Special issue on hepatocellular carcinoma (HCC) and hepatitis B and C as its main causes worldwide
Más datosThe latest studies on the epidemiology of diverse types of cancers have located in the scene the relevance of liver tumors, particularly hepatocellular carcinoma (HCC). HCC is a life-threatening malignancy triggered by chronic exposure to hepatitis B and C viruses, excessive alcohol intake, hepatic lipid droplet accumulation, and aflatoxins that lead to persistent liver damage. The occurrence of such etiological risk factors deeply marks the variability in the incidence of HCC worldwide reflected by geography, ethnicity, age, and lifestyle factors influenced by cultural aspects. New perspectives on the primary risk factors and their potential gene-environment interactions (GxE) have been well-addressed in some cancers; however, it continues to be a partially characterized issue in liver malignancies. In this review, the epidemiology of the risk factors for HCC are described enhancing the GxE interactions identified in Mexico, which could mark the risk of this liver malignancy among the population and the measures needed to revert them. Updated healthcare policies focusing on preventive care should be tailored based on the genetic and environmental risk factors, which may influence the effect of the etiological agents of HCC. Robust regional investigations related to epidemiological, clinical, and basic studies are warranted to understand this health problem complying with the rules of ethnic, genetic, environmental, and social diversity.
Currently, primary liver cancer in the form of hepatocellular carcinoma (HCC) is the sixth most diagnosed neoplastic disease and the third leading cause of cancer-related death worldwide [1,2]. Globally, 906,000 new cases and 830,00 deaths were caused by this malignancy in 2018 [1]. HCC imposes a high disease burden, especially in low and middle-human development index countries or in risk subpopulations that lack systematic surveillance programs and timely diagnostics-to-treatment strategies regardless of socioeconomic level [1]. It is a potentially life-threatening disease curable by several medical procedures if diagnosed at early stages [3–5]. According to geography, it also shows incidence and prevalence variances within populations [1,2,6,7]. Overall, the main etiologies of HCC are chronic infections caused by the hepatotropic hepatitis B (HBV) and hepatitis C (HCV) viruses, alcohol abuse, metabolic fatty liver disease, and exposure to aflatoxin B1-contaminated foodstuffs [8]. Studies on the worldwide epidemiology of HCC show that these multiple etiologies can predominantly act as single risk factors in some regions, but mostly, they have a synergic pro-oncogenic effect relative to HCC [9]. Furthermore, the fraction of contribution of each predisposing risk factor varies from one region to another [2,10]. Therefore, identifying such factors by area and population is relevant to implementing primary prevention, early detection, and feasible management strategies.
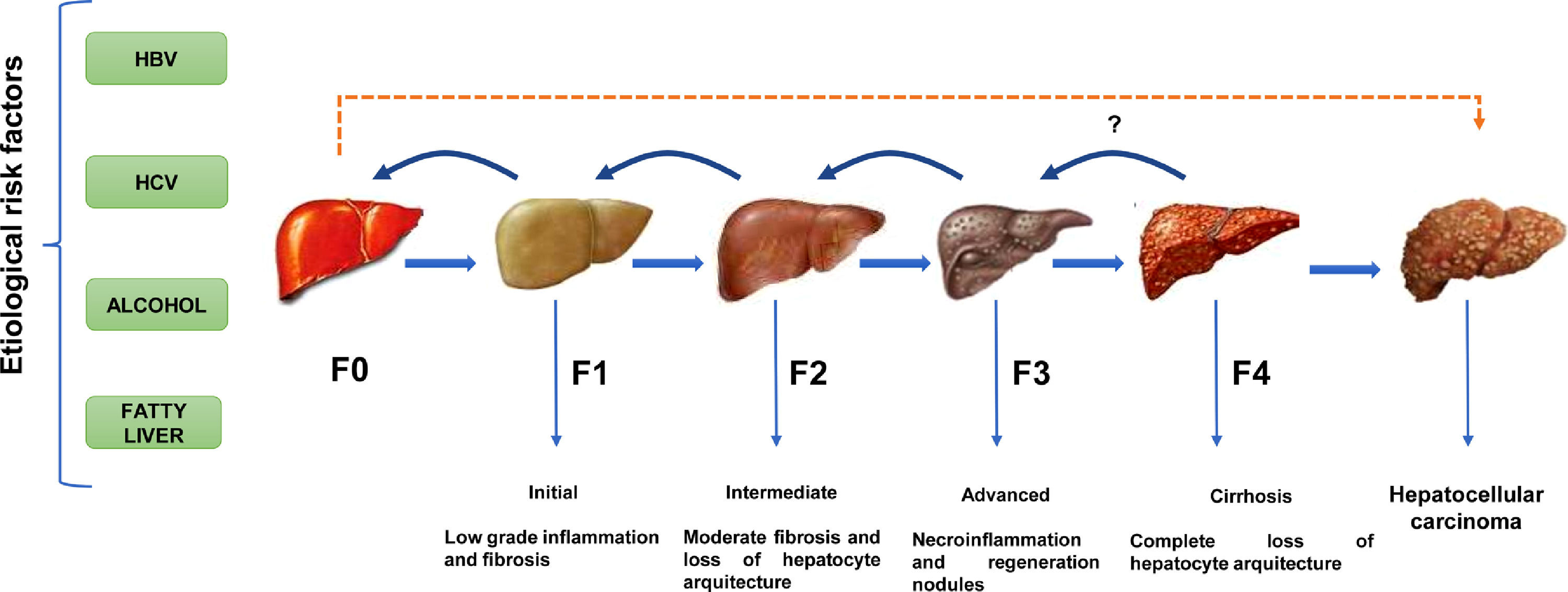
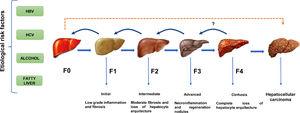
Except for ambient aflatoxins, the HCC canonical causative agents activate direct or indirect pathogenic pathways causing liver injury and cellular inflammation (necroinflammation), leading progressively to multistage fibrosis, cirrhosis, and ultimately HCC [11]. However, 30% of the HCC cases can occur directly without the development of cirrhosis. (Fig. 1). The underlying mechanisms that drive the natural course of liver disease may occur within a lapse of 20 to 30 years since the average age of detection of late-stage HCC is often above the age of 50. The variations in the global prevalence of HCC are also influenced by co-factors such as ethnicity, gender, age, or tobacco smoking [11]. However, the occurrence of HCC will not depend only on the prevalence of each etiological factor but also on the measures taken to limit them. Fortunately, most of them are preventable. For example, the early onset of HCC in children and young adults in Alaska Natives was eliminated by introducing newborn and catch-up vaccination schemes that reduced the number of HBV carriers [12]. In contrast, within the high endemic regions for HBV infection in Asia and Africa, the lack of aflatoxin-neutralizing control measures contributes to higher HCC incidence rates than non-exposed populations [13].
