Special issue on hepatocellular carcinoma (HCC) and hepatitis B and C as its main causes worldwide
Más datosThe majority of studies regarding hepatitis C virus (HCV) prevention, screening, and treatment have been conducted in urban populations, and it is unlikely that their findings are broadly generalizable to nonurban populations. This study aimed to measure the prevalence and risk factors of HCV infection in the rural northeastern United States (US) to provide further clinical guidance for HCV screening.
Materials and methodsThis was a retrospective review of all patients older than 18 years evaluated at an integrated healthcare system, serving northern Pennsylvania and southern and central New York, who received first-time HCV screening from January 2014 to December 2019.
Results30,549 patients were screened, of which 1.7% were HCV antibody positive. From 2014 to 2018, the incidence of positive HCV antibody screening cases per 100,000 population increased two-fold from 18.1 in 2014 to 40.4 in 2018. The age of positive HCV antibody patients peaked at 29.13 (95% CI 26.15–31.77) and 59.93 (95% CI 58.71–61.17). Positive HCV antibody was associated with positive urine drug screen (OR 5.9; 95% CI 3.8–9.3), narcotic use (OR 25.4; 95% CI 8.7–77.8), and overdose (OR 17.5; 95% CI 3.0–184.6).
ConclusionsIn this rural northeastern US population, there is an increasing incidence of positive HCV screening with a bimodal age of distribution. Risk factors associated with opioid use reflect challenges to disease eradication in this population. We propose a one-time screening for persons aged 35 to 40 will aid in earlier HCV infection diagnosis and treatment in rural populations.
Hepatitis C virus (HCV) infection is a significant contributor to morbidity and mortality in the United States (US). It is a common cause of chronic liver disease and is the leading indication for liver transplantation in the adult US population [1,2]. Recent data estimates that approximately 1.7% of all adults in the US population are HCV antibody positive, corresponding to 4.1 million persons [3]. Despite advances in HCV treatment, which have increased sustained virologic response upwards of 97%, many populations continue to have limited access to testing and treatment [4,5]. A recent systematic review of the literature indicated that among those living with chronic HCV infection, approximately 50% were unaware of their diagnosis and only 16% were prescribed treatment [6]. HCV screening is critical to reducing morbidity and mortality in at-risk populations.
In 2016, the World Health Organization (WHO) identified targets to eliminate HCV as a public health threat by 2030 [7]. A recent Markov disease progression model found that a large majority of the US is not on track to meet these WHO elimination targets within this timeframe, with the most difficult goal being the reduction of HCV incidence by 80% [8]. Increased efforts are needed to optimize screening to identify patients with active HCV infection who are at risk of transmitting HCV to others. The Centers for Disease Control (CDC) current guidelines support at minimum a once in a lifetime HCV screening for all adults aged ≥18 in settings where the prevalence of HCV infection is greater than 0.1% [9]. This recommendation expanded on previous guidelines that recommended one-time testing for persons with recognized risk factors or exposures and persons born between 1945 and 1965. Current guidelines, however, do not specify ideal timing for a first-time HCV screen.
Since 2006, the incidence of acute HCV infection among young persons has significantly increased, with annual increases more than two times greater in nonurban settings compared to urban settings [10]. The vast majority of studies regarding HCV prevention, screening, and treatment have been conducted in urban settings, and it is unlikely that their findings are broadly generalizable to nonurban settings. There is a need for research-based guidance on effective strategies for HCV prevention, screening, and treatment in rural settings.
This study aims to measure the prevalence and risk factors of HCV infection and viral coinfection in an integrated healthcare system in the rural northeastern US to provide further clinical guidance for HCV screening.
