nacional contra el SIDA, CENSIDA). In 2011, a prevalence of 19.6% was reported for this age group, and it has been increasing over the years. In 2015, the number raised to 22% and by the first quarter of 2020, it was 45% [7], which shows the urgency of taking preventive measures to halt the spread of HIV infection. Therefore, besides the measures already installed at the public health level, more focused age-group prevention strategies are required to decrease this population's risk of viral infections and progressive liver damage.
Viral hepatitis-related liver cirrhosis and hepatocellular carcinoma are among the main chronic morbidities with deleterious sequelae worldwide [27,28]. Thus, one of the main benefits of reducing the incidence and mortality of viral hepatitis is avoiding long-term progressive liver disease. In this study, 38.3% (750/1959) of patients who were negative to viral infections had at least one altered liver enzyme that increased to 54.0% in those who were positive for anti-HIV and was over 60% in patients with positive serological markers for hepatitis B and hepatitis C. This high rate of patients with abnormal liver enzymes who tested negative for viral infections suggests the presence of other etiologies such as metabolic-associated fatty liver disease (MAFLD) driven by obesity [29] or alcoholic liver disease [30].
On the other hand, in this study, occult B infection (HBsAg negative, DNA-HBV positive in serum) was not assessed due to the lack of anti-HBc antibodies or nucleic acid testing results among the study group. Occult B infection is shared among several risk Mexican populations, including blood donors, native populations, HIV, patients with chronic liver disease, and immunocompromised patients [31, 32]. Therefore, we have suggested that HBsAg, anti-HBc, and nucleic acid testing be performed simultaneously in these cases [21].
In patients who were positive for viral infections, liver fibrosis was widespread, suggesting the influence of the virus-related liver injury and the presence of excess weight or excessive alcohol intake as reflected by the abnormal levels of GGT. These assumptions are based on the current obesity epidemic affecting more than 70% of the Mexican population [33] and that nearly 27.6% (23.5 million people) [34] consume above-average amounts of drinks per occasion (>30 gr alcohol). Therefore, liver diseases can prevail due to a combination of concurring etiological factors that require medical attention and preventive measures. Early diagnosis of liver disease could be a reasonable measure with the parameters used in this study by which patients can be benefited considering that those who had significant liver fibrosis were older compared with patients without this condition.
The WHO's elimination strategy to reduce viral infections inherently impacts the rate of viral infection-related liver disease [1]. Therefore, knowing who is infected can aid in focusing on the target populations that need the most medical attention, resources, and treatments. In this study, the most significant number of tests for viral infections were from Gastroenterology, Prenatal Care, Outpatients, Internal Medicine, and the HIV Unit. However, these were not the central wards with the highest prevalence of anti-HCV, HBsAg, or even anti-HIV positivity. For example, anti-HCV antibodies were mainly detected among older patients (Geriatrics, Thorax & Cardiovascular). These findings agree with the peaked age group above 60 for HCV reported in this study and among prison inmates who are risk groups highly exposed to HCV and HIV [6].
Similarly, HBsAg positivity was detected nearly at the same rate in patients attending Proctology and Rheumatology (3.00%). Interestingly, despite that many services tested for hepatitis B, in more than half of them, no cases were detected (Table 2). In these patients, it may be recommended to seek for occult B infection as mentioned above because other risk factors can mask the clinical presentation of this type of infection [35]. Regarding HIV, anti-HIV positivity was highly detected at the HIV unit as expected; however, it was also detected in other areas such as the Emergency and Proctology departments.
It is noteworthy, that among the highest requests for testing was the Prenatal Care Unit, where expecting mothers are screened for viral infections, thus avoiding mother-fetal transmission of these infections. No positive patients for viral hepatitis were detected during the study period, whereas 0.25% of the tested women were anti-HIV positive. Another aspect was the high rate of positivity found by the GMH Department, where patients are referred to by other services for confirmation of previously undetermined results. Overall, this breakdown analysis reveals that the hospital´s medical services should continue testing for these infections among low-income patients exposed to several predisposing factors for viral infections and liver damage.
In this study, not knowing the status of HBV or HCV infections of the patients with positive serology was a limitation to define chronicity since other markers such as anti-HBc or viral load were not revised. Additionally, liver fibrosis was not verified by direct methods, such as transitional elastography or liver biopsy.. . Therefore, an in-depth analysis of the complete medical records is necessary to estimate the rate of active viremia, degree of liver damage, and other risk factors.
Nonetheless, the results derived from this study underscore the importance of following clinical diagnostic algorithms that enhance the capture of patients who are unaware if they are infected regardless of the specialty of each medical service. Another asset of these findings is that viral infections were detected in various patient categories not belonging to the conventional risk groups generally screened for these illnesses. Therefore, regardless of the attending service and given the combination of predisposing risk factors and socioeconomic status that affect low-income patients, attention is warranted to implement elimination strategies. General awareness campaigns, higher vaccination coverage, screening campaigns among older patients, and promoting lifestyle modifications can significantly reduce the risk of silent long-term injury to the liver.
5ConclusionHigh seroprevalence of HCV, HBV, and HIV infections was found among the studied population. Significant liver fibrosis was detected in negative and positive patients for viral infections. Medical services need to continuously test for viral infections, promote early detection of chronic liver damage and identify target patients for elimination strategies to decrease disease burden.
SL-M is the recipient of a doctoral scholarship by the National Council of Science and Technology (CONACYT) CVUnumber 832033 Application: 2018-000012-01NACF-11092.