The natural course of liver disease is triggered by HBV, HCV, alcohol, or fatty liver. (
) HCC is a long-term, multi-step process involving initial, intermediate, advanced fibrosis, and cirrhosis. Fibrosis may be reversible if the insulting agent is withdrawn. () HCC is known to present without cirrhosis.Latin America (LA), comprising the Spanish and Portuguese-speaking countries of the American continent, is challenged by substantial predisposing factors. In this sense, the efforts put in by the International Agency of Research on Cancer to provide reliable population attributable fractions for HCC depend on the quality of the local cancer registry [2, 7]. This situation is notable in LA, where committed national registries detailing the epidemiology of liver cancer status based on histopathological evidence are lacking; thus, leading to unintended discrepancies compared to the estimations of HCC projected by the Global Cancer Observatory (GLOBOCAN) [14]. Furthermore, a pitfall is a reduced number of robust epidemiological studies (prospective or retrospective) in LA or, in the best of cases, this number is profusely limited compared to remarkable studies conducted in Asia, Europe, or the US.
Despite these shortcomings, modern-day LA populations, rich in biological diversity and multi-cultural heritage, share a common ancestral linage mainly comprised of Amerindian, Caucasian, and African ancestors. These characteristics provide an opportunity to explore the regional gene-environmental (GxE) interactions related to the onset and outcome of complex multifactorial diseases such as cancer [15]. Therefore, it is expected that differences in virus genotypes, the host's genetic variations (single nucleotide polymorphisms, SNPs), as well as regional dietary components or patterns, may influence the outcomes of liver disease and consequently the incidence of HCC. These features set the scene for the complexity of the epidemiology, diagnosis, and treatment of HCC in LA, including Mexico [16].
Currently, novel research revealing the genomic and molecular characterization of HCC is a fundamental aspect to understand the link between causal agents (HBV, HCV, alcohol, fatty liver, toxins), pathobiology, and oncogenesis of HCC. Table 1 summarizes some features that mark the difference between different etiological agents regarding the histological phenotype and clinical outcomes. The relevance of these features is the development of more specific and sensitive diagnostic markers and therapeutic strategies at the early stages of HCC to lower the morbidity and mortality rate in diseased patients. However, the knowledge needed to understand the link between any etiological risk factor and HCC begins by investigating the degree of regional incidence or prevalence of each one of them [17]. This review describes the risk factors for HCC enhancing the GxE interactions identified in Mexico that could mark the risk of this liver malignancy among the population and the measures needed to revert them.
Different clinical features of hepatocellular carcinoma.
| Parameter | Proliferative class | Non-proliferative class |
|---|---|---|
| Etiology | HBV | HCV (genotype 3)Alcohol consumption |
| Alpha-fetoprotein levels | High | Low |
| Clinical data | More aggressive tumors with poor differentiation | Less aggressive with hepatocyte-like cells |
| Epigenetic signature | Global hypomethylation | Hypermethylation |
Reference 44.
A member of the Hepadnaviridae family, human HBV is a relatively small partially double-stranded DNA virus comprising ten distinct genotypes (A-J) worldwide. HBV's genome contains four open reading frames (ORFs) denoted as Pre-S/S, Pre-C/C, P, and X encoding seven viral proteins: small, medium, and large hepatitis B surface antigens (HBsAg), e-antigen (HBeAg), core-antigen (HBc), polymerase (Pol) and X (HBx) [11]. The molecular link between HCC and chronic HBV infection is a multi-step process related to the virus's life cycle. During persistent infection, the natural selection of mutated quasi-species encoding truncated and mutated preS/S protein sequences is produced [18]. These aberrant molecules then activate a series of cellular processes related to the transactivation of transcription factors, upregulation of the immune-inflammatory response, the unfolded protein response, as well as the endoplasmic reticulum stress-dependent and stress-independent pathways [19–21]. Furthermore, integration of the HBx sequence per se causes genetic instability of the hepatocyte's genome, and the HBx product interacts with other proteins, causing transactivation of viral and cellular genes involved in inflammation and proliferation pathways [22,23]. Furthermore, hepatitis B occult infection, defined as the presence of HBV in the liver and/or serum of HBsAg negative individuals, is considered a potential oncogenic co-factor contributing to HCC in different populations worldwide [9].
From a public health standpoint, the impact of HBV infection on the potential development of HCC is mainly analyzed in terms of the endemicity of HBsAg, the age of acquisition, mode of transmission, and HBV genotype [24–26]. As in most countries, hepatitis B prevalence is conventionally reported using HBsAg prevalence despite the bias it causes in the data analysis. Double positive HBsAg and anti-HBc antibody markers are evidence of active infection, whereas testing positive to HBsAg alone does not distinguish between acute or chronic infection. However, in the case of Mexico, another confounding factor related to the allegedly low prevalence of HBsAg is the high prevalence of occult B infection which has been frequently documented among Amerindian (Nahuas, Huichols) and admixed populations in the country [27,28]. Complementary to this fact is the trend of a relatively higher level of the anti-HBc marker, suggesting that past HBV infection may be higher than suspected. With this in mind, we have recommended testing for HBsAg, anti-HBc, and HBV-DNA in risk patients to prevent underdiagnosis in the clinical setting [29].
