South America is one of the regions with the highest rates of non-alcoholic fatty liver disease (NAFLD). This study aimed to assess the prevalence and severity of NAFLD in suburban Argentina.
Patients and MethodsThe study involved a general community cohort of 993 subjects evaluated sequentially with a comprehensive lifestyle questionnaire, laboratory testing, abdominal ultrasound (US) and transient elastography with XL probe. NAFLD was diagnosed according to standard criteria.
ResultsThe prevalence of NAFLD by the US was 37.2% (326/875) overall, 50.3% in subjects with overweight/obesity, 58.6% with hypertriglyceridemia, 62.3% with diabetes/hyperglycemia and 72.1% with all three risk factors. Male gender (OR 1.42, 95% CI 1.03–1.47, p = 0.029), age (50–59 years: OR 1.98, 95 CI 1.16–3.39, p = 0.013 and ≥60 years: OR 1.86, 95% CI 1.13–3.09, p = 0.015), BMI (25–29: OR 2.87, 95% CI 1.86–4.51, p<0.001 and ≥30: OR 9.57, 95% CI 6.14–15.20, p<0.001), diabetes/hyperglycemia (OR 1.65, 95% CI 1.05–2.61, p = 0.029) and hypertriglyceridemia (OR 1.73, 95% CI 1.20–2.48, p = 0.002) were independent predictors of NAFLD. Among patients with steatosis, 22.2% (69/311) had ≥F2 fibrosis (overweight 25%, hypertriglyceridemia 32%, diabetes/hyperglycemia 34%). BMI (OR 5.22, 95% CI 2.64–11.74, p<0.001), diabetes/hyperglycemia (OR 2.12, 95% CI 1.05–4.29, p = 0.04) and hypertriglyceridemia (OR 1.94, 95% CI 1.03–3.68, p = 0.040) were independent predictors of liver fibrosis.
ConclusionsThis general population study from Argentina showed a high prevalence of NAFLD. Significant liver fibrosis was present in 22% of subjects with NAFLD. This information adds to the existing knowledge of NAFLD epidemiology in Latin America.
The definition of non-alcoholic fatty liver disease (NAFLD) includes the demonstration of hepatic steatosis and the exclusion of both secondary causes of hepatic fat accumulation and significant alcohol consumption [1,2]. Major risk factors for NAFLD are obesity, type 2 diabetes mellitus (T2DM), hypertension and hyperlipidemia [1–3]. For this reason, NAFLD is considered the hepatic manifestation of metabolic syndrome [1,2,4]. NAFLD is today the most common chronic liver disease worldwide [3,5]. A meta-analysis based on community surveys from 22 countries using abdominal ultrasound (US) estimated the global prevalence of NAFLD in 2016 at around 25% [6]. The two highest regional prevalences were in the Middle East (31.8%) and South America (30.5%). More recently, three systematic reviews and meta-analyses showed that the overall prevalence of NAFLD worldwide is considerably higher than previously estimated (>30%) and continues to increase [7,8,9]. Reported figures for South America in these studies were 34.5% [7] and 35.7% [8]. The prevalence of NAFLD in Argentina is unknown.
O'Brien is a small town of 2488 inhabitants located in Buenos Aires Province, Argentina (Argentina, I. N. D. E. C. “Censo Nacional de Población, Hogares y Viviendas 2010. The goal of this population-based study was to assess the prevalence, risk factors and severity of NAFLD in subjects from O'Brien.” [10].
