In clinical trials, patients with hepatitis C virus (HCV) genotype (GT)1a infection and baseline resistance-associated substitutions (RASs) at amino acid positions 28, 30, 31, or 93 receiving elbasvir/grazoprevir for 12 weeks achieved lower rates of sustained virologic response (SVR) than those without baseline RASs. SVR rates in patients with RASs were improved when elbasvir/grazoprevir treatment duration was extended from 12 to 16 weeks and administered concomitantly with ribavirin.
Materials and MethodsThis was a retrospective, observational analysis using electronic health record abstraction. Patients with HCV GT1a infection and RASs at positions 28, 30, 31, or 93 who were prescribed 16 weeks of elbasvir/grazoprevir and ≥ 1 prescription for ribavirin were included. SVR was defined as HCV RNA below the lower limit of quantification ≥ 70 days after end of treatment.
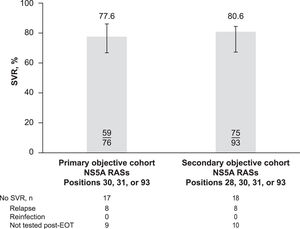
ResultsThe primary analysis included patients with baseline RASs at positions 30, 31, or 93 (n = 76); a secondary analysis included patients with RASs at positions 28, 30, 31, or 93 (n = 93). SVR was achieved by 77.6% (59/76) of patients in the primary analysis and 80.6% (75/93) of those in the secondary analysis. Of the 18 (19.4%) patients in the secondary cohort who failed to achieve SVR, 8 relapsed (4 with treatment-emergent NS5A substitutions) and 10 did not have viral sequencing to distinguish relapse from reinfection.
ConclusionsThis analysis highlights the opportunities in leveraging real-world data to further understand treatment outcomes in smaller, discrete subgroups of patients with HCV infection who cannot be thoroughly evaluated in clinical trials.
Approximately 1% of the US population, or 2.4 million individuals, has chronic hepatitis C virus (HCV) infection [1]. The US Department of Veterans Affairs (VA) is the largest provider of care for HCV infection in the United States, with an estimated 165,000 veterans diagnosed with chronic HCV infection at the beginning of 2014 [2]. By January 1, 2018, more than 100,000 veterans with HCV had initiated treatment with an interferon-free direct-acting antiviral (DAA) medication; more than 77,000 of these patients had achieved a sustained virologic response (SVR) or undetectable HCV RNA levels after DAA treatment [3]. The HCV-infected population within the VA is predominantly male and aged 50 to 69 years; approximately 80% of these patients have HCV genotype (GT) 1 infection [4].
Elbasvir/grazoprevir is a fixed-dose combination product containing elbasvir (50 mg), an HCV NS5A inhibitor, and grazoprevir (100 mg), an HCV NS3/4A protease inhibitor, and was approved in January 2016 by the US Food and Drug Administration primarily as a 12-week regimen for the treatment of chronic HCV GT 1 or 4 infections in adults [5]. Elbasvir/grazoprevir is also indicated for use with ribavirin in certain patient populations, including those with HCV GT1a infection and NS5A baseline resistance-associated substitutions (RASs), and treatment duration is extended to 16 weeks [5].
Post hoc analyses of data from the elbasvir/grazoprevir clinical trial program, however, indicated that among patients with GT1a infection who received elbasvir/grazoprevir for 12 weeks, SVR rates were lower in those with baseline RASs at amino acid positions 28, 30, 31, or 93 compared with those without baseline RASs at these positions (70% vs. 98%) [5]. This post hoc analysis also showed that SVR rates in patients with HCV GT1a infection and RASs improved when elbasvir/grazoprevir treatment duration was extended from 12 to 16 weeks and administered concomitantly with ribavirin [5]. Therefore, although only a relatively small number of patients with HCV GT1a infection and baseline NS5A RASs were included in the elbasvir/grazoprevir clinical trial program, which represents approximately 12% of all US patients with GT1a infection, the approved treatment regimen for this population is elbasvir/grazoprevir plus ribavirin administered for 16 weeks [5]. The efficacy and safety of elbasvir/grazoprevir have been established in a comprehensive phase 3 clinical program [6–13] and have been subsequently confirmed in several real-world effectiveness studies [14–18].
The objective of this study was to evaluate the real-world effectiveness of elbasvir/grazoprevir for 16 weeks when coadministered with ribavirin in a VA cohort of patients with HCV GT1a infection and baseline RASs at NS5A amino acid positions 28, 30, 31, or 93.