2Materials and methodsA retrospective review of all patients older than 18 years evaluated at an integrated healthcare system who received a first-time HCV screening for any reason from January 2014 to December 2019 was conducted. The geographic distribution included north central and northeast Pennsylvania, and southern New York, including the “Southern Tier” and parts of the “Finger Lake” and “Central New York” regions. Positive HCV antibody patients were compared to a matched number of randomly selected negative HCV antibody patients. HCV incidence, HCV prevalence, and HCV genotype were calculated. When available, screening rates of human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis A virus (HAV) were also calculated. Additional data extracted included patient demographic information (age, gender, race/ethnicity, and state of residency), and history of a positive urine drug screen, narcotic use, or overdose. Indications for screening included injection drug use, date of birth between 1945 and 1965, serum alanine aminotransferase (ALT) above the upper limit of normal (>19 U/L for women and >30 U/L for men), or other indications. Examples of other indications include sexually transmitted disease screening or exposures, liver related findings, history of blood transfusion prior to 1992, or other medical tests and results that warrant screening at the provider's discretion.
Descriptive analysis was calculated using GraphPad Prism software (Version 8; San Diego, California, USA). Subgroup analysis was calculated using Welch's t-test and χ2 for independent variables. The Guthrie Institutional Review Board approved this study.
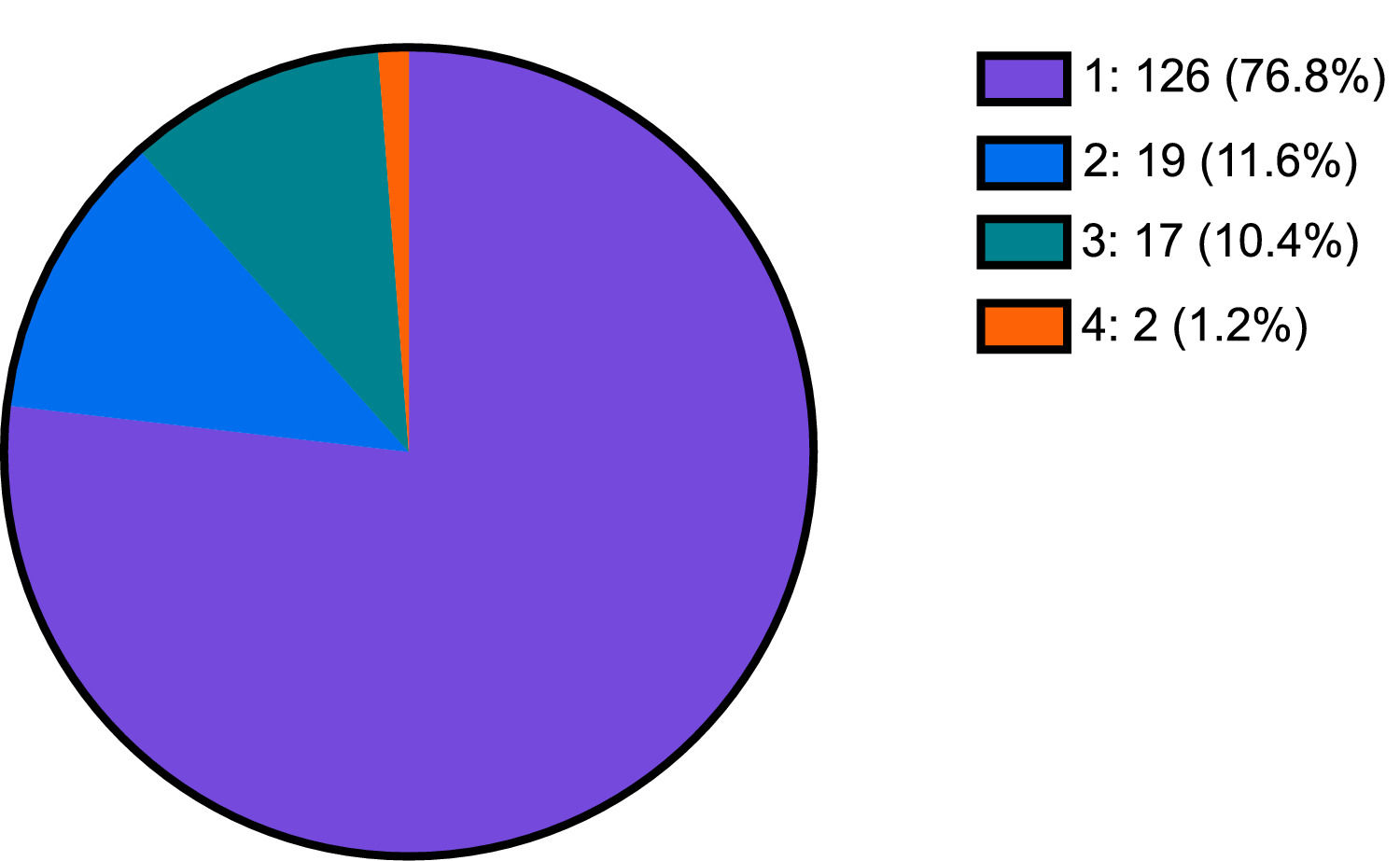
3ResultsThe average total population greater than 18 years old served at this integrated healthcare system from January 2014 to December 2019 was 188,176 persons. 16.2% of this population received a first time HCV screening (n=30,549), of which 1.7% were HCV antibody positive (n=533). From 2014 to 2018, the incidence of positive HCV antibody screening cases per 100,000 population increased two-fold from 18.1 in 2014 to 40.4 in 2018. 30.77% of those who were HCV antibody positive (n=164) received HCV genotype testing. Of the 164 patients who received genotype testing, 126 (76.8%) were genotype 1, 19 (11.6%) were genotype 2, 17 (10.4%) were genotype 3, and 2 (1.2%) were genotype 4 (Fig. 1).
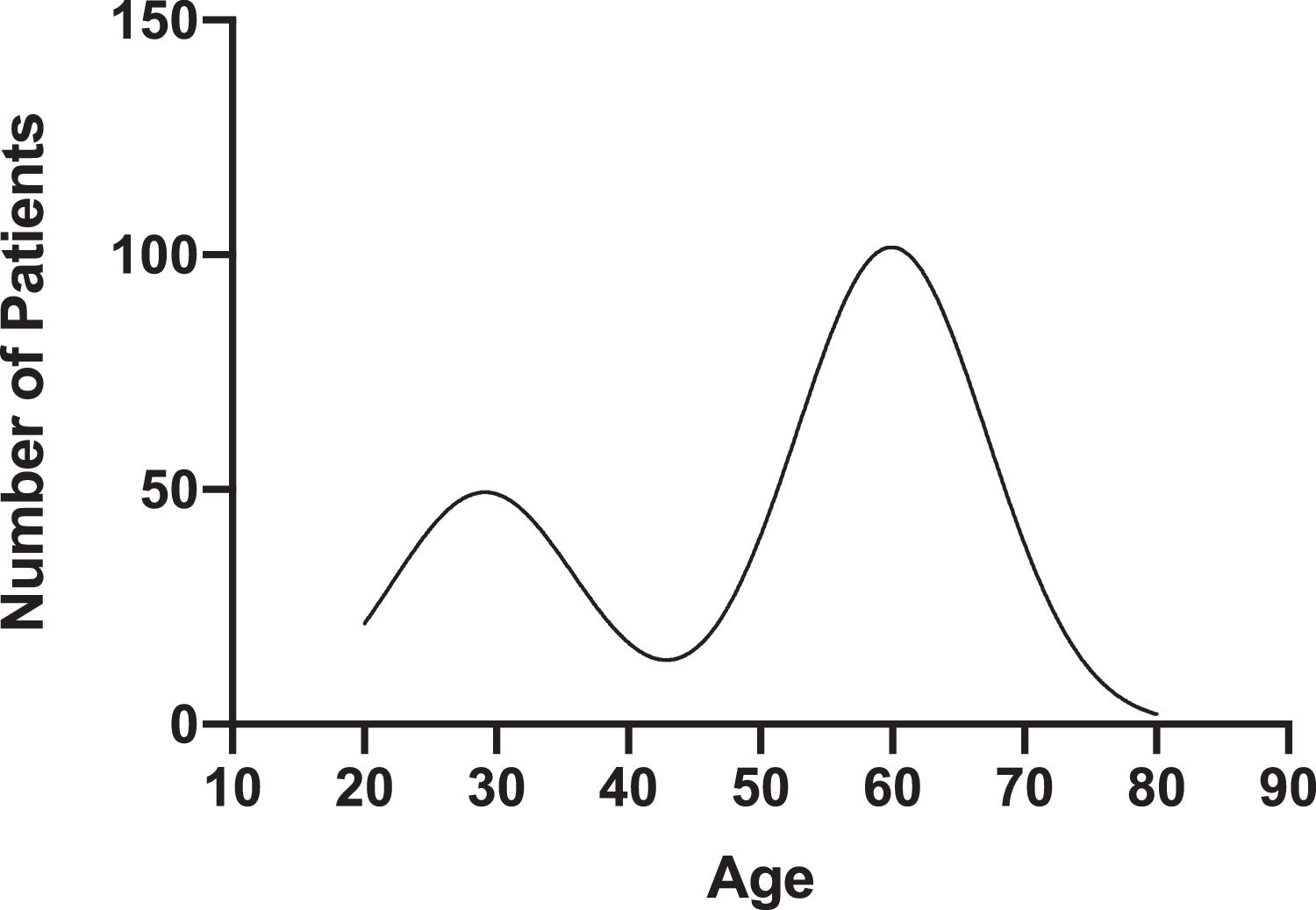
The mean age of HCV antibody positive and HCV antibody negative patients was 53 ± 15.38 and 60 ± 12.37, respectively (p < 0.05) (Table 1). The age of HCV antibody positive patients peaked at 29.13 (95% CI 26.15–31.77) and 59.93 (95% CI 58.71–61.17) (Fig. 2). 300 (56%) of HCV antibody positive patients and 256 (48%) of HCV antibody negative patients were male (p < 0.05). 515 (96.6%) of HCV antibody positive patients and 507 (95.1%) of HCV antibody negative patients were Caucasian (p>0.05). 224 (55.7%) of HCV antibody positive patients and 178 (64.7%) of HCV antibody negative patients resided in Pennsylvania and New York, respectively (p < 0.05) (Table 1).