2Patients and MethodsFrom September 2019 to March 2020, a cross-sectional population study was conducted in the city of General O'Brien (Bragado County) located 267 Km west of Buenos Aires. All investigations were performed at Unidad Sanitaria “Martín Espinel Bavio”, a public primary care facility and the only medical institution in O'Brien. The first part of the study was completed between September and November 2019. Volunteer subjects aged above 12 years were requested to present after an overnight fast of at least 8 h following a predefined schedule. All participants completed in advance a comprehensive lifestyle questionnaire that gathered information on demographics, medical history (T2DM, hypertension, overweight), physical activity, alcohol consumption and medications. The patients themselves answered the questionnaire, except for their alcoholic history which was reassessed by hepatologists at the time of performing the US. Alcohol intake was estimated according to the type and quantity of beverages consumed on a daily basis. Individuals with significant alcohol consumption (>30 gr/day for males and >20 gr/day for females) were excluded from the study. Physical activity was included in the questionnaire only as a Yes/No question and whether participants exercised more than 2 times a week. No objective physical exercise parameters were registered. Venous blood samples were collected to determine fasting glucose, total cholesterol, triglycerides, aspartate aminotransferase, alanine aminotransferase (ALT), alkaline phosphatase and gamma-glutamyltransferase. All laboratory tests were measured by standard techniques utilizing an automated chemistry analyzer (Metrolab 2100, Wiener lab). Hypercholesterolemia was defined as serum levels >200 mg/dL, hypertriglyceridemia >150 mg/dL and hyperglycemia when fasting glucose was >110 mg/dL. When investigating predictors of NAFLD and liver fibrosis, patients with a history of T2DM and those found to have hyperglycemia were grouped. The upper limit of normal for AST was 38 IU/L, ALT 41 IU/L, alkaline phosphatase 400 IU/L and gamma-glutamyltransferase (GGT) 18 IU/l for females and 28 IU/l for males. Elevated liver enzymes were defined as values above these limits. All subjects tested negative for HBsAg and anti-HCV. Drug-induced liver injury was investigated indirectly through the questionnaire that included the list of all medications taken by participants. Other etiologies of liver disease such as autoimmune disorders or iron overload were not investigated in this study.
Immediately after the collection of blood samples anthropometric measurements including height, weight (precision scale) and body mass index (BMI) measured as body weight divided by the square of height in meters were obtained by accredited nurses. BMI between 25 and 29 Kg/m2 was considered overweight and ≥30 Kg/m2 was obesity.
US studies were performed during a 4-day period in December 2019 by a group of seven experienced radiologists who were unaware of the clinical and laboratory data. All participants had a prescheduled time appointment and were requested to have an 8-hour fasting. Instruments used were three MediSono P3, 2 Samsung Medison U6 and 2 Mindray DP-50 portable US scanners with 3.5 MHz convex transducers. The diagnosis of steatosis relied on the combination of increased parenchymal echogenicity, when compared to spleen and kidneys, blurring of hepatic vessels and diaphragm and deep attenuation of the US signal. According to the intensity of these abnormalities, steatosis was subjectively scored as mild, moderate or severe [11].
The final step of the study was simultaneous noninvasive assessment of hepatic fibrosis and steatosis by vibration-controlled transient elastography (VCTE) obtained only in patients diagnosed with fatty liver disease by US. Liver stiffness measurements (LSM) and controlled attenuation parameter (CAP) were evaluated over a 3-day period in March 2020 utilizing two identical equipment with extra-large (XL) probe (Echosens, Paris). VCTE was performed on the right lobe of the liver through intercostal spaces. Reliable LSM was defined as 10 valid shots. The two operators performing VCTE were blinded to clinical and laboratory data. Whenever an unreliable result was obtained the study was repeated by the second operator and considered valid only when ten successful acquisitions were evaluated. Stage of fibrosis by VCTE was selected according to the study reported by Eddowes et al. that included 404 patients using biopsy analysis as a reference standard for NAFLD. Cutoff values were 8.2, 9.7 and 13.6 kPa for F ≥ F2, F ≥ F3 and F4 respectively [12]. The study did not include serum markers or scores for non-invasive assessment of liver fibrosis.
2.1Statistical analysisCategorical data are expressed as frequencies and percentages and continuous data as means and standard deviation for normally distributed variables or as medians and interquartile ranges (IQR) otherwise. Normal assumption was assessed using the Shapiro-Wilk test. Groups were compared using the Chi2 test or Fisher's exact test as appropriate for categorical data and with Student's T test or ANOVA for normally continuous variables and Mann-Whitney U test or Kruskal-Wallis test for no normal variables. Independent predictors of steatosis and fibrosis were assessed using multivariate logistic regression models with the events (steatosis and fibrosis) as dependent variables. The Akaike information criterion was used to select the most parsimonious and informative model. All are two tailed and a p-value <0.05 was considered statistically significant. Statistical analyses were conducted using software R, version 4.0.1 for IOS (R Foundation for Statistical Computing, Vienna, Austria).