2Materials and Methods2.1Study designThis was a retrospective observational analysis of a cohort of VA patients with HCV GT1a infection with baseline NS5A RASs at amino acid positions 28, 30, 31, or 93 (Protocol VEAP 6421). The study began on December 19, 2017, and ended on October 31, 2018. A review of the VA's Corporate Data Warehouse (CDW) data began on January 2, 2018, and concluded on September 28, 2018. Abstraction of electronic health records (EHRs) took place from April 1, 2018, through May 1, 2018, and data analysis was completed by October 31, 2018. This was a noninterventional study using a retrospective review of clinical and administrative data.
2.2Data sourcesThe VA CDW is a large, integrated healthcare information system that includes information from inpatient and outpatient visits from more than 150 VA hospitals in the United States. The VA was one of the first health systems to include elbasvir/grazoprevir on a formulary, so the CDW allowed access to a large cohort of patients treated with elbasvir/grazoprevir. The CDW contains information derived from routine clinical encounters, including diagnosis and procedure codes and demographic, laboratory, and pharmacy information. EHR abstractions were completed by a trained VA research assistant/chart abstractor to confirm the presence of baseline NS5A RAS, selected laboratory results, and outcomes of interest. EHR data were also used to confirm the elbasvir/grazoprevir treatment index date (i.e., date of first dispensed prescription for elbasvir/grazoprevir), duration of treatment, coadministration of ribavirin, and clinical outcomes. Abstractions for all patients who did not achieve SVR and a random sample of patients who did achieve SVR were repeated by a trained clinician for quality control.
2.3Study populationAdult patients with HCV GT1a infection (defined as ≥ 1 positive HCV RNA or HCV GT test after January 1, 2014, and before the treatment index date in the CDW) receiving care at any VA medical center or associated clinic were included in the cohort. The index date was defined as the date of the first dispensed prescription for elbasvir/grazoprevir (50 mg/100 mg, fixed-dose combination) occurring between February 1, 2016, and August 31, 2017. All patients were required to have 16 weeks of prescriptions dispensed for elbasvir/grazoprevir and ≥ 1 prescription dispensed for concomitant ribavirin.
All cohort patients were required to have ≥ 6 months of CDW or EHR history with ≥ 1 inpatient or outpatient visit ≥ 6 months prior to the index date and a minimum follow-up period of ≥5 months after the index date. All patients were also required to have data available to determine the SVR outcome based on EHR abstraction. Patients were also required to have ≥ 1 baseline NS5A RAS at amino acid positions 30, 31, or 93 (for the primary effectiveness outcome) or positions 28, 30, 31, or 93 (for the secondary effectiveness outcome) based on EHR abstraction.
Patients were excluded if they had a pharmacy claim for elbasvir/grazoprevir prescription that was not aligned with either drug's US prescribing information, including previous treatment with a DAA regimen for HCV infection, concomitant use of a contraindicated medication (e.g., phenytoin, carbamazepine, rifampin, efavirenz, atazanavir, darunavir, lopinavir, saquinavir, tipranavir, cyclosporine), and presence of Child-Pugh class B/C cirrhosis (based on International Classification of Diseases [ICD] 9/10 codes and EHR review).
