With the advent of new therapeutic options for patients with hepatocellular carcinoma (HCC) for intermediate or advanced stages of the Barcelona Clinic Liver Cancer (BCLC), regional real-world data regarding prognostic survival factors are of significant importance.
Patients and MethodsA multicenter prospective cohort study was conducted in Latin America including BCLC B or C patients since 15th May 2018. We report here the second interim analysis focusing on prognostic variables and causes of treatment discontinuation. Cox proportional hazard survival analysis was performed, estimating hazard ratios (HR) and 95% confidence intervals (95% CI).
ResultsOverall, 390 patients were included, 55.1% and 44.9% were BCLC B and C at the time of study enrollment. Cirrhosis was present in 89.5% of the cohort. Among the BCLC-B group, 42.3% were treated with TACE with a median survival since the first session of 41.9 months. Liver decompensation before TACE was independently associated with increased mortality [HR 3.22 (CI 1.64;6.33); P<.001]. Systemic treatment was initiated in 48.2% of the cohort (n=188), with a median survival of 15.7 months. Of these, 48.9% presented first-line treatment discontinuation (44.4% tumor progression, 29.3% liver decompensation, 18.5% symptomatic deterioration, and 7.8% intolerance), and only 28.7% received second-line systemic treatments. Liver decompensation [HR 2.9 (1.64;5.29); P<.0001], and symptomatic progression [HR 3.9 (1.53;9.78); P=0.004] were independently associated with mortality after first-line systemic treatment discontinuation.
ConclusionsThe complexity of these patients, with one-third presenting liver decompensation after systemic therapies, underlines the need for multidisciplinary team management and the central role of hepatologists.
The Barcelona Clinic Liver Cancer staging (BCLC) has been adopted worldwide for more than two decades for the treatment-decision-making process of patients with hepatocellular carcinoma (HCC) [1,2]. The BCLC-B stage includes patients with preserved liver function, multinodular intrahepatic tumors without vascular invasion or extrahepatic spread, and the treatment recommendation is transarterial chemoembolization (TACE)[3]. Over the last 5 years, new systemic therapies for advanced-stage HCC have been approved [4–8].
More than eighty percent of HCC tumors occur in the setting of cirrhosis, and liver decompensation is the main competing risk for HCC-specific mortality. On the other hand, not every pattern of tumor progression is associated with the worst survival after systemic treatments [9]. In this complex scenario, the treatment-decision-making process may be different in the real-world setting when compared to clinical trials. Patient preferences, feasibility, and access to treatments are other important factors that may contribute to treatment decisions, referred to as treatment stage migration [9]. Nevertheless, adherence to the BCLC staging algorithm in daily practice has been associated with better survival [10].
Following the approval of new treatments for HCC, regional real-world data are of significant importance. Published data including BCLC B or C patients coming from Latin America are scanty, with survival rates lower than expected [11]. These results could have been explained due to barriers in access to appropriate timely treatments, eligibility criteria, or treatment management. For this reason, with the advent of new systemic treatment options, including immunotherapy that may be seen as a “more complex” treatment, we proposed to answer the following research question as the primary aim. What are the prognostic factors affecting survival in BCLC B or C patients following specific treatments in daily practice?
2Patients and Methods2.1Study design, participating centers, and eligibility criteriaThis was an observational multicenter prospective cohort study conducted in Argentina, Chile, Brazil, and Colombia, including HCC patients at BCLC B or C stages since May 15th, 2018. The results shown in this report correspond to a pre-specified second interim analysis on March 1st, 2022. The final study analysis is scheduled for 31st December 2023. The study protocol was written according to international recommendations for observational studies (STROBE guidelines) [12]. It complied with international ethical statements, and standards of Good Clinical Practice, requiring a signed informed consent and confidentiality agreement in all centers (CIE 18-078).
We included consecutive patients with clinical or histological diagnoses of HCC if all the following eligibility criteria were met:
- •
Adult patients (>17 years of age).
- •
BCLC B or C at study enrollment [2]. Patients could have been in other BCLC stages over their past medical history but at the moment of inclusion, they should be at BCLC B or C.
- •
Radiological, either with Computerized Axial Tomography (CT) or Magnetic Resonance Image (MRI), or histological diagnosis of HCC, according to international recommendations [13–15].
- •
With or without cirrhosis.
Patients were excluded if one of the following criteria were met:
- •
Other BCLC stages than B or C at study enrollment.
- •
Other malignant tumors were present.
All the demographic and exposure variables were included in all subjects on a web-based electronic Case Report Form designed for this study from the Latin American Liver Research Education and Awareness Network (LALREAN, https://www.temasis.com.ar/lalrean-org). The study variables included demographic data, and comorbid conditions such as systemic hypertension, diabetes mellitus, coronary heart, cerebral vascular, peripheral vascular, chronic pulmonary, or kidney diseases (creatinine clearance less than 30 ml/min). Data regarding liver disease at HCC diagnosis was registered including, etiology of liver disease, fibrosis stage (I-IV), prior or last upper endoscopy findings (presence or absence of gastro-esophageal varices or portal hypertensive gastropathy), and Child-Pugh score.