In a meta-analysis study, Roman et al. concluded that HBsAg prevalence had remained steady since 1976 (to 2010) with a prevalence of 0.3% in which anti-HBc ranged from 3.13% (95% CI, 3.01–3.24) in blood donors to 27.7% % among hemodialyzed patients [30]. In contrast, testing for HBV among samples collected for the National Health and Nutrition Surveys from the general population has shown differences. Valdespino J et al. reported a prevalence of 0.21% (95%CI 0.11–0.37) in samples from the year 2000 among adults over 20 years [31] whereas Lopez-Gatell et al. tested for natural immunity against HBV infection with a weighted prevalence of 0.23% (Anti-HBsAg and anti-HBc positive, HBsAg negative) in survey samples of 2012 among a population aged 10-25 years [32]. However, the accumulated trend of new cases of HBV infection registered in the national weekly surveillance reports since 2000 shows an incidence rate of 1.7 × 105 among people between the ages of 25-44 years (https://epidemiologia-salud-gib-mx/anuario/html/incidencia_casos.html).
Furthermore, a recent study by Laguna et al. in West Mexico found 1.0% of HBsAg positivity among outpatients attending a third-level hospital [33], and Jose-Abrego et al. found 28% in HIV patients with low socioeconomic status [29]. These data suggest that HBV is still circulating significantly; thus, chronicity should be confirmed in HBV-infected patients to decide if periodical screening for HCC is necessary.
Most studies conducted in adults show that horizontal transmission is the main route of acquisition through unsafe sexual practices and contaminated biological fluids [30]. In this context, among low socioeconomic populations, parents with risk factors for HBV infection may horizontally infect their children, who are not protected by vaccination, while mother-to-child transmission has not been documented to date in Mexico [34]. In conjunction, focused catch-up immunization campaigns should be considered in several subpopulations throughout the country.
The study of the natural history of HBV infection and the incidence of HCC is narrated conventionally to what occurs in populations outside LA. The Asian HBV/C and the European HBV/D have the worst prognosis, compared to the respective genotypes B and A [26,35]. Also, intermediate-high endemicity rates of chronic infection and mother-to-child vertical transmission are typical in these regions.
Contrarily, the low incidence of HBV-related HCC [36,37] is attributed to a low prevalence of HBsAg in Mexico despite the high rates of anti-HBc antibodies and occult B infection, suggesting that many people (nearly 15 million) may have been exposed to the virus with apparently no severe liver damage [30]. The fact that the HBV/H was hosted by the endemic Amerindian populations allowed for long-term immunogenetic adaptations influencing the natural history of HBV infection in modern-day admixed populations [38,39]. In Sozzi et al. study, HBV/H showed a low replication phenotype compared to genotype HBV/D3 [40]. Similarly, Tanaka et al. observed the absence of inflammation and liver fibrosis in chimeric mice mono-infected with HBV/H after 24 weeks, compared with mice co-infected with HBV/G [41]. Such evidence suggests efffectively that the low general trend of HBV-induced HCC in Mexico coincides with the low risk of liver damage; however, further investigation is required.
Although HBV/H predominantly circulates, other genotypes have been detected. HBV/G prevails among men who have sex with men and patients infected with human immunodeficiency virus (HIV), whereas HBV/A2 and D4 have been identified among chronically-infected patients living in urban areas [29,38]. Interestingly, in patients infected with HIV receiving retroviral therapy, co-infection with heterologous mixtures of HBV genotypes (H/G/D) and high viral loads were associated with an increased risk of liver damage [42] compared to HBV/H mono-infected patients having typical lower viral loads as previously reported [38]. HBV/H mono-infection presents a milder course of disease than dual or triple infections that increase the risk of long-term liver damage. Therefore, the distinct HBV genotypes circulating among the Mexican population may cause different outcomes that impact the risk for HCC.
Another common HBV genotype circulating within Central and South America is HBV/F (F1-F4), the endemic virus among the indigenous populations [38,43]. Specifically, HBV genotype F1b has been associated with HCC in Alaska Natives [25], Argentinians, and recently in Andean Native Peruvians. In this group, a novel global DNA hypermethylation pattern and gene expression signature was reported in opposition to what has been commonly reported [44]. Interestingly, we recently documented the first-time detection of HBV F1b in the Mexican-Native population [29] and in an admixed Mexican patient who presented an occult phase at the end of the acute stage and then recovered [45]. Finally, the role of immune escape, antiviral resistance, and HCC-associated mutants related to HBV/H require more research to continue studying this genotype's dynamic and adaptive behavior among the Mexican population [46].
2.2HCVA member of the Flaviviridae family, HCV is a single-stranded RNA virus encoding a polyprotein precursor cleaved in the hepatocyte by cellular and viral proteases into ten functional viral proteins [11]. Core protein and envelope glycoproteins E1 and E2 are structural proteins required to assemble the virions. In contrast, the remaining non-structural (NS) proteins (p7 viroporin, NS2 protease, NS3-4A complex harboring protease-NTPase/RNA helicase activities, NS4B, NS5A, and the NS5B RNA-dependent RNA polymerase) are involved in replication activities [20, 21]. All HCV encoding proteins contribute to the oncogenic pathways [47].
HCV genome manifests a high degree of inter-host genetic heterogeneity represented by 8 genotypes and 90 subtypes as of 2019 that are distributed among the human populations worldwide [48]. Furthermore, intra-host nucleotide diversity due to the lack of proofreading activity of the viral RNA-dependent RNA polymerase generates in the infected host a quasi-species cloud, a viral "colony" containing many mutated genomes with immune escape capability and baseline resistance-associated substitutions [49]. These intra-host HCV quasi-species have clinical implications related to chronicity that, together with a tumorigenic cellular microenvironment, has been associated with HCC [50]. HCV-induced HCC comprises multifactorial and complex processes between the virus (genotype), the liver immune system, and the host's metabolic status [47]. In this sense, the relationship between lipid structures and metabolism, spontaneous clearance, and chronicity has been considered [51,52] and will be discussed in this review.
HCV infection affects 71 million people worldwide [53], and the availability of the highly effective direct-acting antivirals (DAAs) led the WHO's Global Health Sector Strategy program to target the goal of its elimination by 2030. Undoubtfully, epidemiological data regarding the endemicity among low and high risks populations is required among all nations to conveniently deliver these drugs to all eligible patients regardless of socioeconomic status. However, studies regarding the retrospective or prospective association between HCV and HCC have not been conducted explicitly in LA.
