Special issue on hepatocellular carcinoma (HCC) and hepatitis B and C as its main causes worldwide
Más datosThe images used in the graphical abstract were created using the icons contained in the free web-site https://thenounproject.com/ and are attributed as follows:
- Brain: Brain by BomSymbols from the Noun Project
- Diagnosis: Liver by Chanut is Industries from the Noun Project
- Epidemiology: People by Nithinan Tatah from the Noun Project
- Patient with HCC: Liver by Luis Prado from the Noun Project
- Prognosis: Prediction by Becris from the Noun Project
- Staging: Liver by myiconfinder from the Noun Project
- Surveillance: Surveillance by Alice Design from the Noun Project
- Treatment: Get treatment by Lima Studio from the Noun Project and Surgery by Justin Blake from the Noun Project
- Writing: Writing by Becris from the Noun Project
If you want to go fast, go alone. If you want to go far, go together.
The Italian Liver Cancer (ITA.LI.CA) study group was born in 1998, during the annual meeting of the Italian Association for the Study of the Liver (AISF), as an informal and friendly collaboration between seven hepatologists aimed at collecting the data of patients with hepatocellular carcinoma (HCC) managed in their real-world clinical practice. This cooperation would have permitted to analyse a much larger patient cohort than that the one collected by each centre alone, and the ITA.LI.CA founders had correctly guessed that, thanks to the growing diffusion of digitalisation and informatics, the era of “big databases” had begun even in Medicine. Indeed, the analysis of large databases can provide solid and reproducible results, also allowing to perform sub-analyses able to deliver reliable indications to guide clinical decision-making processes across the patient journey. Big databases are particularly useful for producing insights on prevalence, incidence and mortality rates, temporal trends, disparities (between centres, geographic areas, gender, ethnicity, and income-related), inputs for cost-effectiveness and treatment benefit modelling, and also for monitoring rare diseases. They also help formulating hypotheses for causal relations (risk factors), predicting future scenarios, and designing new prospective studies.
On the other hand, big databases generated by the real-world practice inevitably have structural drawbacks regarding quality of data (missing data, incorrect data-entry, lack of standardisation), misclassification risk, low control of data integrity and security vulnerability. These imperfections are well known by reviewers of scientific journals who tend to overemphasise the inherent biases of non-randomised studies, underestimating the complementary importance of the information coming from the everyday clinical practice, i.e. the so-called real-world evidence[1]. This attitude makes more difficult to publish results of studies reporting data on real-world evidence in highly reputed journals compared to those coming from randomised controlled trials (RCT), therefore contributing to generate a lower level of consideration for real-world evidence in the decision-making processes. Nonetheless, the ITA.LI.CA founders accepted this “publication risk”, being firmly convinced that the road towards advancements in disease knowledge and management should rely on not only the results of RCTs but also on those provided by real-world studies. Indeed, RCTs are – and remain – the gold standard for generating evidence finalised to the approval and world-wide dissemination of new preventive or diagnostic procedures as well as of innovative therapies, but their results should be interpreted on the basis of – and integrated with – real-world data so as to improve and personalise patient management. This need arises as a substantial proportion of patients observed in clinical practice have demographic and/or clinical characteristics (e.g., age, sex, ethnicity, comorbidities, co-treatments, familial and social conditions, etc.) that prevents their access to RTCs (designed to maximise the probability of success) or, alternatively, generates sub-groups too small to achieve robust evidence regarding efficacy and safety by post-hoc analyses of these trials. Furthermore, RCTs are resource-consuming, so that they are generally designed and carried out in high-income areas of the world and in expert centres, excluding economically-deprived regions which, instead, could complementarily contribute to the medical progress as huge sources of real-world data. For these reasons, RCT results cannot be automatically generalised to the real-world practice, and evidence is needed to validate their results in a more generalised clinical setting. Real-world testing can, in fact, reveal positive (i.e., extension of the proven benefit to patient categories excluded from, or under-represented in, RCTs) as well as negative aspects (e.g., poor applicability, low physician uptake of the procedure/treatment, low patient adherence, unexpected risks, etc.) missed by RCTs, which are specifically designed and conducted to optimise the probability of success. Therefore, real-world evidence complements RCT results offering insights on the external validity of what is observed in “ideal” conditions and “ideal” patients. In other words, field-practice data are essential to transform the efficacy attested by RCTs into effectiveness, which is an indispensable information not only for those who take care of patients but also for stakeholders and third-party payers.
