Abstracts of the 2024 Annual Meeting of the ALEH
Más datosNo
Introduction and ObjectivesClinical trials evaluating the efficacy of first line systemic immune therapies for patients with advanced hepatocellular carcinoma (HCC) have recruited a lower proportion of patients with cirrhosis. In this group of patients, immune related adverse events (irAEs) may lead to decreased prognostic outcomes. The aim of this study was describe the incidence rate of irAEs and its impact on survival.
Patients / Materials and MethodsA multicenter prospective Latin-American cohort study was conducted including HCC patients who received A+B since its regional approval, either as first or sub-sequent systemic lines, to March 15, 2024. Overall survival since A+B, and survival since date of irAE was compared between patients developing and not developing irAEs (date since A+B), through Cox proportional hazard analysis (Harrell's c-index).
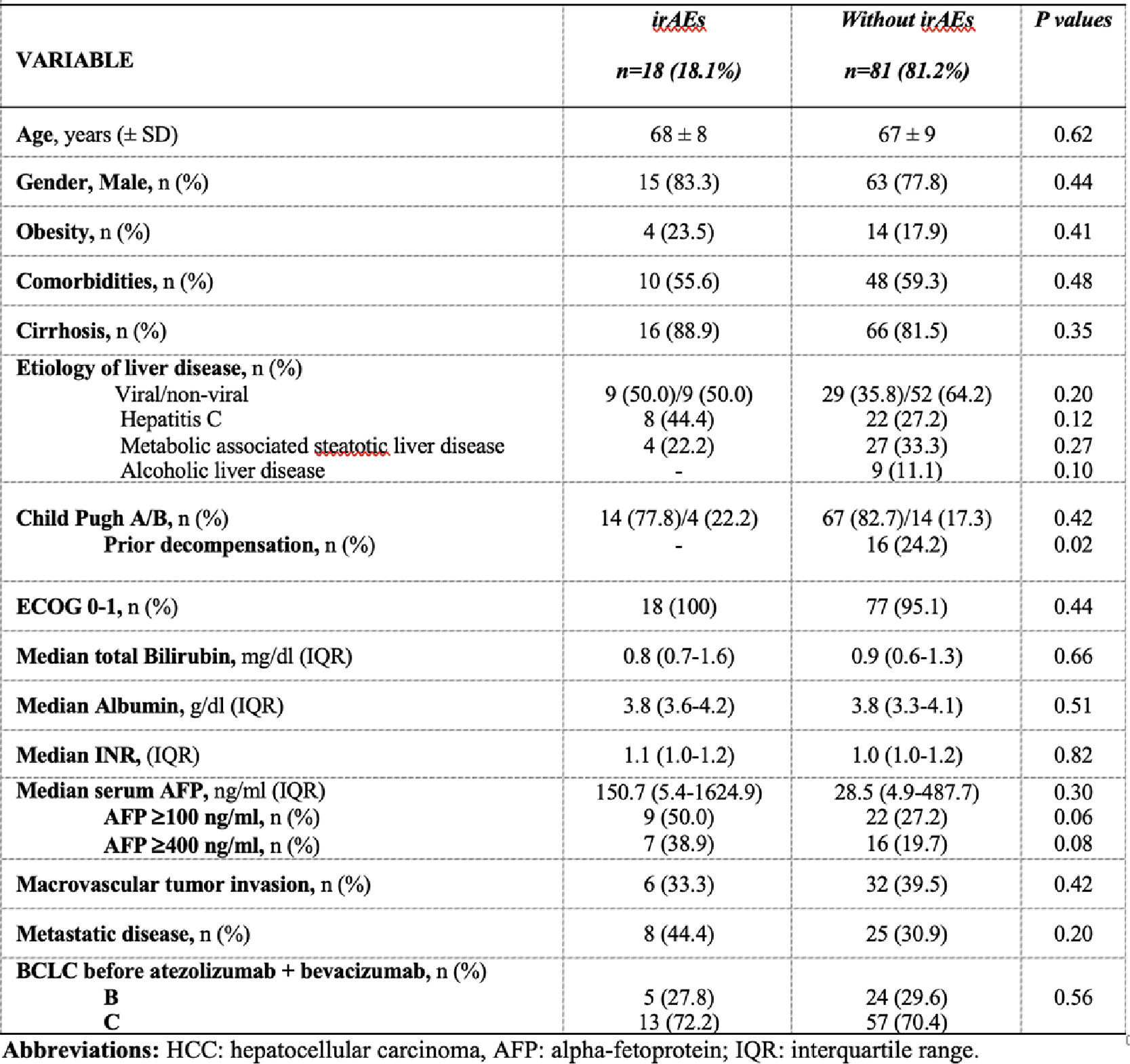
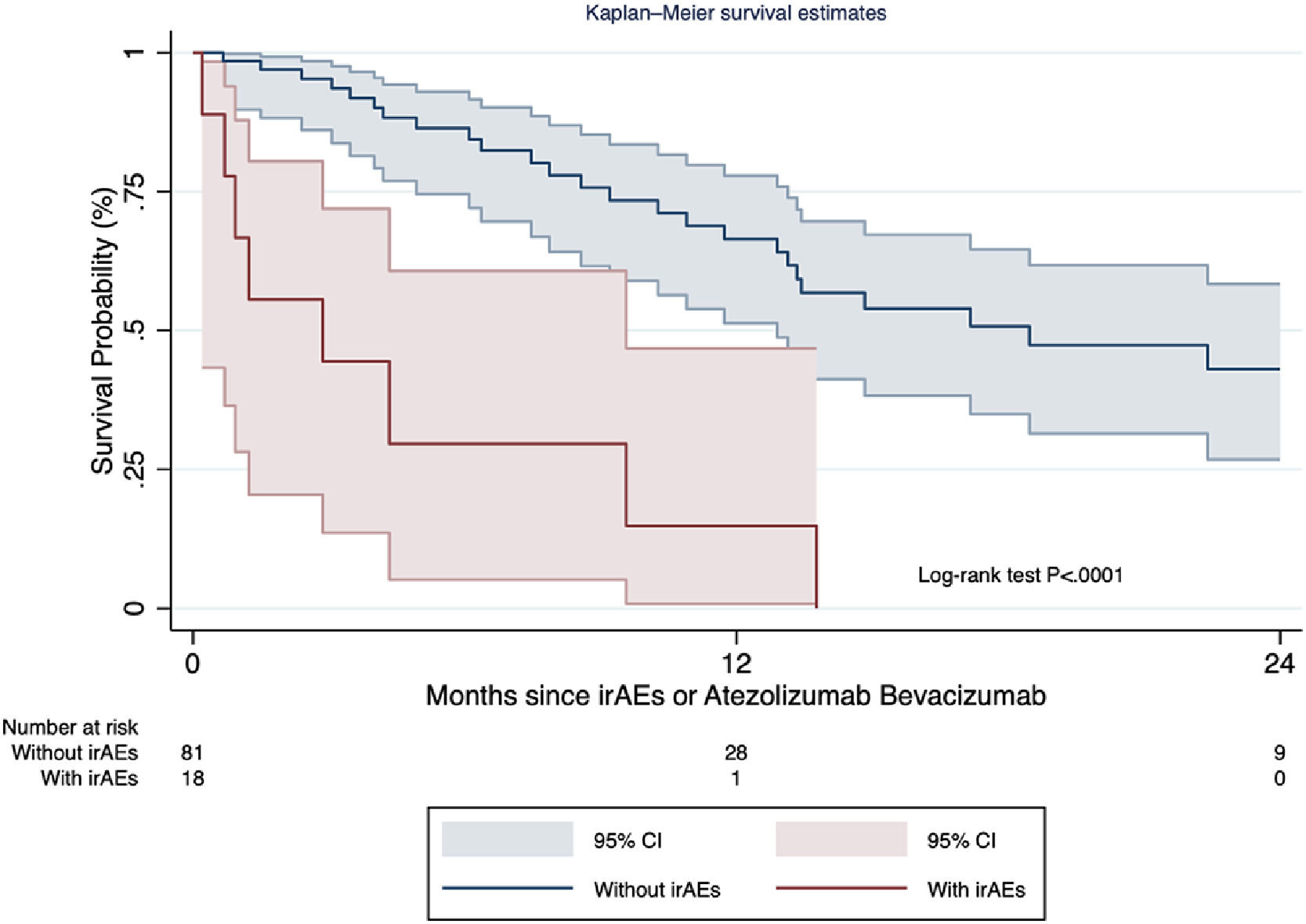
Results and DiscussionOverall, 99 patients treated with A+B were included (n=8 received it as second line post sorafenib), 82.3% presented cirrhosis. The median treatment duration was 6 months [number of cycles 5 (range 3-11.5)], with a median overall survival of 17.0 months (range 12.6-19.8). Over a median follow-up of 7.7 months (range 4.5-17.2), the irAE incidence rate was 2.1 cases per 100 persons-months [cumulative incidence 18.1% (95% CI 11.1-27.2%); n=18]. Median time to irAE was 2.3 months (range 1.4-4.8), most frequently hepatitis (n=6), thyroiditis (n=5), and 8/18 required steroids (Table). Follow-up and treatment duration times were similar regardless irAEs occurrence. On multivariable Cox regression model, AFP values before A+B >400ng/ml [HR 2.9 (95% CI 1.1-7.6)], adjusted for HCC diffuse intrahepatic pattern was associated with irAE development (c-statistic 0.66). Patients developing irAEs presented decreased overall and post-irAE survival [median 2.9 months vs 18.5 months; HR 6.2 (95% CI 2.7-14.2); P<.0001] (Figure).
ConclusionsCautions management in patients with irAEs is of relevant importance in our region, highlighting the role of onco-hepatologists in the clinical-decision making process of these patients.