Characteristics of subjects according to HCV antibody status. P-values calculated using Welch's t-test and chi-square test for independence.
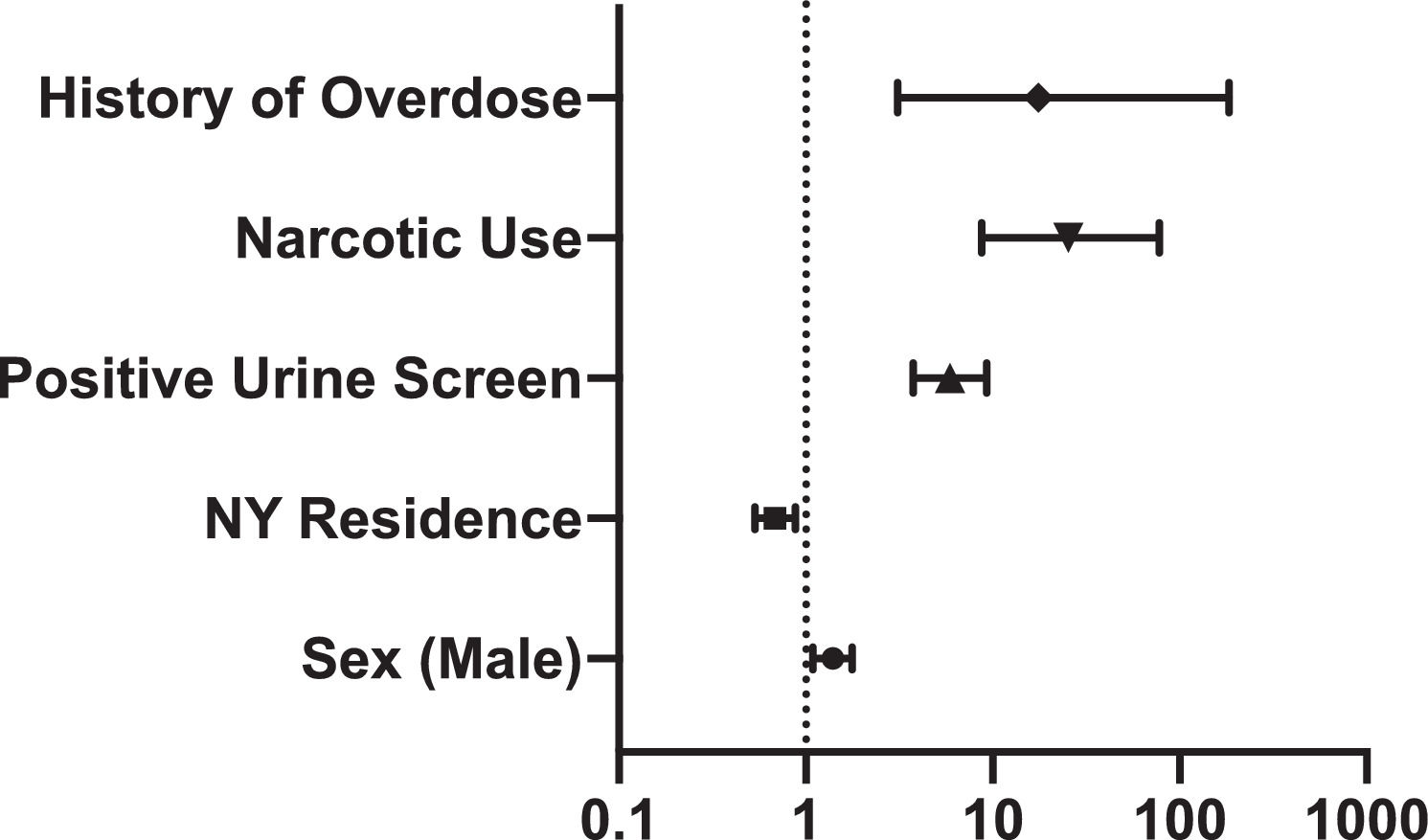
Indication for screening in HCV antibody positive patients was 18.6% injection drug use, 11.3% elevated serum ALT, 49.7% born between 1945 to 1965, and 20.5% other indications, compared to 0.5%, 3.6%, 74.6%, and 21.4%, respectively, in HCV antibody negative patients (p < 0.05) (Table 1). Positive HCV antibody was associated with history of positive urine drug screen (OR 5.9; 95% CI 3.8-9.3), narcotic use (OR 25.4; 95% CI 8.7–77.8), and overdose (OR 17.5; 95% CI 3.0–184.6). (Figure 3).
Screening rates for HIV, HBV, and HAV in HCV positive and HCV negative patients were 45% (n=241) and 25% (n=133), 56% (n=300) and 25% (n=134), and 32% (n=169) and 6% (n=30) respectively (p < 001) (Table 2). Of those screened for coinfection, prevalence of HIV infection, HBV immunity, and HAV immunity was 0.667% (n=2), 21.6% (n=52), and 25.4% (n=43) in HCV antibody positive patients and 1.5% (n=2), 23.2% (n=31), and 13.3% (n=4) in HCV antibody negative patients.