Data regarding patient characteristics, laboratory values, and tumor characteristics were registered at HCC diagnosis and reviewed before each treatment performed to re-assess the BCLC or clinical status on a longitudinal analysis. Laboratory values at HCC diagnosis, at each BCLC stage, and before each treatment included platelet count, total bilirubin, international normalized ratio (INR), serum albumin, and alpha-feto protein (AFP). The date of treatment initiation and definite suspension, number of locoregional therapies, and number of immunotherapy cycles, among other variables, were registered in all cases. The hepatoma arterial embolization prognostic score (HAP score) was registered before TACE [16].
2.3. Study end-points and statistical analysisWe longitudinally registered the following events throughout the follow-up since the date of each treatment initiation: treatment suspension, causes of the suspension including liver deterioration or decompensation (defined as worsening on Child-Pugh score equal or higher than 2 points), tumor progression, the pattern of tumor progression, intolerance due to adverse events, and symptomatic progression (worsening on ECOG performance status from 0-1 to 2 or higher).
Tumor progression or progressive disease (PD) was defined using the modified Response Evaluation Criteria In Solid Tumors (mRECIST) [17]. We shared a systematic mRECIST automatized calculator, assessed at each center. The patterns of progression were also registered to evaluate treatment decisions across centers [9]. Time to PD was registered from the date of each treatment initiation to the date of radiological progression. Adverse events following each treatment were registered following the CTCAE criteria (version 4) [17].
The sample size estimation was conducted for an expected mortality rate at 2 years of follow-up between 50% and 65% [11]. A two-tailed statistical value (log-rank test), alpha and beta errors of 5% and 20% (80% power) were included for sample size estimation. A minimum sample size per each BCLC stage of 170 patients (a total of 340 patients) was needed to present 146 primary events following the Freedman method estimation.
Time-event survival analysis was conducted with death as the primary or failure event (date of death) and censored observations in the absence of death registered at the last date of follow-up. Overall survival was defined from the date of each treatment initiation until death or censoring. Post-treatment discontinuation survival was defined as the survival period from the date of treatment discontinuation to death or the date of the last follow-up (censored). Kaplan Meier survival curves were compared using the log-rank test (Mantel-Cox), from the date of each treatment initiation to the failure event or date of censoring. A multivariable Cox proportional hazards regression analysis was conducted to identify independently associated variables with the main outcome, estimating hazard ratios (HR) and 95% confidence intervals (95% CI). Variables from the crude analysis showing P-values <.10 were further included in the multivariable model in a step-by-step process, evaluating confounding effect (change in HR estimates more than 20%). The proportional hazard assumption was evaluated through the statistical Schoenfeld residual test and graphically through distributions of these residuals and log-log curves. Data were analyzed with STATA 17.0 BE perpetual license (StataBE, Texas, USA).
2.4Ethical statementThe study protocol complied with international ethical statements, and standards of Good Clinical Practice, requiring a signed informed consent and confidentiality agreement in all centers (CIE 18-078). Patient consent statement: The study protocol required a signed informed consent (CIE 18-078).
3ResultsOf a total of 413 patients, 390 patients with intermediate (55.1%) and advanced (44.9%) stage HCC were enrolled. Twenty-three patients with BCLC stage A were excluded (Supplementary Fig. 1). Table 1 describes the characteristics of the included population. Of these, 79.2% were enrolled from Argentina, 12.0% from Colombia, and 6.9% from Brazil. The median follow-up was 13.1 months (IQR 4.5-28.4), with a median survival after HCC diagnosis of 27.2 months (Supplementary Fig. 2). The main associated etiology was chronic hepatitis C (36%), followed by non-alcoholic fatty liver (26%) and alcoholic liver disease (15%). Comorbid conditions were present in 50.8% (n=198) of the patients, including diabetes mellitus (n=134), non-active coronary heart disease (n=14), prior cerebral vascular disease (n=5), other peripheral vascular disease (n=2), chronic obstructive pulmonary disease (n=4), renal insufficiency (n=7), other malignancy (n=12), and other comorbid conditions (n=20).
Baseline patient and tumor characteristics (n=390).