2.2Ethical statementWritten informed consent was obtained from each patient included in the study. Parents consented to the participation of children under 18 years of age. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in prior approval by the Bragado Secretary of Health of the Bragado Committee.
3ResultsThe number of participants enrolled was 993, after excluding 77 subjects (7.2%) due to alcohol consumption above the pre-established limits. The Median (IQR) age was 44 (29–59) years with 10% of participants <18 years. There were 595 females (59.9%) and 398 males with a median (IQR) BMI of 26.9 (23.1–30.9) Kg/m2.
3.1Metabolic risk factors in the general population cohortMedical history, as self-reported in the questionnaire, included T2DM in 78 subjects (7.9%), hypertension in 194 (19.5%), dyslipidemia in 360 (36.2%) and overweight in 316 (31.8%). Among the 535 participants who referred to some level of physical activity, 452 (84%) exercised at least two times a week. Laboratory examination showed fasting hyperglycemia in 113 (11.4%), hypercholesterolemia in 490 (49.3%) and hypertriglyceridemia in 258 (26%). Overall, a history of DM was recorded in 7.9% of participants, hyperglycemia in 11.4% and either one in 14.8%. Liver test abnormalities were infrequent with increased alkaline phosphatase in 63 subjects (6.3%), gamma-glutamyltransferase in 27 (2.7%) and ALT in 117 (11.8%). Most individuals with elevated alkaline phosphatase values were adolescents with normal levels of gamma-glutamyltransferase. Among subjects with elevated ALT, median values were 53 (45–66) IU/L.
Anthropometric measurements revealed overweight (BMI ≥25) in 629/993 (63.3%) participants of which 295 (29.7%) were obese (BMI ≥30). Metabolic risk factors and overweight/obesity in the general population increased significantly with age (Table 1). In contrast, the proportion of individuals who referred physical activity decreased significantly with advancing age.
Medical comorbidities, anthropometrics and laboratory tests variables.
BMI: body mass index (Kg/m2); ALT: alanine aminotransferase.
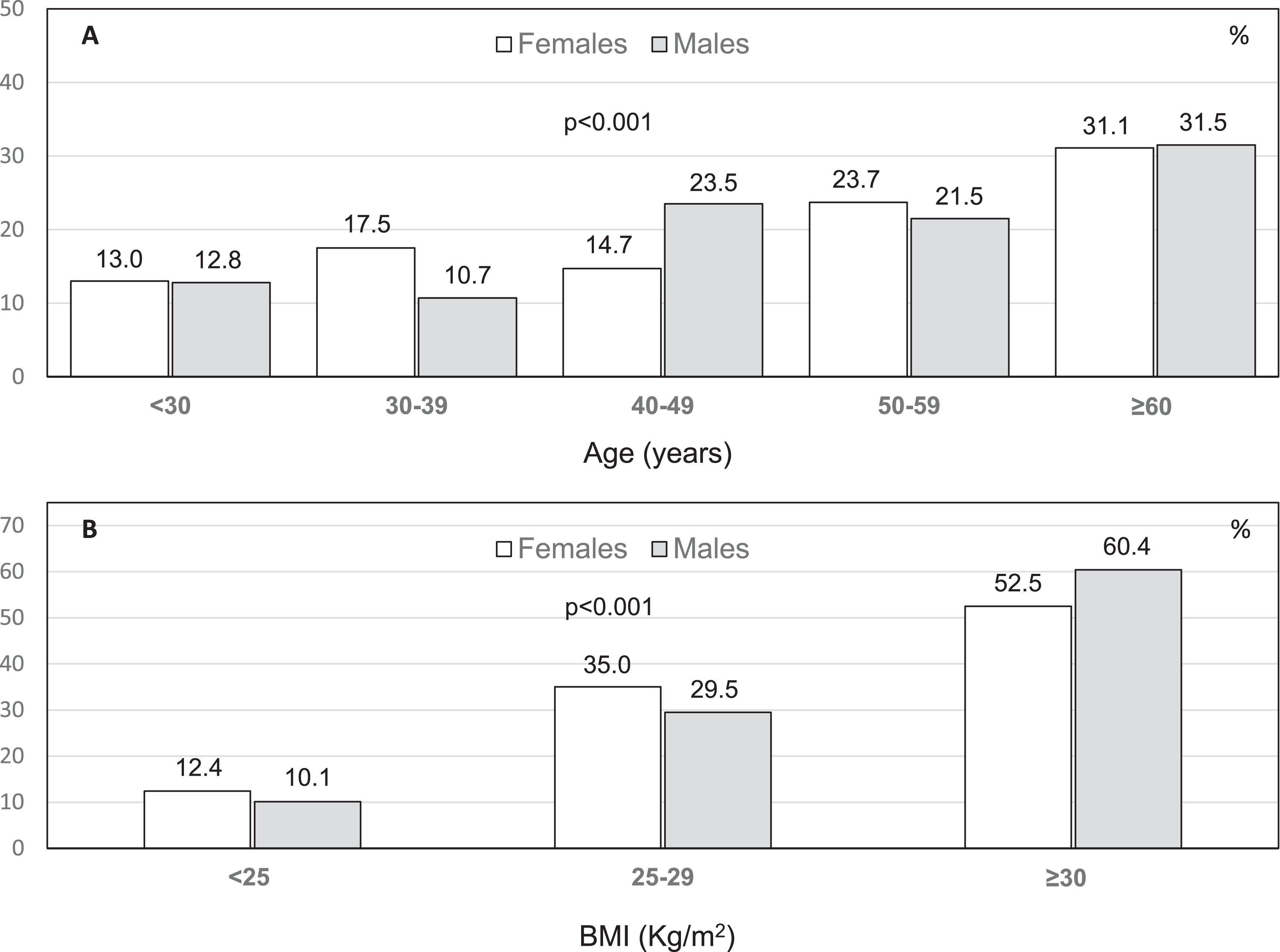