4DiscussionOur large, five-year retrospective study shows a substantial increase in the incidence of positive HCV screening from 2014 to 2019 in the rural northeastern US. In 2018, the CDC reported 1.2 acute HCV infection cases per 100,000 population nationwide, while our rural community reported 40.2 cases per 100,000 population [11]. Approximately 16% of our population received a first time HCV screening. National trends in HCV screening for the general population vary from 0.7% to 48%, with higher screening rates noted among individuals aged < 40 years [12]. We demonstrate a bimodal age distribution of positive HCV antibody patients with a nadir of age 40 to 45, reflecting the changing epidemic of HCV infection in the US. From 2006 to 2012, surveillance data from multiple Appalachian states demonstrated a 364% increase in acute HCV infections among persons ≤ 30 years old [13]. Over the same period, increases in the number of acute HCV infections in persons with similar demographic characteristics were reported in Wisconsin and upstate New York [14,15]. The number of incident HCV infection cases reported in the US via the National Notifiable Diseases Surveillance System has increased among adults of all ages, with the most marked increases seen in persons aged 20 to 39 years [3,11]. The current CDC guidelines are not adequately tailored to capture the burden of HCV infection in rural communities. We propose that a one-time screening for persons aged 35 to 40 will aid in earlier HCV diagnosis and treatment in rural populations.