| Variable | Value |
|---|---|
| Age, years (± SD) | 65 ± 10 |
| Male gender, n (%) | 295 (75.6) |
| Comorbidities, n (%) | 198 (50.8) |
| Cirrhosis, n (%) | 349 (89.5) |
| Etiology of liver disease, n (%) | |
| Hepatitis C | 134 (36.2) |
| Non-alcoholic fatty liver | 98 (26.5) |
| Alcoholism | 57 (14.4) |
| Cryptogenic | 39 (10.5) |
| Hepatitis B | 19 (5.1) |
| Under surveillance, n (%) | 101 (25.9) |
| BCLC B/C at study enrollment, n (%) | 215 (55.1)/175 (44.9) |
| *Characteristics at HCC diagnosis | |
| Median total bilirubin, mg/dL (IQR) | 1.1 (0.7–1.8) |
| Median serum albumin, g/dl (IQR) | 3.5 (3.0–3.9) |
| Median INR, (IQR) | 1.1 (1.0–1.3) |
| Mild ascites, n (%) | 75 (20.3) |
| Gastro-esophageal varices, n (%) | 72 (19.4)/106 (28.6) |
| ECOG 0-1, n (%) | 351 (90.7) |
| Median number of HCC nodules at diagnosis, (IQR) | 1 (1–3) |
| Median largest size of HCC nodules at diagnosis, cm (IQR) | 3.1 (0.8–5.4) |
| Presence of vascular tumor invasion at diagnosis, n (%) | 72 (18.5) |
| Presence of extrahepatic metastasis at diagnosis, n (%) | 42 (10.8) |
| BCLC at diagnosis, n (%) | |
| 0 | 9 (2.4) |
| A | 95 (25.3) |
| B | 138 (36.7) |
| C | 121 (32.2) |
| D | 13 (3.5) |
| Unknown | 14 (3.6) |
| Median AFP, ng/ml (IQR) | 27.3 (4.8-347) |
AFP: alfa-fetoprotein; BCLC: Barcelona Clinic Liver Cancer staging; HCC: hepatocarcinoma, INR: international normalized ratio; IQR: interquartile range.