Abdominal US was obtained in 875/993 (89.9%) subjects enrolled and showed hepatic steatosis in 326 (37.2%). The amount of fat was considered as mild in 183 (56%), moderate in 107 (33%) and severe in 36 (11%). The prevalence of steatosis increased progressively with age and BMI, both in females and males (Fig. 1).
Significant risk factors for NAFLD by univariate analysis were male gender, age, history of hypertension, overweight/obesity, no physical activity, T2DM/hyperglycemia, elevated ALT, hypercholesterolemia and hypertriglyceridemia (Table 2).
Risk factors for non-alcoholic fatty liver disease by univariate analysis.
BMI: body mass index; ALT: alanine aminotransferase.
Male gender, age, BMI, diabetes/hyperglycemia and hypertriglyceridemia were independent predictors of NAFLD (Table 3).
Risk factors for non-alcoholic fatty liver disease by multivariate analysis.
BMI: body mass index; ALT: alanine aminotransferase.
The prevalence of NAFLD by US was 50.3% (288/572) in patients with overweight/obesity, 58.6% (137/234) in patients with hypertriglyceridemia and 62.3% (81/130) in those with T2DM/hyperglycemia and increased significantly (p<0.001) according to the presence of one (38.4%), two (59.9%) or three (72.1%) of these risk factors respectively. The prevalence of NAFLD was only 6.3% in individuals aged <18 years (5/79) and 11% in those with BMI <25 Kg/m2.
3.3Prevalence and risk factors for liver fibrosis in patients with steatosisVCTE was obtained in 311/326 (98.4%) patients with steatosis on US. Fibrosis stage was F0-F1 in 242 patients (77.8%), F2 in 49 (15.7%), F3 in 14 (4.5%) and F4 in six (1.9%). LSM (kPa) were 5.0 ± 1.3, 8.6 ± 0.7, 12.3 ± 1.2 and 37.7 ± 26 respectively. Overall, 78% of patients with NAFLD were found to have minimal or no fibrosis (F0-F1), 22% with some degree of fibrosis (≥F2) and 6.4% advanced fibrosis (F3- F4). Significant risk factors for liver fibrosis on univariate analysis were a history of hypertension, overweight/obesity, no physical activity, T2DM/hyperglycemia, elevated ALT and hypertriglyceridemia. BMI, diabetes/hyperglycemia and hypertriglyceridemia were independent predictors of liver fibrosis (Table 4).