In Mexico, 0.27% of anti-HCV antibodies were detected in samples taken among the general population for the 2012 National Health and Nutrition Survey [54]. In an updated meta-analysis (2008-2019), anti-HCV positivity ranged from 0.77% to 2.5% among low-risk Mexican groups compared to 11.8% to 39.6% in high-risk groups that included prison inmates, injection drug users, and dialyzed patients [55]. In a hospital-based study in West Mexico, 3.9% of anti-HCV antibodies were detected [33]. Genotyping assays performed during the same study period found genotypes 3a, 4a/c/d, and 5a. This data indicates that a re-shaping of the molecular epidemiology landscape has impacted the regional genotype distribution in the last decade. Some studies have suggested that the propensity to develop HCC is related to HCV genotype [56]; however, the role of HCV genotypes remains debatable. Nonetheless, the predominant prevalence of the HCV genotype 1 and the emerging of 3a in Mexico may become a risk for HCC development in chronically-infected HCV patients if synergistic co-factors are prevalent [57]. These changes are mainly due to the emerging parenteral transmission of HCV by injection drug use and unsafe tattooing that has increased in the last decade among incarcerated people and the low-risk general population [55].
Regarding the role of the host's genetic susceptibility for the clearance or chronicity of HCV infection, another critical factor is the influence of IL28B gene polymorphisms, mainly in response to pegylated interferon plus ribavirin antiviral therapy in HCV genotype 1-infected patients during the pre-DAAs era [58]. In the relationship between IL28B SNPs and HCV clearance, the role of ethnicity has been underscored. Currently, the new DAAs are considered pan-genotypic, minimizing the role of HCV genotypes. However, IL28B (rs12979860) T risk allele was associated with liver cirrhosis in Egyptian HCV-infected patients treated with DAAs but was not a predictor for HCC [59]. In the case of Mexico, the rate of carriers with the IL28B (rs12979860/rs8099917) T/G risk haplotype is higher among Mexican Amerindian populations than in admixed Caucasians [60]. However, it is unclear if treated patients are at higher risk for cirrhosis or HCC. Further studies are needed to clarify the relationship between HCV´s molecular evolution and quasi-species dynamics, and the genetic susceptibility for HCV-induced HCC in chronically-infected patients.
2.3.Alcohol-related liver diseaseAlcohol-related liver disease is a nosological spectrum generated by excessive chronic alcohol consumption causing liver injury. The pathological accumulation of lipids into the hepatocytes (steatosis) followed by chronic inflammation (steatohepatitis) are the two cell processes that activate the fibrogenic and oncogenic pathways towards cirrhosis and HCC respectively [61]. Alcohol-induced liver cirrhosis is a leading hallmark for HCC in countries in which HBV or HCV infections are at low prevalence; however, it is a synergistic oncogenic factor in the setting of chronic viral infections [62].
Liver diseases are the fourth cause of death in Mexico; alcoholic liver disease accounted for about 35% of liver disease mortality in 2019 [63], with an incidence rate of 3.2 × 105 inhabitants [64]. However, the amount of alcohol per capita per year, alcoholic liver disease, and alcohol-induced HCC may not be linearly related. This paradox may be explained by a combination of genetic and environmental factors that may increase the risk for liver cirrhosis in some populations.
The WHO's Global Health Observatory reported in 2016 an alcohol consumption of 5.6 L of pure alcohol per capita that raised to 15.6 L among drinkers in Mexico [64], lower than countries such as Russia or even France. However, the pattern of alcohol consumption consisting of light to moderate beer drinking on weekends during youth can gradually raise to daily consumption of hard spirits (tequila, mezcal) in the lapse of one or two decades [65]. Heavy drinking (300 g/occasion) was associated with a relatively higher frequency of the DRD2/ANKK1 (rs1800497) A1/A1 risk genotype among Amerindian groups [66] and the novel TAS2R38 (AVV/AVV) risk haplotype associated to drinkers compared to non-drinkers among mestizos [67]. In relation to liver damage, early onset of liver cirrhosis in alcoholic patients was associated with dyslipidemia and being a carrier of the APOE, e2 allele [68]. Likewise, ADH, ALDH, and CYP2E1 are highly polymorphic genes encoding alcohol-metabolizing enzymes modulating acetaldehyde production. Interestingly, the risk or protective alleles of these genes are inherited differentially among Amerindian and mestizos Mexicans in the form of slow-, intermediate- and fast-metabolizer profiles [69] that may influence the course of alcohol-induced liver damage and cause early death due to complications of cirrhosis rather than the development of HCC, thus not reaching the stage of overt cancer malignancy [37] .
In light of this, the Latin American Association for the Study of the Liver (ALEH) endorses the need for future studies to implement strategies for managing alcohol-related liver disease based on regional variances in genetic susceptibility and cultural determinants of alcohol consumption [70]. This proposal could spark the implementation of systematic multi-center and collaborative studies between regional researchers and medical specialists to decipher the role these risk factors have on alcohol-induced liver disease and HCC.
2.4Metabolic-related liver diseaseIn the last decade, the world trends of hepatitis B and C virus infections have decreased due to vaccination and more effective treatment strategies. However, an increasing incidence of HCC related to obesity and fatty liver has emerged, and with the global obesity epidemic, the incidence is unlikely to improve [71]. Non-alcoholic fatty liver or steatosis (once excessive alcohol intake is discarded) and the subsequent oxidative stress leading to steatohepatitis are important hallmarks for HCC, acting as synergistic co-factors in patients with viral hepatitis or alcohol-related liver disease. The mechanistic link between metabolic steatohepatitis and HCC has been widely studied. Insulin resistance and dyslipidemia are conditions that trigger inflammatory, fibrogenic, and oncogenic pathways. In this context, imbalanced dietary intake or unhealthy dietary patterns leading to dyslipidemia and obesity may play an essential role in the long-term development of HCC pathogenesis.
2.4.1Diet and liver diseaseThe association between regional dietary patterns containing specific risk or protective nutrients and the development of HCC has been intensively studied, coinciding with the role of fat content [72]. In this regard, most traditional diets of LA contain regional staple foods that are considered healthier than the modern-day globalized diets. In Mexico, traditional diets containing dishes prepared with prehispanic ingredients are viewed as nutritionally balanced because they include cereals, legumes, fruits, vegetables, seeds, nuts, and fiber that provide plant-based proteins and fats, low-glycemic complex carbohydrates, adequate vitamins and minerals with a lower content of animal fats or high-fat meats. Formerly, these dietary ingredients were obtained by traditional husbandry techniques and contained fewer processed components [73]. Currently, there is an epidemiological transition, leading to the coexistence of two opposite nutrition-related concerns: malnutrition and overweight/obesity. Similarly, as mentioned for the other risk factors, this situation is a serious concern in LA due to the increasing global incidence of non-communicable diseases, including cancer [74,75].