Within patients enrolled in stage BCLC-B (n=215), the most common treatments performed were TACE (42.3%), systemic treatment (22.8%), percutaneous ablation or alcoholization (6.0%), radioembolization (5.6%), surgical resection (5%), and liver transplantation (3.2%). A minor group of patients did not receive HCC treatment at the time of study analysis (15.1%).
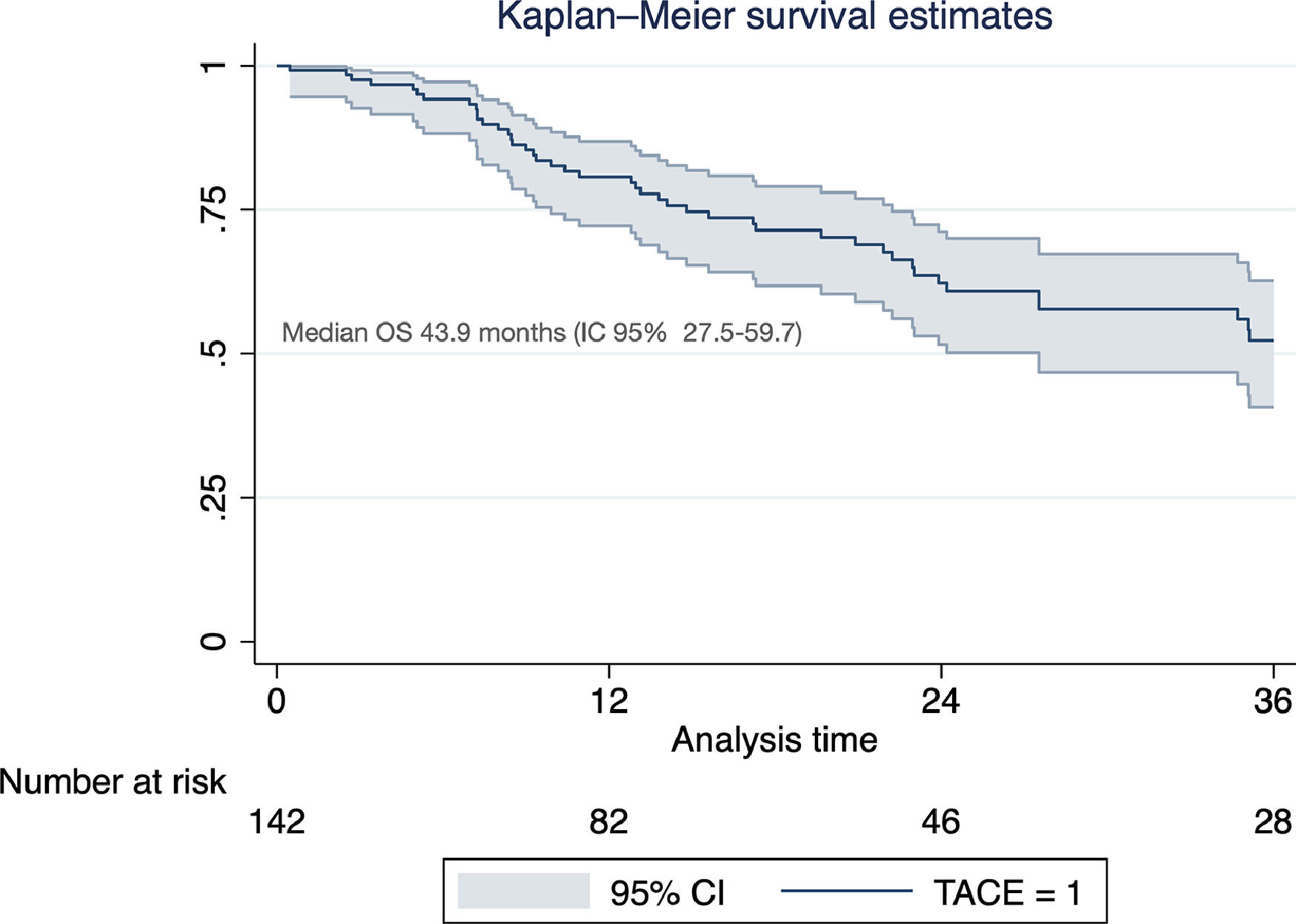
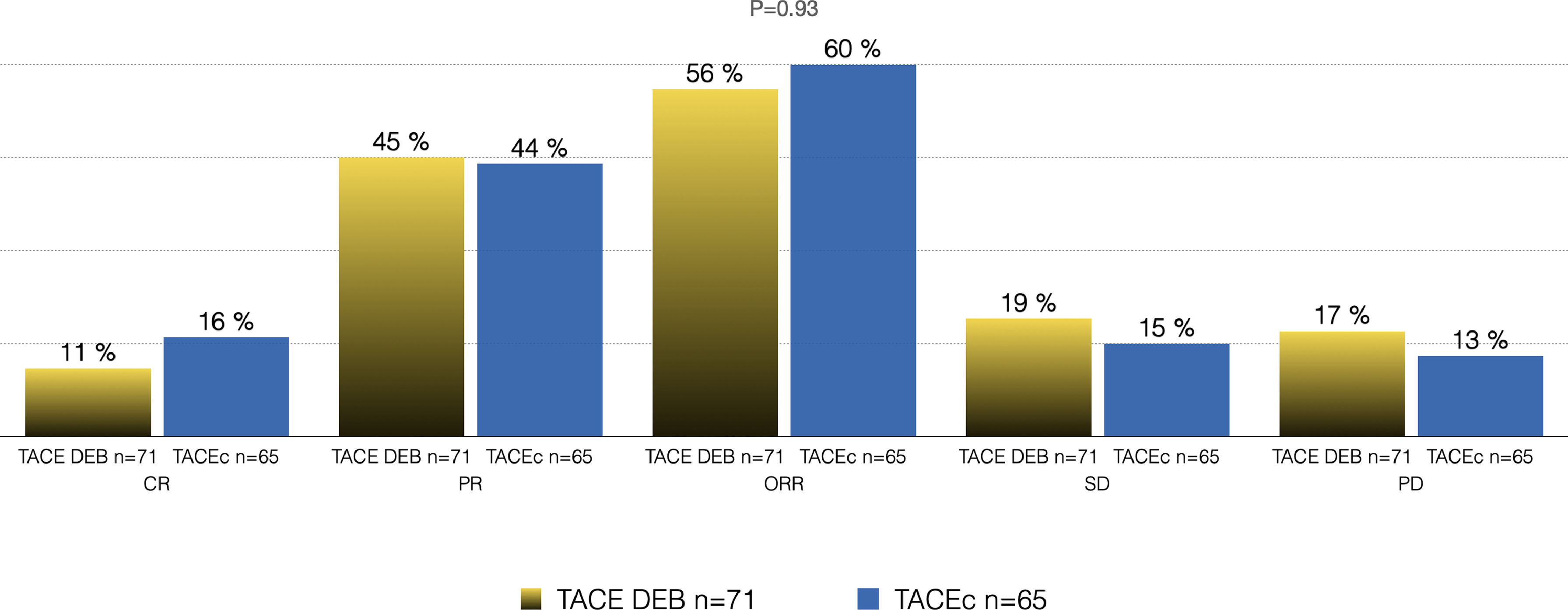
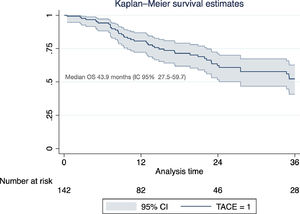
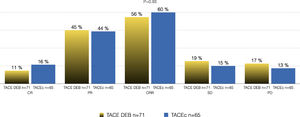
Of the total cohort, 142 patients were treated with TACE with a median number of 2 sessions (IQR 1-2) (Table 2). Conventional TACE was performed in 47.4%, with drug-eluting microspheres (DEB-TACE) in 51.8%, and 7% were treated with "bland" embolization (without chemoembolization). Pre-TACE BCLC stage was B in 63.4%, while 9.1% had a BCLC stage C (TACE was performed in 2 patients with invasion of the sub-segmental branch of the right portal vein, and 2 patients with lung disease). The HAP score distribution was A 38.7%, B 34.3%, C 20%, and D 7.1%. The median survival from the first TACE was 43.9 months (95% CI 27.5-59.7) (Fig. 1). Fig. 2 describes the radiological response by mRECIST after the first TACE session according to the conventional modality or DEB-TACE. Patients presenting objective response rate (either partial or complete response) after the first TACE session showed no significant better OS compared to the group of patients with stable disease or progressive disease [HR 0.57 (95% CI 0.32–1.03); P=0.06].