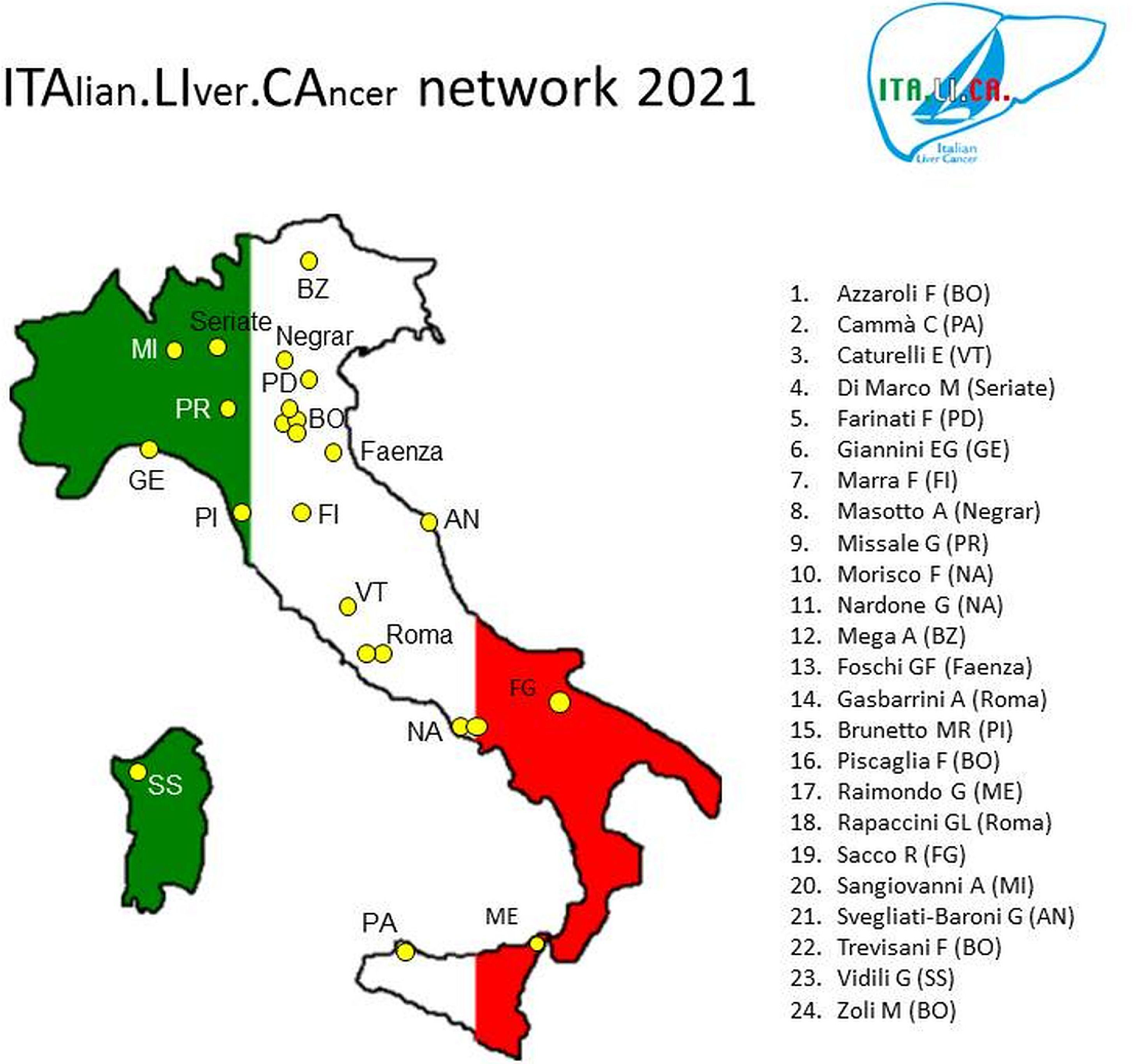
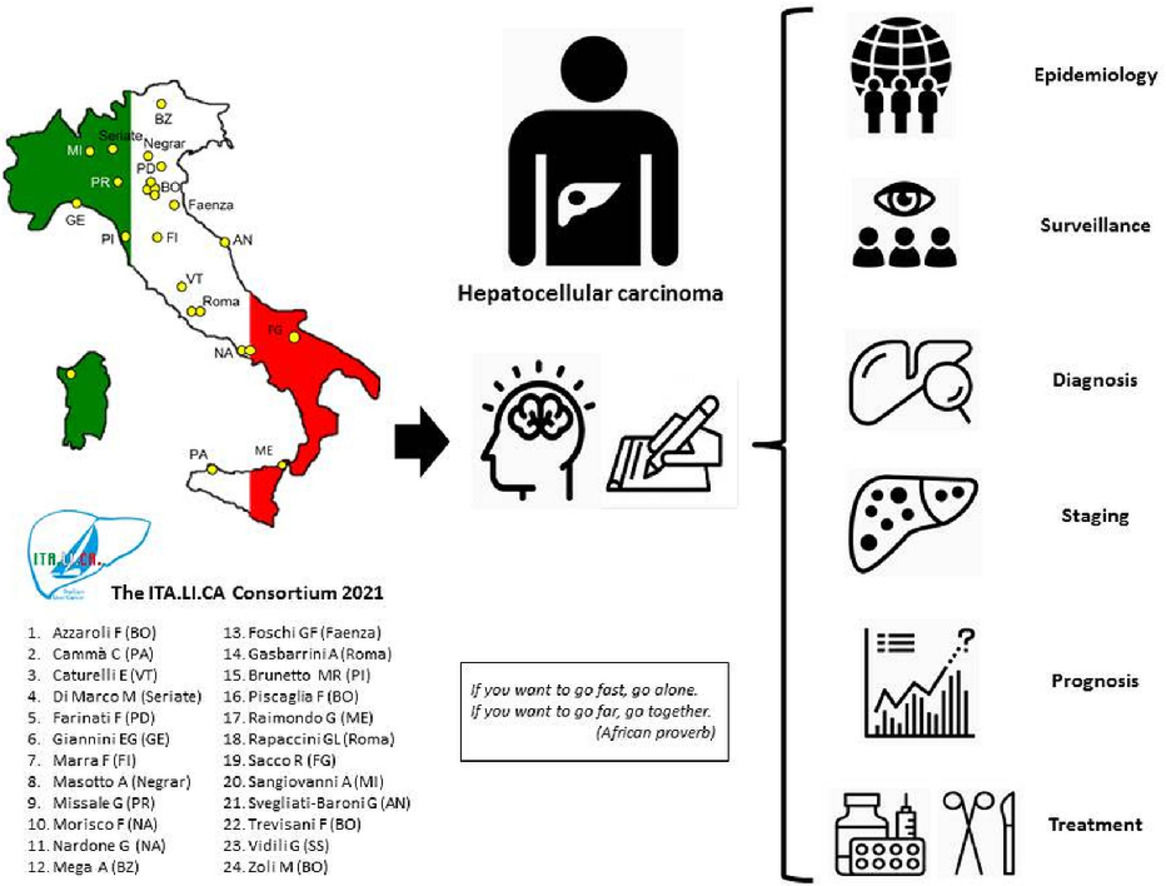
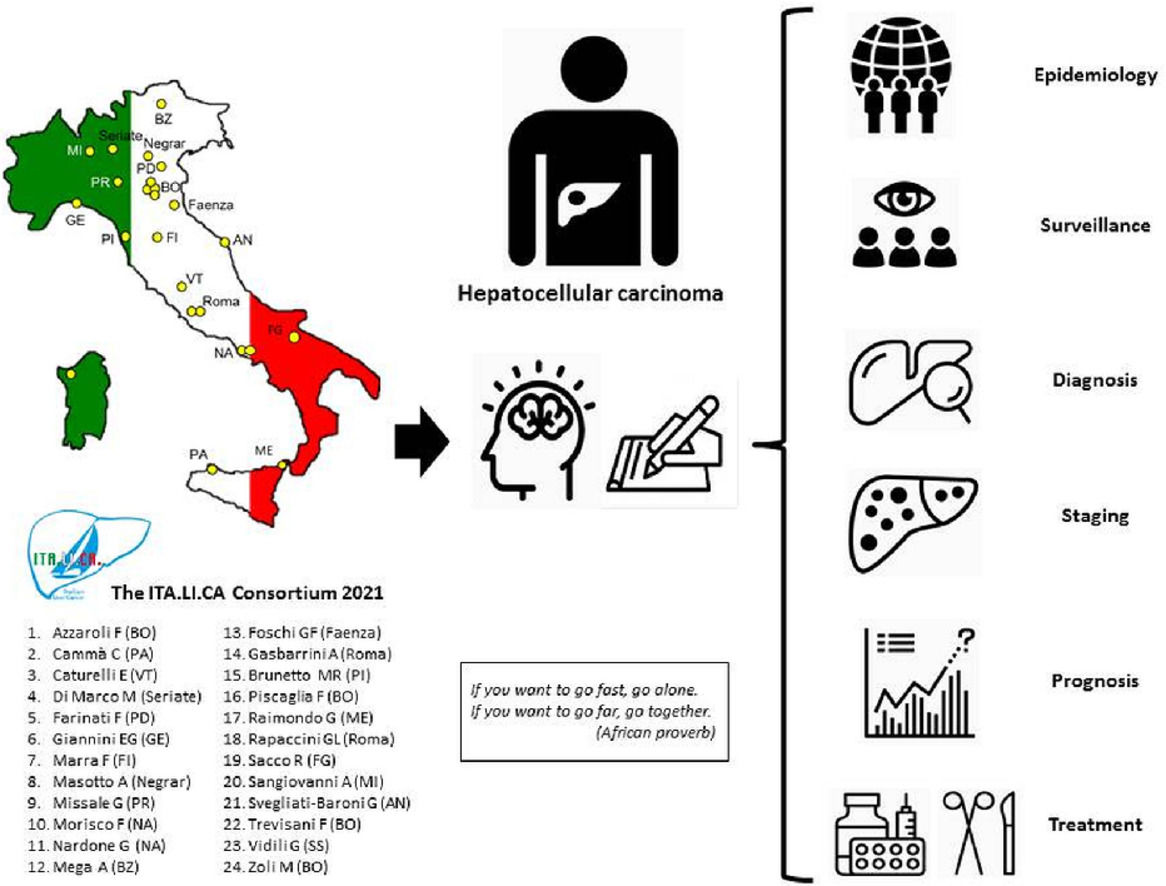
2The ITALICA consortium and its databaseThe initial group of seven ITA.LI.CA founders progressively expanded over time, so that in 2006 the group included nine centres, and in 2016 the centres had become twenty-three. Currently, the consortium includes twenty-four centres distributed throughout the Italian territory (Fig. 1).