According to the 2020 National Health and Nutrition Survey, 36.0% of the adult population, 18.6% of children (5-11 years), and 17.0% of adolescents (12-19 years) are obese [75]. Regional genetic and environmental factors in Mexico may be contributing to this alarming rise of obesity and obesity-related co-morbidities. Mainly, a high prevalence of dyslipidemias, hypertriglyceridemia (49%), and hypercholesterolemia (26.1%) have been reported nationwide, which relates to the increase in obesity, type 2 diabetes, liver steatosis, and cardiovascular diseases [76].
Furthermore, several host genetic alleles modulating liver disease have been identified in patients with chronic liver disease. For example, the oral fat-sensing taste receptor CD36 (rs1761667) G>A polymorphism is associated with liver fibrosis and consuming high dietary fat and cholesterol in patients infected with HCV [77]. In contrast, a protective role of the APOE e4 allele related to less liver damage in patients with spontaneous clearance and hypercholesterolemia [78] has been reported. Interestingly, cholesterol and its derivatives show immunomodulating effects on the course of HCV infection related to spontaneous clearance [79]. Additionally, increasing evidence shows that liver lipid dynamics are modulated by the PNPLA3 (rs738409) C>G polymorphism. More importantly, this polymorphism has a differential global allele distribution that impacts the outcomes of alcohol and metabolic-related cirrhosis and even HCC [70,80].
Interestingly, the HCV viral particle containing a lipid membrane envelope has a hypolipidemic-like effect on plasmatic lipids and concomitantly induces an intrahepatocyte lipid-rich micro-environment to enhance replication [35,79]. Regarding this point, we were able to characterize the effect of the adherence to a typical diet containing more than 4.9% of poli-unsaturated fats and less than 21.5 gr/d of fiber associated with lower viral load in patients with HCV infection [81]. These findings may have implications for the nutritional management of both treatment-naive and post-treated patients.
Further insights on the characteristics of the hepatopathogenic diet frequently consumed by young and obese Mexicans show that an unbalanced ratio of saturated/unsaturated fats and cholesterol, among other nutrients, is associated with fatty liver and histologically confirmed steatohepatitis [82]. Therefore, diet composition may play a key role as a modifiable lifestyle factor to avoid chronic liver disease [83].
With this evidence, we have proposed consuming the GENOMEX diet containing traditional food components (nutrients) consistent with the Mexican adaptive gene polymorphisms and food culture [73]. Patient adherence to the GENOMEX diet significantly improved anthropometric and metabolic parameters, including weight, lipid profile, and insulin resistance in a 24-week intervention study [84]. This diet was tailored to provide regional nutritional support in Mexican patients with chronic disease to avoid relying on foreign dietary strategies [85].
Nonetheless, further regional large-scale and prospective studies are needed to provide evidence regarding the long-term effect of this diet on the onset and progression of metabolic abnormalities related to obesity and other co-morbidities, including HCC.
2.4.2Cholesterol and HCCHigh lipid diets, particularly those enriched in cholesterol, are critical determinants in HCC as tumor inducers and promoters. HCC requires excessive lipid content because they are needed as building blocks for new membranes in the proliferative process, fuel supply, and intermediaries for post-translational modification of essential proteins such as Ras. Therefore, it is common to find a high dependence on free fatty acids and cholesterol in HCC tumors [86].
Studies carried out in mice models have proved that dietary cholesterol accelerates the hepatocarcinogenic process by a mechanism dependent on the generation of reactive oxygen species (ROS), DNA damage (judged by increased levels of 8-hydroxyguanine), and the decrement in the expression of the leading DNA repair enzymes such as p53, ATM, CHK1, CHK2, among others [87]. Mitochondria impairment also seems targeted by cholesterol overload, as previously proved [88]. Furthermore, controlling the cholesterol content by experimental therapeutic approaches using GDF11 improved mitochondrial functionality, increased ATP synthesis, and ROS decrement. These features were associated with a lesser aggressivity phenotype in human HCC cell lines [89, 90]. These data show that dietary cholesterol is a significant risk factor for the initiation of HCC. Although the role of cholesterol in the expression or activation of these genes remains elusive, it is clear that GxE interactions are acting at the carcinogenic level.
We recently proved that cholesterol-enriched diets, similar to the westernized or high cholesterol diet (1.0% cholesterol), conditioned the development of a subtype of HCC tumors with high aggressivity and poor prognosis using massive RNA sequencing analysis in a mouse model [91]. The specific signature revealed 62 genes differentially expressed and conserved between experimental diets. Additionally, human HCC signatures showed significant enrichment in HCCs with the following features: poor survival, hepatoblast traits, activation of c-Met/HGF, and late TGF-β signaling pathways. Even more, a comparison with a specific group of human HCCs (n= 101) revealed a significant decrease in overall survival for patients included in the cluster with the cholesterol-associated signature compared with cholesterol-independent HCCs (p= 0.01).