Characteristics before chemoembolization (TACE).
| VARIABLE | TACE n=142 (36.4%) |
|---|---|
| Age, years (± SD) | 66 ± 9 |
| Median total bilirubin, mg/dL (IQR) | 1.0 (0.7–1.5) |
| Median albumin, g/dl (IQR) | 3.5 (2.9–3.9) |
| Median INR, (IQR) | 1.1 (1.0–1.3) |
| Mild ascites, n (%) | 22 (15.5) |
| Gastro-esophageal varices small/large, n (%) | 72 (19.4)/106 (28.6) |
| ECOG 0-1, n (%) | 137 (96.5) |
| Median number of HCC nodules, (IQR) | 2 (1–2) |
| Median target lesion diameter, cm (IQR) | 4.5 (3.3–6.0) |
| Median AFP, ng/ml (IQR) | 11.6 (4.3–139.0) |
| BCLC pre TACE, n (%) | |
| 0/A | 38 (26.7) |
| B | 90 (63.4) |
| C | 10 (9.1) |
| HAP score pre TACE, n (%) | |
| A | 29 (20.7) |
| B | 59 (42.1) |
| C | 33 (23.6) |
| D | 19 (13.6) |
AFP: alfa-fetoprotein; BCLC: Barcelona Clinic Liver Cancer staging; HCC: hepatocarcinoma, INR: international normalized ratio; IQR: interquartile range.
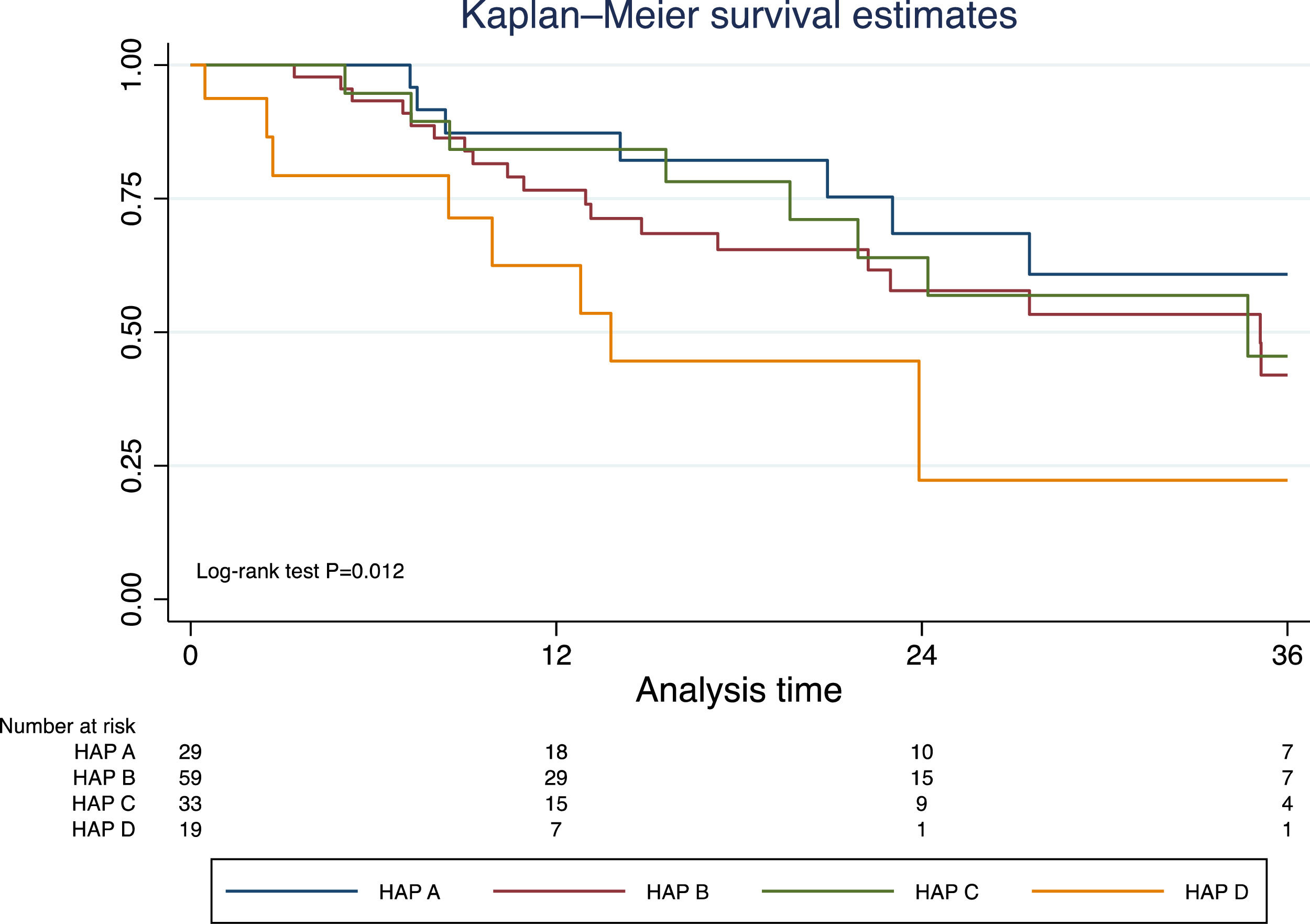
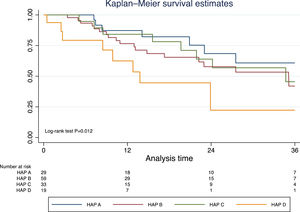
Excluding those who received TACE before liver transplantation (n=17), the median survival was 41.9 months (95% CI 30.3–50.2). In this group of patients, survival was significantly lower in those with a HAP D score (Fig. 3). In a prognostic model of patients who were treated with TACE, the only pre-TACE variable independently associated with increased mortality was the presence of decompensated cirrhosis [HR 3.22 (95% CI 1.64;6.33); P<.001], adjusted for ECOG performance status and HAP score (Table 3).