The ITA.LI.CA consortium includes specialists in Gastroenterology and Internal Medicine operating in secondary and tertiary referral hospitals for HCC in order to make the results derived from the analysis of our database as much as possible representative of the epidemiology and management of this cancer in our country. To further limit recruitment distortions, the consortium does not include surgery and oncology centres which suffer from the inherent bias of principally collecting and managing patients “polarised” towards the extreme, opposite ends of the HCC spectrum (early or advanced stage, respectively).
In July 2010, our organisation was legally recognized as “no-profit association” according to the Italian law. An official statute and a regulatory charter were edited and signed by four ITA.LI.CA founders (FT, FF, GR, and MZ). The charter defines the rules governing the access to and the permanence in the ITA.LI.CA consortium, the modality of database use, the timing of mandatory database updating (every 2 years), the approval process of new studies, and the authorship of articles (Table 1). These well-defined rules, their endorsement and their respect by all ITA.LI.CA members form the backbone of the longevity and progressive growth of the consortium.
The approved rules of the ITA.LI.CA consortium.
| Requirements for the access to and stay in the consortium |
| The entry of a new member has to be approved by all ITA.LI.CA members |
| The initial data entry must include data of at least 100 HCC patients |
| All consecutively observed patients have to be regularly followed-up and registered |
| Data collection of all patients after entry is mandatory |
| Data updating every 2 years at pre-established dates (December 31 of odd years) is mandatory |
| Study projects |
| A copy of the study project has to be sent to the coordinator of the consortium* |
| For “external” researchers the use of the database is restricted to the approved study project |
| Articles |
| A draft must be sent to all ITA.LI.CA members for comments and final approval |
| The article must specify the origin of data |
| Authorship |
| Authors: all people who participated in the article conception and writing, followed by one author per centre ⁎⁎ |
| Appendix: must mention centre coordinators excluded from the authorship (see previous point) and all collaborators (data entry, patient management, etc). |
In 2011, an on-line web-site (www.progettoitalica.it) for data entry was created. The data are collected in a semi-anonymous way (i.e., each patient is identified by a number, and the combination patient-number is only known by the reference centre). The access to the web-site needs a password, and each centre can digit, control and modify only its own data. The coordinating centre (Semeiotics Unit, Alma Mater Studiorum - University of Bologna) has instead an unlimited access to data, allowing the ITA.LI.CA data-manager to check the quality and consistency of data after each update. If clarifications or additional information are needed, the data-manager contacts the centres requesting data check. After final approval of the data check by the guarantor (FT), an anonymised Excel version of the database is made available for statistical evaluation not only to ITA.LI.CA members but also to “external” researchers who have presented to the consortium a study project that has been approved by all official members. Indeed, over the years, several external researchers have produced reports based on the ITA.LI.CA registry [2–23].
It is also worth noting that the database contains the patients’ data collected at the time of each HCC treatment (i.e., of the naïve tumour as well as of its post-treatment persistence or recurrence), considering “locked” the clinical status in between. Nevertheless, even for patients whose data are locked, the survival is checked at the time of each database updating. This pragmatic choice, suggested by the knowledge that “The best is the enemy of the good” (Voltaire), was preferred to a more stringent updating (i.e., at each visit, or annually) because a more demanding task could have increased the risk of drop-out among members of the consortium, who are clinicians heavily involved with clinical activities. We feel that this choice is another backbone of the ITA.LI.CA longevity. Based on the above-described modalities, the registry data are collected prospectively, updated every 2 years and, after final approval by the guarantor, it can be retrospectively analysed by internal and external researchers.
The management of the ITA.LI.CA database conforms to the Italian legislation on privacy. According to the Italian law, no specific patient approval is needed for retrospective analyses, but all patients provided written informed consent for any diagnostic and therapeutic procedure, as well as for having their clinical data anonymously recorded in the ITA.LI.CA database. All ITA.LI.CA studies are conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. The Ethical Committee or Institutional Scientific Board of each participating centre approved the creation of the ITA.LI.CA registry and its use for scientific research.