Risk factors for significant fibrosis by univariate and multivariate analysis.
BMI: body mass index; ALT: alanine aminotransferase.
The prevalence of fibrosis was 25% (69/276) in patients with overweight/obesity, 31.8% (41/129) in patients with hypertriglyceridemia and 34.2% (26/76) in those with T2DM/hyperglycemia and increased significantly (p<0.001) according to the presence of one (17.4%), two (22.1%), or three (51.2%) of these risk factors respectively.
Among the 311 subjects with steatosis on US who underwent VCTE, CAP values were 284 ± 73 dB/m. Significant differences were observed between patients with mild (n = 173, 257 ± 71 dB/m), moderate (n = 102, 304 ± 62 dB/m) or severe (n = 36, 353 ± 43 dB/m) steatosis as assessed by US (p<0.001). However, there was significant overlapping between steatosis grades (Fig. 2)
No patient with NAFLD below 18 years of age or without overweight had significant fibrosis on VCTE.
4DiscussionNAFLD is highly prevalent in all continents but with significant geographic variability. A meta-analysis reported by Younossi et al. in 2016 showed an overall global prevalence of 25% and identified South America as a region with one of the highest rates of NAFLD [6]. Three systematic reviews reported in 2022 showed that the prevalence of NAFLD worldwide is now >30% and continues to increase [7,8,9]. The prevalence in South America also increased from 30.5% [6] to 34.5%−35.7% [7,8] although in all studies it was stated that the results should be interpreted with caution because of the small number of US studies obtained from samples of the general population. A meta-analysis recently reported by Ortega Rojas et al. including 19 reported series from Latin America showed a pooled prevalence of NAFLD of 24% [13]. However, only 11 studies were based on US data and almost all studies estimated the prevalence of NAFLD in high-risk populations such as patients with T2DM, obesity, metabolic syndrome or coronary artery disease. Consequently, prevalence data from South America is still sparse and heterogeneous. Only two US-based studies, one from Brazil and one from Colombia were included in the reported meta-analyses with NAFLD prevalence of 35.2% and 26.6% respectively resulting in the 30.5% pool regional estimate reported by Younossi et al. (6). The study from Brazil, reported by Kamikowski et al., included only 139 individuals aged 55 or older recruited to undergo health screenings [14]. The study from Colombia by Perez et al. was restricted to 263 males of the Colombian Air Force [15]. In addition, a larger study from Chile on 832 individuals living in urban areas and randomly selected by a computer program showed a NAFLD prevalence of 23.4% [16]. In contrast, the O'Brien Project allowed the estimation of NAFLD prevalence at a general population level. The ethnicity of O'Brien inhabitants resulted from the interaction of native populations with European colonizers, mostly from Spain and Italy, and is similar to Argentina's ethnicity overall. Their principal occupation is the textile industry, with no related toxic exposures, and to a lesser degree rural work. For these reasons, we believe that the prevalence of NAFLD in O'Brien may be extrapolated to the country overall.
The first strength of this study is that almost half of O'Brien inhabitants volunteered to participate and showed high compliance across the different steps of the protocol, such that 90% of the enrolled subjects who completed clinical and laboratory evaluation underwent US and 98% of those with hepatic steatosis were screened for liver fibrosis with VCTE. The 37.2% prevalence of NAFLD found in O'Brien was not surprising considering the high rates of overweight, T2DM/hyperglycemia and hyperlipidemia in the community, especially in people older than 50 years of age. The prevalence of risk factors for NAFLD such as overweight (63.3%) and T2DM/hyperglycemia (14.3%) found in O'Brien are very similar to those previously reported for the whole country (66.1% and 12.7% respectively) [17]. In agreement with previous studies, age [18,19], male gender [19–21], overweight/obesity [22–25], T2DM/hyperglycemia [25–27] and hypertriglyceridemia [23–25] were independent predictors of NAFLD on multivariate analysis. A bidirectional association was found between risk factors and prevalence of NAFLD, being 50.3% in patients with overweight/obesity, 58.6% in hypertriglyceridemia, 62.3% in those with T2DM/hyperglycemia and as high as 72.1% when the three comorbidities were present. Of note, steatosis was found in only 6% of individuals aged <18 years and 11% of those without being overweight.