Prognostic baseline pre-TACE variables. Cox regression analysis.
| Variable | Crude HR | P | Adjusted HR | P |
|---|---|---|---|---|
| (95% CI) | (95% CI) | |||
| Age, years | 0.98 (0.96-1.01) | 0.95 | ||
| Male gender | 0.84 (0.46-1.52) | 0.56 | ||
| ECOG 2 vs 0-1, | 2.81 (1.00-7.89) | 0.049 | 2.02 (0.70-5.82) | 0.19 |
| Number of nodules | 0.95 (0.78-1.16) | 0.61 | ||
| Total bilirubin, mg/dL | 1.30 (1.07-1.59) | 0.009 | ||
| Albumin, gr/dl | 0.88 (0.75-1.06) | 0.18 | ||
| Target lesion diameter, cm | 1.00 (0.99-1.02) | 0.13 | ||
| Liver decompensation* | ||||
| No (n=103) | Ref | - | Ref | - |
| Yes (n=22) | 3.71 (1.95-7.07) | <.0001 | 3.22 (1.64-6.33) | 0.001 |
| AFP, ng/ml | 1.01 (1.01-1.02) | 0.001 | ||
| HAP score, n (%) | ||||
| A | Ref | - | Ref | - |
| B | 1.78 (0.86-3.65) | 0.12 | 1.41 (0.66-2.98) | 0.37 |
| C | 0.94 (0.40-2.17) | 0.88 | 1.19 (0.49-2.87) | 0.70 |
| D | 3.62 (1.41-9.31) | 0.007 | 2.08 (0.75-5.76) | 0.16 |
| Number of TACEs | 0.95 (0.78-1.16) | 0.61 |
Note: Analysis excluding 17 treated patients with TACE as a bridge before liver transplantation.
AFP: alfa-fetoprotein, HCC: hepatocellular carcinoma; HR: hazard ratio; TACE: trans-arterial chemoembolization.
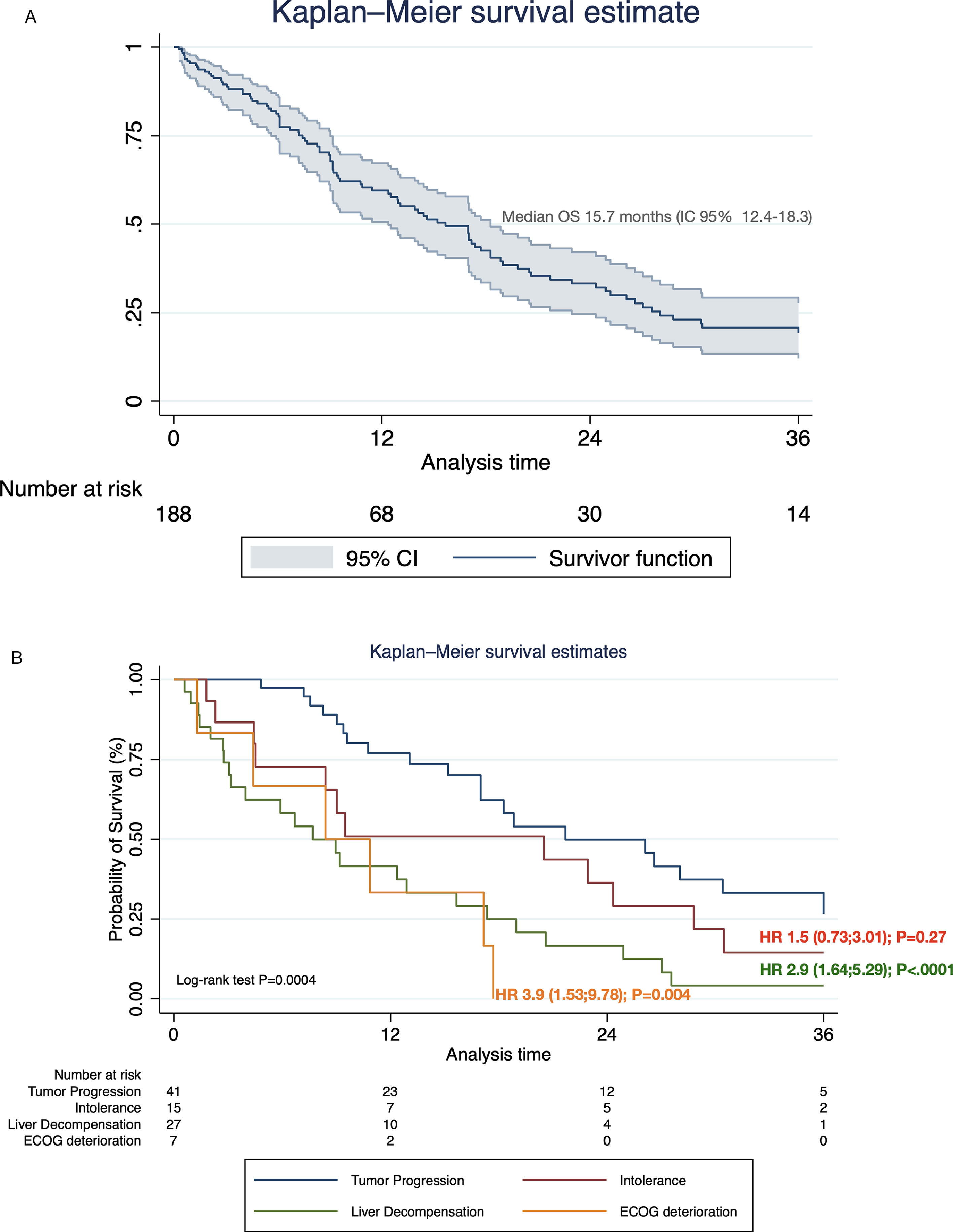
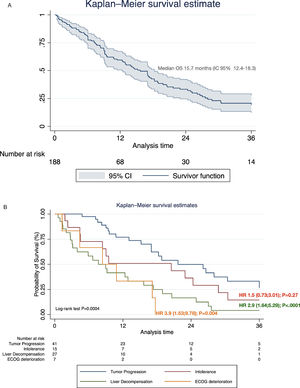
Systemic treatment was initiated in 48.2% of the cohort (n=188) (Table 4). Of these, 28.7% and 3.2% continued with second and third-line systemic treatments, respectively. The proportion of pharmacological agents used in different therapeutic lines is shown in Supplementary Table 1. The median survival after initiating first-line systemic therapy was 15.7 months (95% CI 12.4–18.3) (Fig. 4A). In regard with HAP score before TACE, among HAP A group, 62.1% continued with systemic therapy upon tumor progression, whereas 42.4%, 42.2% and 42.1% of HAP scores B, C, and D could initiate systemic treatments (P=0.32).
BCLC stages before initiation of systemic therapies (n=188).
| Variable | Global n=188 (48.2%) | BCLC-B | BCLC-C |
|---|---|---|---|
| Sorafenib, n (%) | 140 (74.5) | 37 (26.4) | 100 (71.4) |
| Atezolizumab + bevacizumab, n (%) | 33 (17.5) | 6 (18.2) | 27 (81.8) |