3The scientific journey of the ITA.LI.CA consortium: simply not going from (stage) A to (stage) BThe first ITA.LI.CA article, showing that diagnosis of HCC with ultrasound surveillance in patients with compensated cirrhosis was associated with an improved survival as compared to incidental or symptomatic diagnosis, was published in 2002 by the American Journal of Gastroenterology, after seven rejections by other prestigious journals of Medicine, Gastroenterology, Hepatology, and Oncology [24]. These numerous refusals likely reflected the widespread hostility, at that time, of the research community against surveillance that had been generated by an antecedent, single-centre prospective study published in the New England Journal of Medicine that had failed to demonstrate a survival benefit of surveillance [25]. Thus, our paper was one of the first that contributed to demolish a “conceptual barrier” expanding the use of surveillance for patients at risk of HCC development in clinical practice, a tenet that was thereafter recommended by all guidelines for HCC management. Since then, 84 ITA.LI.CA articles and letters-to-the Editor have been published in peer-reviewed international journals.
The subsequent part of this review will follow the journey of the ITA.LI.CA publications along the HCC pathway, from epidemiology, to surveillance, diagnosis, staging, prognostication and treatment (Fig. 2). It will depict how the ITA.LI.CA studies contributed to innovate the management of HCC in the course of the last decades, and how real-world evidence served as a support to guidelines recommendations, and to inform policy-makers decisions.
Due to its nature that encompasses the inclusion of both secondary and tertiary referral centres distributed throughout the country, the ITA.LI.CA database allowed to draw a comprehensive picture of HCC patients in Italy, and how epidemiological trends evolved along more than 20 years [26–28]. Furthermore, it permitted the characterisation of uncommon conditions such as cryptogenic HCC as well as non-cirrhotic patients with HCC [29,30]. It was shown that patients with cryptogenic HCC resemble those with non-alcoholic fatty liver disease, having higher platelet counts, lower aminotransferases, and being less frequently diagnosed during surveillance as compared to patients with hepatitis C virus (HCV) infection, therefore leading to the detection of larger lesions with a lower amenability to curative treatments [2,29]. The evaluation of non-cirrhotic HCC patients showed that this is an infrequent condition in the Western world (encountered in approximately 2% of HCC patients), and even less frequent is the finding of HCC patients without any chronic liver disease (i.e., 19.2% of patients without cirrhosis). Interestingly, despite approximately 40% of these patients could be treated with curative intention, overall median survival was low (26 months) due to a high prevalence of macrovascular invasion (9.6%) and extra-hepatic diffusion (7.8%), as the majority of patients without cirrhosis were diagnosed outside any surveillance program, mainly due to the unawareness of their at-risk background condition [30].
The opportunity of systematically updating the database along the years allowed to describe how aetiology and demographic characteristics of HCC patients changed over time [26–28]. Overall, we observed a progressive aging of patients at diagnosis, with a mean age at diagnosis currently approximating 70 years, a progressive relative expansion of non-viral cases – in particular those associated with metabolic conditions – with a concurrent decrease in HCV-related cases (even before the direct acting antiviral agents era) as well as in hepatitis B virus (HBV) infected cases [26-28,31,32]. Noteworthy, among virus-unrelated HCCs, we showed that alcoholic aetiology of liver disease affects survival through its negative effects on secondary prevention and cancer stage at diagnosis rather than through a greater cancer aggressiveness or worse treatments results, while in patients with non-alcoholic fatty liver disease-associated HCC we observed that metabolic factors characterising this liver disease do not influence overall patients survival [33,34]. Lastly, as far as demographic data are concerned, in another article we showed that life expectancy of patients was unaffected by age above 70 years, and that in elderly subjects – likewise younger ones – prognosis was mainly dictated by cancer stage and, hence, by the potential access to curative treatments [35].
3.2Surveillance and diagnosisIn 2002, the seminal paper of the ITA.LI.CA group demonstrated, in a retrospective analysis including 1,051 HCC patients, that semi-annual or annual surveillance with ultrasound (with/without alpha-fetoprotein measurement) was associated with a more favourable stage at the time of cancer diagnosis, greater amenability to curative or palliative treatments, and to an increased lead-time adjusted survival as compared to patients in whom HCC had been diagnosed incidentally or in a symptomatic phase [24]. Moreover, it was observed that this benefit was mainly confined to patients with compensated cirrhosis, particularly when access to liver transplantation was limited or precluded [24]. These results were consistent with evidence emerging from studies carried out in Eastern countries, where chronic liver disease background was different, and they were subsequently confirmed by a study focused on elderly patients [36–38]. All these results provided solid support to the recommendations proposed by International guidelines about this issue.
The role of liver dysfunction on surveillance results was analysed more in detail in another study whose results indicated that the survival advantage determined by this procedure disappeared in patients with decompensated cirrhosis (Child-Pugh C) – unless they are candidates for liver transplantation – due to an exceedingly high cirrhosis-related competing mortality and to the unfeasibility of any treatment for HCC in most of these cases [39]. Conversely, the survival benefit offered by surveillance programmes was maintained in mildly decompensated patients (Child-Pugh class B) [39]. Both findings were endorsed by HCC guidelines.