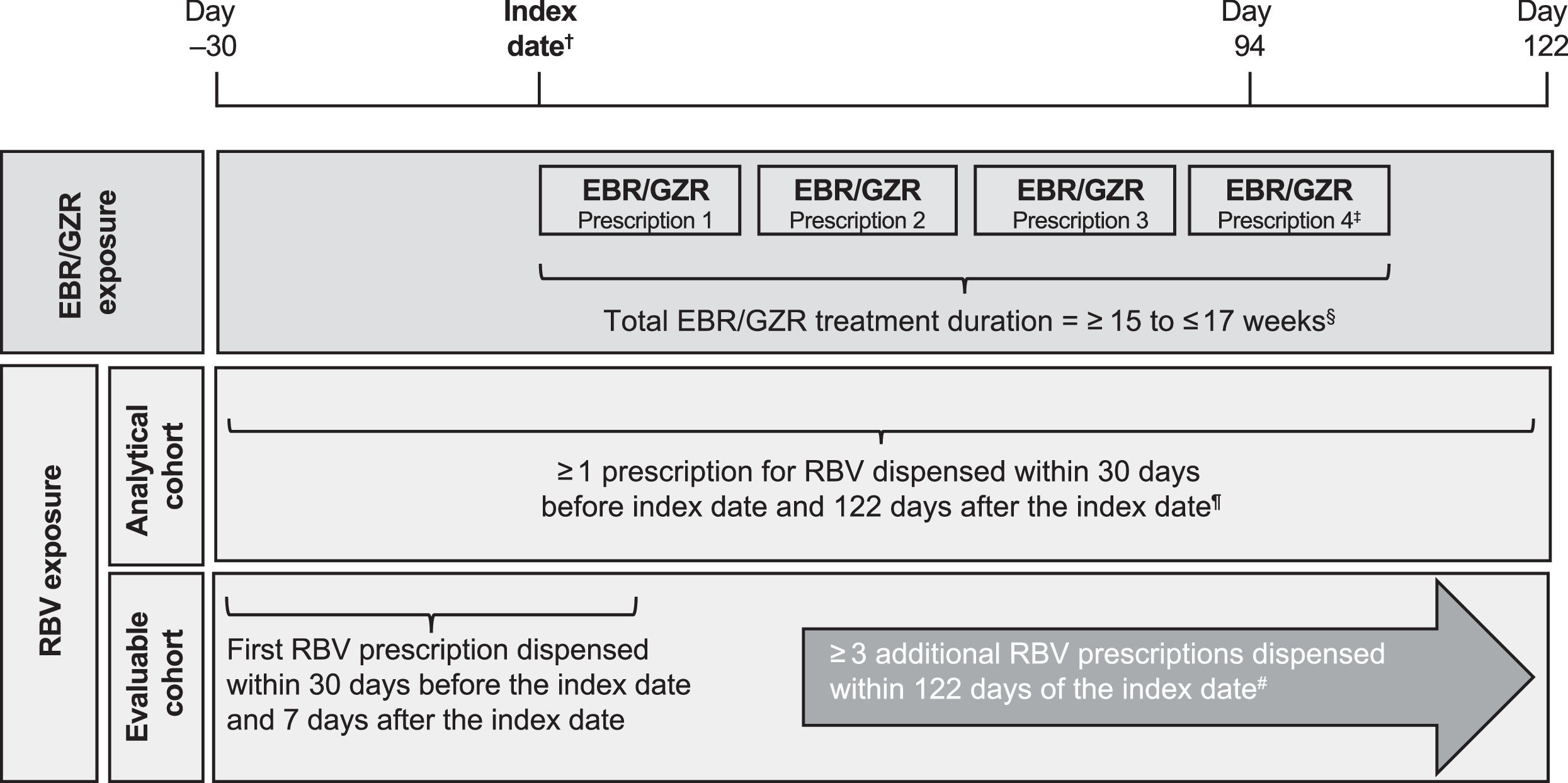
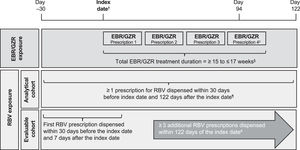
2.4Drug exposure assessmentA 16-week regimen of the elbasvir/grazoprevir fixed-dose combination tablet was defined as an elbasvir/grazoprevir regimen with a duration of ≥ 15 and ≤ 17 weeks, corresponding to 4 consecutive elbasvir/grazoprevir prescriptions dispensed every 28–30 days (based on recorded number of days supplied for the dispensed prescription) for a period of 4 consecutive months (Fig. 1). A cumulative gap of 10 days was permitted between refills, and the last prescription was required to have been dispensed by day 94 from the index date. Patients who were prescribed > 13 weeks of an elbasvir/grazoprevir regimen in the CDW were included in the EHR data abstraction review process to confirm the required 16-week elbasvir/grazoprevir regimen.
Study design. †Index date defined as first dispensed prescription for elbasvir/grazoprevir 50 mg/100 mg occurring during the exposure period of February 1, 2016, to August 31, 2017. ‡Final elbasvir/grazoprevir prescription must have been dispensed by day 94 after the index date. §Cumulative allowable gap of 10 days between elbasvir/grazoprevir refills. ¶Ribavirin coadministration for ≥ 4 weeks confirmed during EHR review. #Each prescription provided 28- to 30-days’ supply of ribavirin, and ribavirin coadministration for 16 weeks was confirmed during EHR review. EBR, elbasvir; EHR, electronic health record; GZR, grazoprevir; RBV, ribavirin.