In our rural population, patients with a history of a positive urine drug screen, narcotic use, or overdose had increased odds of HCV infection. Additionally, injection drug use (IDU) was the indication for screening in more HCV antibody positive patients than antibody negative patients. National surveillance data from 2004 to 2014 report IDU as the most frequently cited risk factor for acute HCV infection, with IDU implicated in more than 80% of acute case reports in 2014 [16]. Between 2014 and 2015, more than 180 individuals in rural Scott County, Indiana, tested positive for HIV, with 88% reporting injection of oxymorphone and 92% co-infected with HCV [17]. This outbreak was notable for the minimal availability of harm reduction strategies to prevent IDU associated HCV and HIV infections. These findings highlight the vulnerability of the growing numbers of people who inject drugs (PWID) in rural communities to HCV transmission.
The social factors and transmission routes that place persons at risk for acquiring HCV, HBV, and HIV are similar. In multiple Appalachian states, where an increased incidence of HCV infection was associated with a concurrent increase of IDU, incident cases of acute HBV infection also increased among non-Hispanic whites aged 30 to 39 years who reported IDU as a risk factor [18]. A systematic review found only 34 studies examining IDU, HIV, and HCV in rural areas of the US between 1990 and 2016, of which only two addressed HCV testing, and none addressed HIV testing [19]. Similarly, in our study, overall provider awareness of coinfection screening for HIV, HBV, and HAV was low. Of those screened for coinfection, the prevalence of HBV and HAV immunity was low. Regional demographic analysis characterizing the availability and barriers to coinfection testing in rural settings may assist coinfection surveillance efforts.
The use of electronic medical record (EMR) reminders and best practice alerts has been shown to improve adherence to screening recommendations. A study implementing EMR prompts for primary care physicians to perform first-time HCV screening for persons born between 1945 and 1965 showed an increase in screening from 7% to 72% within a year of implementation [20]. EMR-based cohort screening has been shown to be 2.6 to 8 times more effective than risk-based screening [20]. Implementing EMR prompts for coinfection screening in patients with positive HCV screening may aid in HIV diagnosis and assessment of hepatitis immunity.
At our integrated healthcare system, initiating HCV treatment and monitoring response to treatment is performed solely by hepatologists. An effective HCV service delivery model that may increase access to healthcare for PWID supports the concept of task-shifting to non-specialist providers who can initiate HCV testing and treatment [21]. In our current national environment, the utilization of telemedicine in viral hepatitis care is a significant opportunity to increase healthcare access and reduce geographic barriers to HCV treatment in rural areas. The opioid epidemic in rural areas remains a challenging issue that stresses health systems’ ability to eradicate HCV and will require unique interventions to address them.
This study was limited by its retrospective design and use of EMR for data collection. Populations at risk for HCV infection (uninsured, incarcerated, and homeless persons) with limited healthcare access are likely to be underrepresented.
5ConclusionsIn this rural northeastern US population, there is an increasing incidence of positive HCV screening with a bimodal age of distribution consistent with national trends of the HCV epidemic. Risk factors associated with opioid use reflect persistent challenges to the eradication of HCV infection in this rural population. Centralized healthcare and inadequate provider awareness for coinfection screening also represent barriers to improved public health. These findings support the need for expanded HCV screening recommendations in rural communities. We propose a one-time screen for persons aged 35 to 40 will aid in earlier HCV infection diagnosis and treatment in rural populations. Regional demographic and socioeconomic analysis may assist specific HCV screening recommendations.
None declared.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.