The increasing number of centres joining the consortium allowed to explore in detail the prognostic improvement achievable with different surveillance interval, and a study published in the Journal of Hepatology in 2010 showed that, in patients with compensated or mildly decompensated cirrhosis, semi-annual surveillance was superior to the annual schedule in improving patients survival, mainly due to a greater amenability to curative treatments [40]. These findings, once again inspiring current guidelines for HCC management, were not taken for granted at the time, since some studies suggested that ultrasound surveillance for HCC had several pitfalls (mainly related to a process failure rather than to a failure of the institute of surveillance) and that the quality of evidence supporting surveillance was too low to support its widespread implementation in clinical practice, also considering that the potential “harms” of serial screening had never been taken into account [41–44].
Some operative aspects of surveillance, such as its cost-effectiveness, the propensity to detect tumours with a slow growth rate (e.g., length-time bias), and the impact of lead-time bias were covered in additional ITA.LI.CA reports showing that: 1) semi-annual surveillance is cost-effective in patients with compensated cirrhosis when the annual incidence of HCC is above 3.2%, as well as in patients with decompensated cirrhosis pending amenability to liver transplantation; 2) the impact of length-time bias in retrospective studies may be limited; 3) the benefit of surveillance has to be tested over an appropriate length of follow-up in order to make the lead-time bias disturb negligible and, thereafter, to demonstrate an actual increase in survival [3–5]. This last point has been confirmed in a more recent analysis of the database, showing that surveillance increased early-stage diagnosis and, therefore, the applicability of curative treatments, thus determining an independent greater likelihood of improved long-term survival [45].
The role of alpha-fetoprotein in surveillance and the factors associated to its diagnostic performance have long been a matter of debate and were also analysed by the ITA.LI.CA group [46–51]. As a fact, the potential diagnostic capability of elevated serum alpha-fetoprotein turned out to be minimal, with a sensitivity of 54%, and this oncomarker demonstrated an even lower utility in detecting single tiny (≤2 cm) HCCs in compensated patients without viral aetiology of liver disease or with cured viral infection, thus without the background noise of hepatic necro-inflammation that contribute the elevation of alpha-fetoprotein levels [49,50]. However, despite all the shortcomings of this oncomarker, a novel use of alpha-fetoprotein as surveillance marker has been proposed, exploiting the negative predictive power of change over time of its levels for the early detection of HCC [47,51].
Lastly, the potential determinants of ultrasound surveillance failure were considered, revealing features that can guide its use when conditions limiting the yield of ultrasounds are present. Pertinently, indicators of an aggressive HCC behaviour such as elevated alpha-fetoprotein levels, infiltrative pattern, macro-vascular invasion and extra-hepatic spread accounted for approximately half of the failure of ultrasound surveillance, and a sub-analysis of patients with available body mass index (BMI) data showed that a BMI ≥25 kg/m2 independently predicted the failure of surveillance [50,52]. This finding raises some concerns about the utility of ultrasound surveillance for monitoring patients with non-alcoholic liver disease who frequently show high BMI and low serum alpha-fetoprotein [53].
3.3Staging, treatment, and prognosisAppropriate staging is essential to assess patient prognosis and to establish the most useful therapeutic approach [54]. In this regard, the availability of a large database including patients with a wide disease spectrum and treated with various approaches was instrumental to assess the influence of patients’ characteristics on prognosis, to evaluate the yield of treatment modalities – and to assess the role of treatment multi-modality – and to design and validate a prognostic system. To this end, the collaboration between the ITA.LI.CA consortium and external researchers with specific interest and expertise in HCC staging led to studies exploring the association among some commonly available laboratory parameters, such as gamma-glutamyltranspeptidase, bilirubin, and platelet count, and prognosis. These analyses, recognised a phenotypic presentation of HCC, characterised by increased cholestasis indexes, elevated platelet counts, larger lesions, showing a worse prognosis [7–14]. Furthermore, the ITA.LI.CA group was also able to elaborate on the relevance of peculiar features of cirrhosis, such as the development of oesophageal varices, on the prognosis of patients with HCC, highlighting the independent negative impact of their presence on overall patients’ survival [55].
An international collaboration between ITA.LI.CA consortium and Liver Cancer in Human Immunodeficiency Virus (HIV) group allowed to describe how HIV infection can negatively impact patients’ prognosis independently of cancer stage and treatment received [56]. Likewise, interrogation of the database delineated how HCC prognosis, especially when the tumour is diagnosed in the course of surveillance, may be independent of patients’ gender and aetiology of liver disease but, at the same time, how HBV infection may portend a worse prognosis in advanced HCC [57–60]. Moreover, in patients with small HCC identified during surveillance and treated with curative intent, alpha-fetoprotein levels did not show a prognostic relevance, while in HCV patients with successfully treated HCC, decompensation of liver disease represented the main driver of prognosis, thus calling for early antiviral treatment in such patients aimed at preserving – or even improving – liver function, without worries about (unproved) antiviral treatment-related increase in HCC recurrence [61–67].