Two cohorts of patients were constructed for the analysis according to ribavirin prescriptions dispensed. The analytical cohort included patients who dispensed ≥ 1 prescription for ribavirin (each prescription providing sufficient drug for 4 weeks), and the evaluable cohort included patients who dispensed ≥ 4 ribavirin prescriptions (≥ 16 weeks of ribavirin exposure) during the elbasvir/grazoprevir treatment period. For the analytical cohort, the fill date of the first ribavirin prescription was required to be ≤ 30 days before or ≤ 122 days after the elbasvir/grazoprevir index date (112 days = 16 weeks of elbasvir/grazoprevir + 10-day cumulative allowable gap between prescriptions). For the evaluable cohort, the fill date of the first ribavirin prescription was required ≤ 30 days before or ≤ 7 days after the elbasvir/grazoprevir index date, and ≥ 3 additional ribavirin prescriptions were required to be dispensed within 122 days of the index date. In both cohorts, the duration of ribavirin coadministration was confirmed during EHR review.
2.5Effectiveness outcomesSVR was defined as HCV RNA less than the assay lower limit of quantification ≥ 70 days after the end of treatment (EOT) with elbasvir/grazoprevir plus ribavirin. All patients were followed from EOT with elbasvir/grazoprevir through the end of available data and for ≥ 12 weeks to permit assessment of SVR. Effectiveness was defined as the proportion of patients with HCV GT1a who achieved SVR in the analytical and evaluable cohorts. Therefore, our results reflect the evaluable cohort and align with the US prescribing information for elbasvir/grazoprevir. SVR was determined using CDW data and confirmed based on EHR abstraction.
Where data were available, patients who did not achieve SVR were further defined as those experiencing a virologic relapse or reinfection. Virologic relapse was defined as detectable HCV RNA after EOT with the same GT detected at baseline and during follow-up. Reinfection was defined as detectable HCV RNA after EOT with a viral GT that differed from the GT present at baseline.
2.6StatisticsWith a minimum target sample of 60 patients with HCV GT1a infection and ≥ 1 baseline NS5A RAS at positions 28, 30, 31, or 93, we anticipated that ≥ 1000 patients treated with elbasvir/grazoprevir would be required to yield approximately 100 patients who met the criteria for inclusion in this study. The assumption that approximately 10% of patients with HCV GT1a infection would have baseline NS5A RASs at amino acid positions 28, 30, 31, or 93 was based on estimates derived from integrated data from the elbasvir/grazoprevir phase 2/3 clinical trials, which indicated that 12% (37/309) of patients with GT1a infection had ≥ 1 NS5A RAS. Approximately 50% of these patients had RASs at position 28, and progressively fewer patients had NS5A RASs at positions 30, 31, or 93. In addition, a feasibility assessment conducted at the start of the study identified approximately 100 patients from the VA CDW who potentially met the cohort inclusion criteria for this analysis.
The nature of this study was purely descriptive, so no formal hypotheses were tested. The primary analysis of SVR was conducted in the VA cohort of patients with NS5A RASs at amino acid positions 30, 31, or 93, and the secondary analysis of SVR was conducted in patients with NS5A RASs at amino acid positions 28, 30, 31, or 93. Thus, the patient cohort for the secondary analysis of effectiveness was larger than the population used for the primary analysis. SVR rates were examined overall and in various subgroups.
2.7Ethical statementThe study was approved by the VA Central Institutional Review Board with a waiver of informed consent because patient identity and medical records were not disclosed (IRB Number: VA CIRB 13–40).