| Lenvatinib, n (%) | 23 (12.2) | 6 (26.1) | 17 (73.9) |
| Regorafenib, n (%) | 22 (11.7) | 4 (18.2) | 18 (81.8) |
| Nivolumab, n (%) | 4 (2.1) | 3 (75.0) | 1 (25.0) |
| Pembrolizumab, n (%) | 7 (3.7) | 7 (100) | |
| Cabozantinib | 3 (1.0) | 3 (100) | |
| Ramucirumab, n (%) | 1 (0.5) | 1 (100) | |
| Other, n (%)* | 4 (2.1) | 4 (100) |
Notes: *Olaparib, abnova viscum.
Among the patients who received sorafenib (n=140), 70.9% started with 800 mg per day, 87.1% reached that dose during follow-up, and 41.7% required dose reduction due to adverse events. Grades I-II adverse events were observed in 65.7% of the patients (n=51), and grades III or higher in 9.3% (n=13). The most commonly reported adverse events were fatigue (40%), diarrhea (32.9%), hand-foot skin syndrome (25%), and rash (16.4%). Median survival after initiation of sorafenib was 9.2 months (95% CI 7.6–13.1). In patients receiving sorafenib-regorafenib sequencing therapies (n=22), the median duration of regorafenib treatment was 4.9 months with a median survival since sorafenib of 21.7 months (95% CI 17.0–28.1).
Patients treated with atezolizumab and bevacizumab (n=33), received a median number of cycles of 5 (IQR 3-9) for both components, and 36.4% had a cycle or one component interruption. Adverse events of grades I-II and III or higher were observed in 69.7% and 15.1%, respectively. The most frequent adverse events were arterial hypertension (9.1%) and proteinuria (6.1%). The incidence of immune-mediated adverse events was 9.1%, and 6.1% were registered as serious adverse events (1 patient with HCV-associated purpura vasculitis, 1 patient with thyrotoxicosis, and 1 patient with nephritis). Median survival following atezolizumab and bevacizumab initiation was 17.2 months (95% CI 12.9-Not Reached).
Twenty-three patients received lenvatinib, 87% developed at least one adverse event, and 17.4% grade III or higher. The most frequently reported adverse events were fatigue (56.5%), arterial hypertension (34.8%), and diarrhea (26.1%). The most common starting dose was 12 mg every day in 63.6%, and 34.8% required dose reduction. The median survival since lenvatinib initiation was 9.0 months (95% CI 4.0-16.0).