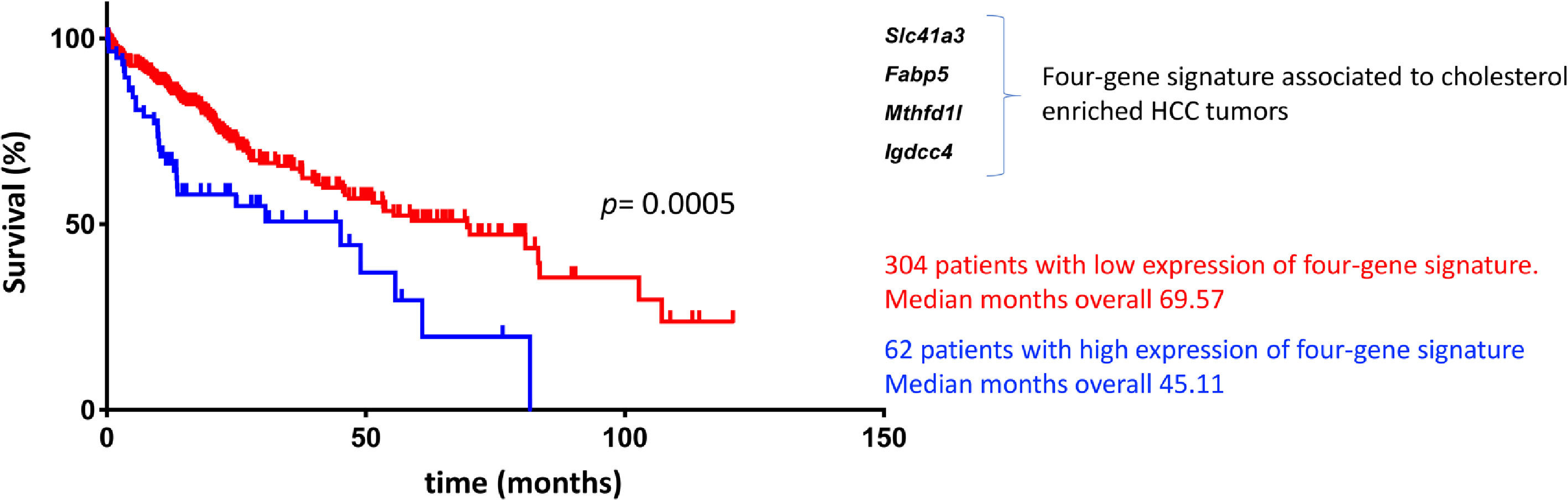
Based on the GxE interactions, the mouse model study discovered four remarkable genes, Slc41a3, Fabp5, Mthfd1, and Igdcc4, with high predictable strength (Fig. 2). A close view of these genes reveals, as expected, a profound impact on metabolic control. Slc41a3 is a mitochondrial cation transmembrane transporter involved in Mg2+ homeostasis and is required to meet the need for aberrant proliferation in cancer [92]. Fabp5, fatty acid-binding protein 5, transports intracellular lipids for storage purposes, providing building blocks for membrane construction and energy supply, both highly required in proliferating cells. It is also involved in epithelial-mesenchymal transition [93]. Expression of folate cycle enzyme Mthfd1l, methylenetetrahydrofolate dehydrogenase 1 like, has been associated with poor prognosis in HCC coffering metabolic advantages due to the relevance of folates in purine synthesis and translation initiation [94]. Finally, Igdcc4 encodes the neighbor of Punc E11, also known as Nope. It is barely detected in the adult liver, and it is a sensitive marker of HCC associated with stemness. Nope protein has been demonstrated to be a confident marker for HCC in the clinic because it could be highly detected in AFP-positive and AFP-negative tumors [95].
Cholesterol-associated four-gene signature defines a subtype of human liver cancer with a poor prognosis. Overall survival, according to Simoni-Nieves et al. 2021 [91].
Cholesterol overload in the liver also elicits this specific four-gene signature associated with a poor prognosis in patients with HCC (n=371) compared with non-tumor liver tissues (n=50). Also, a comparison between cholesterol-associated HCC and non-cholesterol-associated HCC showed significant differences in median months of overall survival (45.11 vs. 69.57 mo) [91]. This signature could be helpful in the clinical setting for making decisions in terms of diagnosis, prognosis, and treatment (Fig. 2). The study provides clear evidence of GxE interactions favoring HCC progression and aggressivity. This scenario is particularly relevant in societies undergoing nutrition transition where dietary changes consist of high-lipid diets, particularly cholesterol.
2.5Ambient toxins2.5.1AflatoxinsMycotoxins are secondary metabolites produced by certain fungi of the Aspergillus family, particularly A. flavus, A niger, or A. parasiticus, contaminating crops such as corn, peanuts, and walnuts, among others and food derived from these. Among these, mycotoxins fumonisins, zearalenone and aflatoxins are particularly relevant as contaminants. Mycotoxins could be produced during harvesting or storage. In LA, contamination of foods by fungi is favored by tropical and subtropical environmental conditions, such as constant warm temperature and humidity [96].
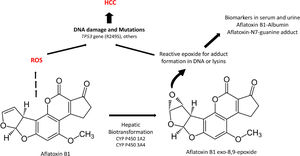
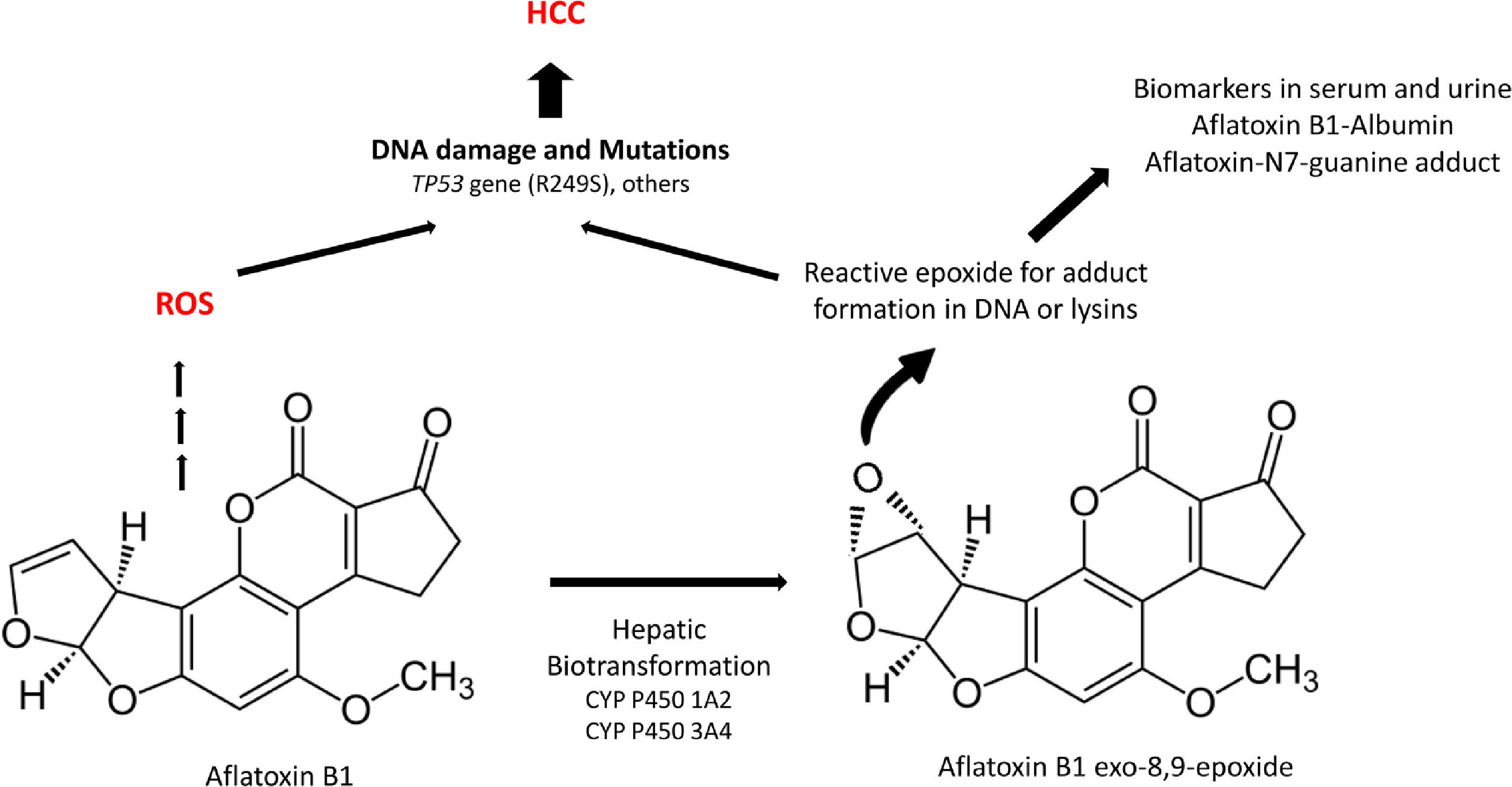