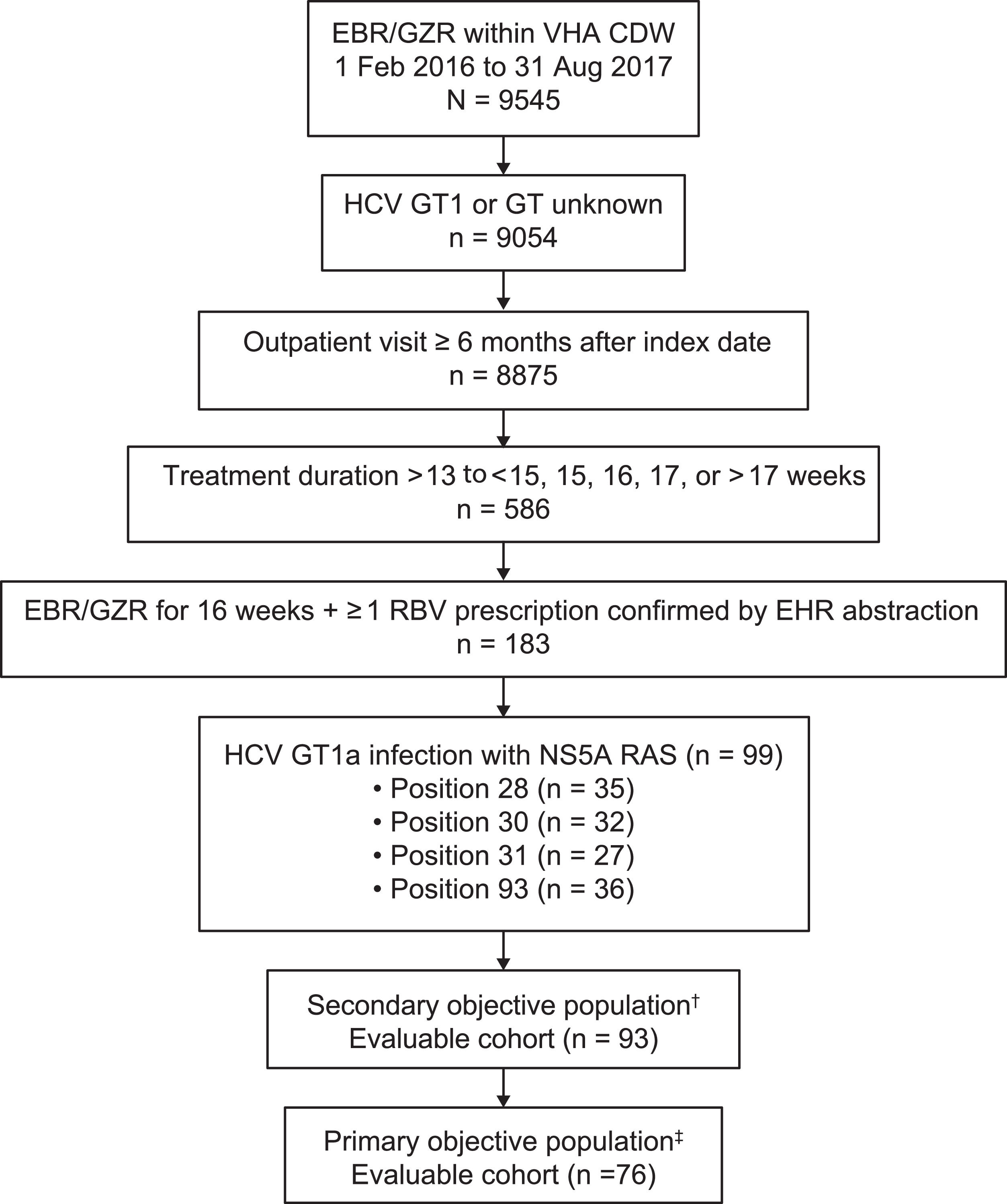
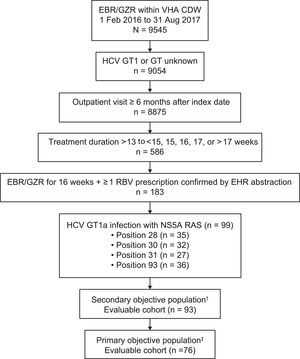
3ResultsThe VA CDW contained 9545 patients with ≥ 1 prescription for elbasvir/grazoprevir; of these patients, 9054 had GT1 or GT unknown infection and had detectable HCV RNA between January 1, 2014, and the elbasvir/grazoprevir treatment index date (Fig. 2). A total of 586 patients had treatment durations of > 13 weeks; 183 patients had a confirmed treatment duration of 16 weeks with ≥ 1 prescription for ribavirin confirmed by EHR chart abstraction, and 99 patients had baseline RASs at positions 28, 30, 31, or 93.
Patient flow diagram. †Secondary objective cohort included treatment-naive and treatment-experienced patients with HCV GT1a–infection and ≥ 1 baseline NS5A RAS at amino acid positions 28, 30, 31, or 93. ‡Primary objective cohort included treatment-naive and treatment-experienced patients with HCV GT1a infection and ≥ 1 baseline NS5A RAS at amino acid positions 30, 31, or 93. CDW, corporate data warehouse; EBR, elbasvir; EHR, electronic health record; GT, genotype; GZR, grazoprevir; HCV, hepatitis C virus; RAS, resistance-associated substitution; RBV, ribavirin; VHA, Veterans Health Administration.