It is well known that the prognostic assessment of HCC patients is a very complex multifactorial task that fundamentally relies on cancer characteristics (size and number of lesions, presence of vascular invasion or extra-hepatic spread, alpha-fetoprotein production), peculiar features of liver cirrhosis (degree of liver dysfunction and presence of portal hypertension) and general clinical conditions (Performance Status). Hence, the availability of a large and heterogeneous series of patients enabled ITA.LI.CA researchers to provide a prognostic “weight” to specific cancer features, such as size and macro-vascular invasion, the latter being usually associated with the advanced stage [68–70]. It was also noted that the size of single HCC is prognostically relevant when these patients undergo surgical resection so that, patients with a single nodule >5 cm have a significantly poorer prognosis as compared to those with smaller lesions [68]. This result makes questionable the Barcelona Clinic Liver Cancer (BCLC) staging system that does not prognostically differentiate single tumours >2 cm in size. Of note, patients classified as “very early” according to the BCLC classification (single lesion up to 2 cm in size and preserved liver function) had a similar prognosis, regardless of the treatment received, suggesting that additional factors should be taken into account when assessing prognosis of patients with these tumours [69]. At the opposite edge of the disease spectrum, the ITA.LI.CA registry allowed to single out prognostic differences among patients sharing an apparently homogeneous condition, i.e. macro-vascular invasion by cancer. In fact, location and extension of vascular invasion dictates the prognosis and is intertwined with treatment selection [70].
The prognostic role of liver disease was evaluated in another study assessing whether the presence of a clinically significant portal hypertension – identified by the presence of oesophageal varices or the indirect parameters proposed by the BCLC classification – affected the survival of patients with single small HCC [71]. This study, at odds with common indications, showed that patients with clinically significant portal hypertension undergoing surgical resection had similar survival compared to those without this condition.
The use of various HCC treatments and their outcomes were also thoroughly evaluated in ITA.LI.CA patients in order to assess the degree of adherence to guidelines recommendations and how much it translates into clinical practice effectiveness. A longitudinal analysis provided evidence that, since the publication of studies demonstrating the efficacy of transcatheter arterial embolization (TACE), the refinements of patient selection and technical progresses led to an improved outcome of transarterial treatments [72–74]. A real-world evaluation of prognostic determinants of patients undergoing TACE was also provided, and helped evaluate the most appropriate method to predict survival in patients undergoing this palliative treatment and to recalibrate the existing prognostic models according to the median survival observed in their own series of previously treated patients [75–78]. Furthermore, the outcome of radiofrequency thermal ablation was compared to that of a Japanese series, showing that refinement of patients selection is the key to obtain the best outcome [79,80].
Liver transplantation for HCC in field-practice has represented another important topic of search. It was observed that the median percentage of patients undergoing transplantation is as low as about 3% and remained virtually unmodified over 20 years of data accrual [15,16,81]. Moreover, Vitale et al. in an article published in Lancet Oncology provided innovative criteria for selecting patients describing the “transplant benefit” (i.e., the survival advantage offered by transplant as compared to the other possible treatments) across the different BCLC stages [16].
Likewise, the survival benefit of surgical resection was assessed across various BLCLC stages, showing that surgery is superior to loco-regional treatments, provided that liver dysfunction and poor performance status are absent [17].
After systemic treatment with proven efficacy for HCC was made available, the ITA.LI.CA database provided field-practice data to assist clinicians better tune sorafenib use by identifying on-treatment predictors of response, assessing the discriminatory prognostic power of various models and, more recently, providing a nomogram to predict prognosis of HCC patients treated with this drug [82–86]. Moreover, collaboration with other groups allowed to test the usefulness of systemic treatments for HCC outside the setting of industry-sponsored trials, providing a solid support to evaluate the usefulness of metronomic capecitabine treatment for advanced HCC in both first- and second-line setting [87].
Another field of study was HCC staging and its role in the treatment choice. Namely, we explored the performance of the most commonly used staging system -– the BCLC – in clinical practice, how much its stage-dictated treatment approach was followed by ITA.LI.CA clinicians and, eventually, which was the outcome of upward and downward therapeutic approaches. As a matter of fact, within the most heterogeneous BCLC stage, the intermediate one, curative treatments (upward approach) were feasible in 26% of cases and, as compared to patients treated with TACE (the BCLC therapeutic indication for this stage), they offered the chance of improved survival even after adjustment for confounding factors [88,89]. Our data, collected across 20 years of patients accrual, indicated that TACE cannot be considered a priori the ideal approach for these patients, mainly due to the great heterogeneity of patients included in the intermediate stage. This heterogeneity has been confirmed by the use of the ITA.LI.CA database to assess the prognostic validity of a sub-classification of intermediate stage proposed by a group of experts, but whose utility had never been tested in clinical practice [90]. Indeed, this sub-classification was able to predict the prognosis of intermediate patients with untreated HCC, thus providing data to assess the potential survival advantage achievable with different treatments in the sub-stages [91]. Furthermore, ITA.LI.CA researchers disentangled the prognostic determinants of patients with advanced HCC (BCLC stage C), another very heterogeneous patient group. In fact, characteristics used to include patients into this stage, such as macrovascular invasion, extra-hepatic spread and a Performance Status >0 identified different sub-populations with different prognosis and who should be treated in a tuned personalised way in order to optimize the outcome rather than with systemic therapy alone, as suggested by the BCLC algorithm [92,93].