3.3. Causes of systemic treatment discontinuation and impact on survivalOf the total number of patients who started first-line systemic therapy with sorafenib, lenvatinib, or atezolizumab-bevacizumab, 48.9% (n=92) had definitively discontinuation. Of these, 44.5% (n=41) discontinuation was due to PD, 29.3% (n=27) due to liver decompensation, 18.5% (n=17) to intolerance, and 7.6% (n=7) symptomatic progression. Among these, considering PD as a reference value, liver decompensation [HR 2.9 (95% CI 1.64;5.29); P<.0001], and symptomatic progression [HR 3.9 (95% CI 1.53;9.78); P=0.004], were associated with increased mortality after treatment discontinuation (Fig. 4B).
4DiscussionIn this multicenter prospective cohort study from South America, including patients at intermediate or advanced BCLC stages, the median survival since treatment initiation is consistent with international standards. Median survival from the first TACE was greater than 3 years. Although the HAP score could not adequately stratify the risk of post-TACE mortality, the HAP D group might not benefit from such treatment. Likewise, both in the intermediate and advanced stages, the presence of liver decompensation, whether at baseline before TACE or following systemic therapies, was independently associated with a worse prognosis. Median survival after systemic treatment was comparable to those reported in other cohorts and may even be comparable to those reported in clinical trials [3–8]. From a regional perspective, these outcomes are outstanding.
The BCLC staging is accepted worldwide for therapeutic decision-making [1,2]. This staging has been modified over the last decades, being the one we have used for this study the last updated version before the year 2018 [2]. The last 2022 updated version, for temporality reasons, was not included in this study [18].
In recent years we have seen great progress in the systemic treatment for HCC. We anchored as a comparison a historical cohort from Argentina, observing that the median survival since sorafenib initiation was much lower than expected [11]. With the advent of sorafenib-regorafenib sequencing therapy, and later on other treatment options [5–8], we evaluated real-life outcomes in a new prospective cohort. We tried to assess whether the results would be different from that historical cohort, systematizing data recording, decision making, and outcome evaluation. This second interim analysis confirms that these results have been improved, not only by the inclusion of new therapeutic options but also by better management of these patients.
Indeed, this second interim analysis has been carried out after a brief period of regional approval of atezolizumab and bevacizumab [8], underlying that only one out of every 3 treated patients could be treated with second or third-line systemic therapies. Moreover, the median survival in patients who received TACE was higher than that previously reported in the historical cohort [11], reaching recommended standards [19].
In patients at intermediate and advanced stages, radiological tumor progression does not always impact survival. In this regard, it has been shown that not all patterns of PD impacts survival [9]. Although in this interim analysis we did not report the effect on the survival of patterns of PD, we hope to have a sufficient number of events per each pattern of PD for the final analysis. However, we observed that liver decompensation, whether before TACE or after systemic therapies, is an independent event associated with a worse prognosis.
There is little information in the region about the daily management of patients in intermediate and advanced stages. It is interesting to evaluate the time to radiological progression, the type of progression, and the access to the different treatments. This is why the report of this study is relevant not only in the regional context but also worldwide, after the advancement of new health technologies. On the other hand, improving access to such novel treatments should be a global perspective [10]. Finally, there have been recent international cohort studies evaluating post-progression survival following immune checkpoint inhibitors [20]. These reports were focused on radiological patterns of tumor progression and their prognostic role in post-PD. Interestingly, not all patterns of tumor progression defined treatment discontinuation. One out of four patients with tumor progression continued immune checkpoint inhibitors beyond progression [20]. However, contrary to sorafenib [9], in that cohort study, intrahepatic growth was an independent prognostic factor for post-progression survival [20]. In our interim analysis, we did not focus on patterns of progression due to a low number of events per stratum. We showed that liver decompensation frequently occurs and it is one of the worst prognostic factors of post-treatment discontinuation [21].
This study has limitations common to all observational studies. On the one hand, the inclusion of patients was not strictly centrally regulated, which is why we had to exclude patients at the time of analysis. Second, the radiological response and the outcome of PD were not centralized due to logistical issues. However, we applied known objective criteria (mRECIST), assessed at each center through an automatable file. Third, the median follow-up was relatively short, but it must be taken into account that this analysis was pre-specified in the protocol and, on the other hand, we are dealing with patients with a life expectancy fewer than two years. Finally, we did not register delays in authorizations by providers/payers that impacted the evolution of these patients. This could have enriched the study.
5ConclusionsIn conclusion, liver decompensation before chemoembolization identifies a subgroup of patients with a poor prognosis, who should be excluded from sequential therapies and systemic treatments. On the other hand, clinical progression including liver decompensation or symptomatic performance status deterioration, are determinant events for systemic treatment suspension. Indeed, 1 out of 3 patients progressed to liver decompensation and only a third accessed second-line systemic therapies. As a consequence, it is of crucial importance to avoid any treatment option promoting liver decompensation, such as unnecessary over repetitive TACE procedures in patients without objective response, or in those presenting with high tumor burden. On the other hand, definite withdrawal of systemic therapies must be done in case of decompensation, such as new development of jaundice or ascites. The complexity in the management of these patients underlines the need for multidisciplinary management and the central role of hepatologists.
Data availability statementThe data shown in this article are available from the corresponding authors upon a reasonable request.
FundingThis research received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
We would like to thank all other the co-authors who participated in this study. All the authors approved the final version of the manuscript.