Another strength of this study was noninvasive assessment of liver fibrosis with VCTE in a large cohort of non-selected subjects with NAFLD diagnosed incidentally by US during the population-based study. VCTE showed some degree of fibrosis (≥F2) in 22% of patients and advanced fibrosis or cirrhosis in 6.4%. Due to its high negative predictive value, VCTE is accepted as a useful screening tool to differentiate patients with low versus the high risk of advanced liver disease [1,2,28,29]. Fibrosis is the most important histological feature of NAFLD due to its correlation with liver-related outcomes and mortality [30–32]. In the study by Angulo et al. on 619 patients who underwent liver biopsy, fibrosis staged at F2 or greater at the time of NAFLD diagnosis in 25% [30]. This rate of fibrosis is similar to the 22% observed in O'Brien although it may not be appropriate to compare stages of fibrosis between VCTE and liver biopsy and prevalence data between population-based studies and cohorts preselected based on a diagnosis of NAFLD [28]. The presence of fibrosis in O'Brien patients was significantly associated with the same risk factors as for NAFLD, namely overweight/obesity, T2DM/hyperglycemia and hypertriglyceridemia, as has been previously reported [1,2,8,19,20,33,34]. Rates of fibrosis progressively increased with the number of comorbidities from 17% to 51% when all three predictors were present.
Overweight/obesity was the most common and significant risk factor for the presence of steatosis (OR 2.87–9.57) and liver fibrosis (OR 5.22, CI 2.64-11.74). Other risk factors such as hypertension, elevated ALT values and low levels of physical activity were significantly associated with NAFLD and liver fibrosis but only by univariate analysis. Although fatty liver is today the commonest cause of incidentally discovered elevated aminotransferases, most patients have normal values as observed in 80% of subjects from O'Brien [21,35,36].
CAP values were available only in patients with steatosis on US who underwent VCTE. Therefore, we could not evaluate the utility of this method to assess the prevalence of NAFLD.
The major limitation of this series is that prevalence of NAFLD was investigated in a small city that may not be representative of the epidemiology of the whole country. New population-based studies are required to establish the true prevalence of NAFLD in Argentina.
5ConclusionsThis general population cohort study performed in a small city in Argentina showed a high prevalence of NAFLD with overweight as the major phenotype. In agreement with previous publications, our results suggest that South America is among the regions of the world with the highest frequency of NAFLD. Non-invasive screening with VCTE in patients with steatosis revealed that around 20% of patients had significant fibrosis (≥ stage F2). This strategy may be useful to identify high-risk patients who may require a liver biopsy.
FundingThe study was supported by Fundación para la Docencia e Investigación de las Enfermedades del Hígado(FUNDIEH, www.fundieh.org.ar) and by Fundación Sayani (www.sayani.org.ar), two non-profit foundations supporting research and education in liver diseases. Bragado County provided laboratory equipment and health care personnel and covered also accommodation and transportation of investigators. No participant received any type of financial reward.
Author contributionsFederico Guillermo Villamil: study concept and design, study supervision, analysis and interpretation of data, drafting of the manuscript; Manuel Barbero: acquisition of data, statistical analysis, critical revision of the manuscript; Nancy Elena Massenzio, Paula Andrea Cocco: acquisition of data (laboratory), study supervision; Shigeru Kozima, Claudia Mabel Riboldi: acquisition of data (ultrasound); Fernando Mario Cairo, Rodrigo Agustín Belloni, Mercedes Rodriguez Gazari: acquisition of data (elastography); Javier Mariani: statistical análisis; Sandra Mónica Giani, Sonia Carolina Gallardo, Patricia Eugenia Gallardo: study supervision, administration.
Data availabilityData from the study is available by request to the corresponding author.
We are grateful to other physicians who participated in the study including hepatologists (Paola Pegoraro, María del Carmen Puente, Luciana Navarro, María Ayelen Trillo, Ignacio Roca) and radiologists (Fernanda Cardinali, Fabiana Prado, German Espil, Hugo Altieri, Paula Perroni, Sebastián Solano).