Aflatoxins are potent hepatocarcinogens, particularly aflatoxin B1 (AFB1), acting through DNA oxidative damage and adduct formation (Aflatoxin-N7-guanine adduct); this is particularly relevant in the liver because it is the main site for its biotransformation producing the aflatoxin epoxide with a high affinity for DNA and protein modifications (lysine adducts, for example, and aflatoxin-albumin, which is a clinical biomarker) (Fig. 3).
A significant association between dietary AFB1 and the development of HCC has been reported, particularly in countries of LA with high production of peanuts, such as Brazil, Argentina, Uruguay, and Paraguay. Still, aflatoxin contamination is more significant in Colombia and Ecuador [96], and aflatoxin contamination in corn is particularly relevant in Venezuela and Guatemala. Recent studies in Guatemala, a country with alarming HCC incidence and death in LA, have revealed a positive association between aflatoxin exposure and cirrhosis [97] and elevated albumin-aflatoxin levels among adults. This was closely related to tortilla consumption rather than maize [98]. Some retrospective case-control studies evidenced the GxE interactions between AFB1-albumin and AFB1-DNA adducts and genetic variants of the RecA/Rad51 family DNA repair enzymes [99, 100].
The well-known tumor-suppressive protein p53 has been widely associated with AFB1-induced liver cancer. A 53% of HCCs studied in patients from areas with high exposure to AFB1 have revealed mutations in the p53 gene (TP53) [101]. AFB1 directly targets TP53 inducing G:C→T:A transversion in the third base of codon 249 as shown in vitro studies [102]. In LA, there seems to be some conflicting evidence; for example, a recent study showed no evidence of TP53 variant in 69 HCC samples (0/69 cases) [103], but a pioneering study conducted in the mid-90s in the state of Nuevo Leon in Mexico explored a limited cohort of HCC samples (n=21), finding only 3/21 cases with the mutational hotspot at codon 249 in the TP53 gene [104]. Recently, a cross-sectional study conducted in Guatemala in HCC tissues (n=91) revealed 47% of the tumoral tissues had a TP53 mutation, being more prevalent the variant R249S mutation (24%) [105].
Nonetheless, it is important to emphasize the fact that chronic HBV infection and AFB1 exposure act synergistically in HCC [97,106].
In addition to TP53, more evidence of the GxE interactions in AFB1-induced HCC arrives from the biotransformation pathways of the aflatoxins. Phase I and II detoxification routes transform the AFB1, and the exploration of critical genes in these pathways could show relevant information. Genetic variation in epoxide hydroxylase and glutathione S-transferase were contrasted with AFB1-albumin in HCC; data showed that both genes were significantly repressed in patients with HCC [107]. These findings leave clear that genetic susceptibility to environmental AFB1 could play a synergistic role with hepatotropic viruses or cholesterol overload, mainly due to the consumption of the hepatopathogenic diet mentioned before.