Inasmuch as the available HCC staging systems had pitfalls and are not reflective of the real-world practice, the consortium researchers compared the prognostic ability of several prognostic scores, and eventually formulated a new integrated prognostic score which was externally validated [18,19,94,95]. Namely, HCC stages were assembled using only tumour characteristics (i.e., largest tumour diameter, nodule number, intra- or extra-hepatic macroscopic vascular invasion, extra-hepatic spread) and, thereafter, an ITA.LI.CA integrated prognostic score was devised by means of a parametric multivariable survival model that was used to calculate the relative prognostic weight of the ITA.LI.CA (above described) tumour stage, Performance Status, Child-Pugh score, and alpha-fetoprotein [94]. The performance of the ITA.LI.CA prognostic system was validated in a Taiwanese population of HCC patients and was compared with those of other integrated systems [BCLC, Hong Kong Liver Cancer (HKLC) staging, Model to Estimate Survival in Ambulatory HCC Patients (MESIAH), Cancer of the Liver Italian Program (CLIP) score, and Japanese Integrated Staging (JIS) score] [94]. Moreover, the ITA.LI.CA system was externally and independently validated in another Italian HCC population [95]. Lastly, the cooperation with another Italian HCC database, the EpaHCC project, allowed researchers to validate the performance of ITA.LI.CA model for the re-staging of patients after initial treatment, a topic often neglected but of utmost relevance given the potential to modify the therapeutic trajectory. This collaboration led to a “dedicated” ITA.LI.CA restaging model that includes among variables the response to treatment, the Model for End-Stage Liver Disease at restaging, and the actual amenability to additional (non-surgical) treatment [18].
The ITA.LI.CA prognostic system was also assessed in conjunction with treatment selection and survival outcome of HCC patients, “closing the circle” describing the utility of a staging system [21]. This analysis demonstrated that a “therapeutic hierarchy” (in term of efficacy) was maintained in each ITA.LI.CA stage, thus once more questioning the “stage-dictated” treatment of HCC patients and moving towards the “therapeutic hierarchy-dictated” approach that better accomplishes the application of Precision Medicine principles even in this oncologic setting [21,96,97].
4Use of the ITA.LI.CA database as a benchmark to evaluate the utility of treatment, and to guide drug policies decision-making processesThe function of a large database recording epidemiological, therapeutic and prognostic data can provide sound figures exploitable to assess the relative gain in life expectancy determined by procedures and drugs and, hence, to inform decisional processes. In this regard, the ITA.LI.CA database was analysed to know how much HCC affects patients’ life expectancy and how much the advancements in HCC management along the years have reduced its grim impact [6]. It was found that this cancer leads to an average of 11.5 years-of-life lost, and advancements in its management were able to reduce this loss from 12.6 years in 1986-1999, to 10.7 in 2000-2006, and to 7.4 years in 2007-2014.
Furthermore, the assessment of the natural history of 600 patients with untreated HCC provided the stage-specific figures of survival that can be used as benchmark to test the benefit achievable in each HCC stage by a treatment modality [98].
Lastly, as the therapeutic landscape of HCC is continuously expanding and currently includes effective but very expensive drugs with a certain toxicity, the ITA.LI.CA database was used to assess the potential eligibility in field-practice to treatment with checkpoint inhibitors, alone or in combination with other drugs, of patients with advanced HCC [99,100]. More in detail, applying the inclusion/exclusion criteria of registration studies, we assessed the amenability rate to nivolumab and pembrolizumab in both the first- and second-line setting, as well as the eligibility rate to the combination of atezolizumab and bevacizumab as front-line treatment [99–103]. All in all, the reported amenability rates to these novel treatments identified exploiting the ITA.LI.CA database can be used by National Drug Agencies and policy makers to inform decisions, and may serve the clinicians establish the yield of new drugs availability in clinical practice.
To conclude, this flight over the scientific production originating from the ITA.LI.CA database and its impact on the management of patients with HCC lends support to the utility of large registries collecting real-world data generated by multicentre co-operations as instruments that flank RCTs in fuelling the continuous improvement of patients care.
This article was read and approved by all the current coordinators of ITA.LI.CA centres (reported in alphabetic order): Francesco Azzaroli, Maurizia Rossana Brunetto, Calogero Cammà, Eugenio Caturelli, Maria Di Marco, Fabio Farinati, Francesco Giuseppe Foschi, Antonio Gasbarrini, Fabio Marra, Alberto Masotto, Andrea Mega, Gabriele Missale, Filomena Morisco, Gerardo Nardone, Fabio Piscaglia, Giovanni Raimondo, Gian Ludovico Rapaccini, Rodolfo Sacco, Angelo Sangiovanni, Gianluca Svegliati-Baroni, Gianpaolo Vidili, Marco Zoli.