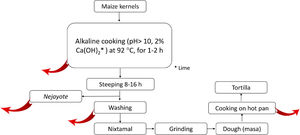
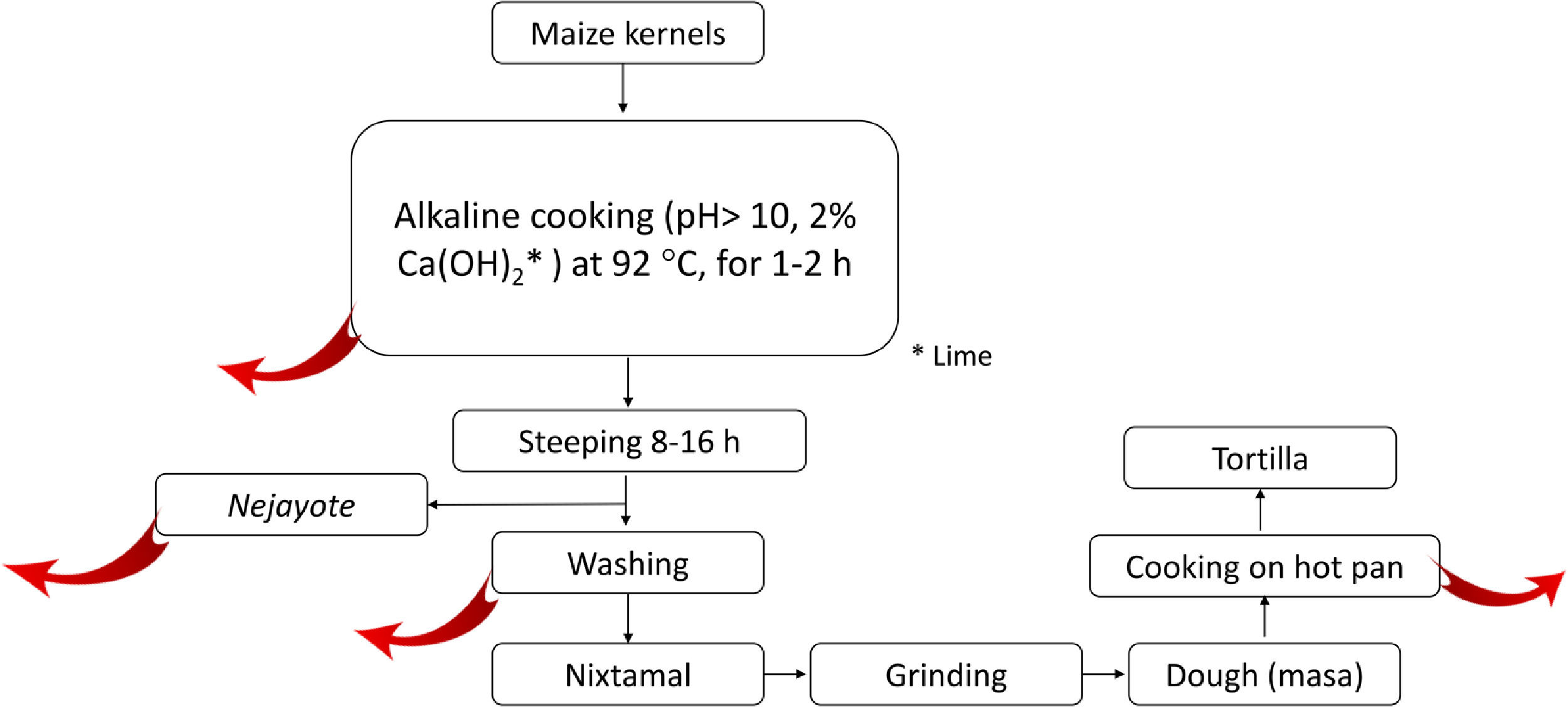
2.5.2Nixtamalization and aflatoxins changesNixtamalization is the alkaline cooking process of maize kernels traditionally implemented in LA, particularly in countries of the ancient Mesoamerica: Mexico and Central America [37,108] and recorded in historical documents (Fig. 4). Nixtamalization consists of cooking the dry maize kernels with 2% lime (called "cal"; Ca(OH)2, reaching pH > 10) at an average temperature of 92 °C for 1-2 h, followed by a wash with water [108]. This process eliminates the fungi contamination and aflatoxins (particularly in the lime broth or Nejayote). Aflatoxins are also subjected to physical and (bio)chemical transformation, reducing the molecule, significatively decreasing its toxicity and carcinogenic potential. Aflatoxin's inactivation could also be done in the process of cooking the flat disk of dough in a hot pan (Fig. 5, red curve arrows indicate potential exit or chemical transformation of aflatoxins). This procedure may relate to the low rate of aflatoxin-induced HCC registered in Mexico, although further studies are warranted [37].
Scheme of the steps in the typical process of nixtamalization of maize kernels to produce "tortillas." Red arrows indicate the potential points of elimination or (bio)chemical transformation of Aflatoxins. Nejayote is the liquid or "lime broth ashes" discarded after steeping cooked maize containing aflatoxins. Ca(OH)2 is in the form of lime.
This review described relevant GxE interactions modulating the role of the main risk factors related to HCC in Mexico. HBV/H is the endemic genotype of Mexico, showing a low risk for chronic infection, cirrhosis, and HCC among the general population. Nonetheless, HIV patients with co-infection with genotype mixtures containing non-H genotypes display high viral loads and liver damage. HCV genotypes 1 and 3a are the main genotypes circulating to date in the country, although the former has risen due to an increase in injection drug use, and HCV prevalence remains high among incarcerated people and drug addicts. Additionally, non-endemic genotypes 4 and 5 have been detected. Alcoholic liver disease remains a risk factor for cirrhosis due to interactions between genetic susceptibility related to alcoholism and cultural determinants influencing the pattern of drinking among the population.
Just a decade ago, the leading causes were HCV and alcoholic liver disease, and, to date, the prevalence of metabolic liver disease driven mainly by the obesity pandemic is moving upward as the leading risk factor for HCC development, even in the absence of cirrhosis. Metabolic liver disease will increase if measures are not taken to revert the escalating prevalence of overweight and obesity in children, adolescents, and adults. This liver disease is a critical condition for a worse tumor presentation in terms of aggressivity and prognosis due to the high content of lipids (rich-cholesterol) found in westernized dietary patterns that are representative of the nutrition transition occurring in the country. Dietary aflatoxin remains a risk factor in many countries of LA, although, in Mexico, the process of maize nixtamalization seems to curb the risk of aflatoxin-induced HCC. Lastly, an important reminder is that these etiological factors can silently cause liver disease for years until detected and act synergically. Therefore, strategies for early diagnostics in risk patients are necessary.
Decreasing the burden of diseases is a complex process. However, in the case of HCC, the first step is vaccination against HBV and antiviral therapy for HCV. It is of utmost importance to re-enforce health policies, prevention strategies, and awareness programs for hepatitis viruses of Mexico [109]. More updated medical and nutritional educational health programs at all levels of society are required to offset the marketing favoring excessive alcohol drinking and the consumption of unhealthy foods.
In conclusion, updated healthcare policies focusing on the needs of preventive care should be tailored based on the genetic and environmental risk factors that influence the effect of the etiological agents related to the incidence and prevalence rate of HCC. Robust regional investigations related to epidemiological, clinical, and basic studies are warranted to understand this health problem complying with the rules of ethnic, genetic, environmental, and social diversity.
FundingNo funding was granted for this work.











![Cholesterol-associated four-gene signature defines a subtype of human liver cancer with a poor prognosis. Overall survival, according to Simoni-Nieves et al. 2021 [91]. Cholesterol-associated four-gene signature defines a subtype of human liver cancer with a poor prognosis. Overall survival, according to Simoni-Nieves et al. 2021 [91].](https://static.elsevier.es/multimedia/16652681/00000027000000S1/v3_202303011411/S1665268121003513/v3_202303011411/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)