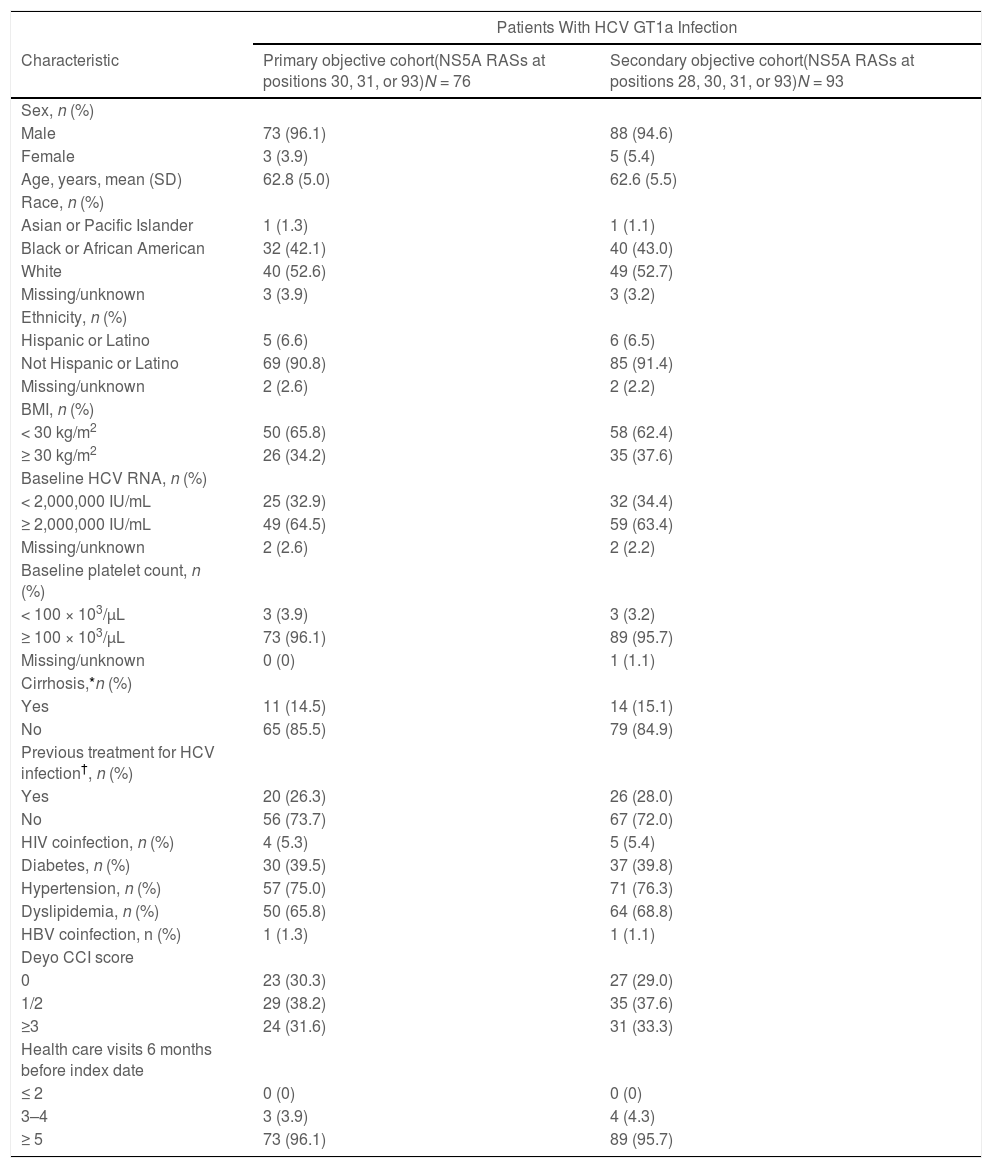
The primary objective evaluable cohort included 76 patients with elbasvir/grazoprevir prescriptions for 16 weeks, ≥ 4 prescriptions for ribavirin, and baseline RASs at positions 30, 31, or 93. Patients with RASs at positions 30, 31, or 93 were included in the primary analysis, irrespective of the presence or absence of a RAS at position 28.
The secondary analysis cohort included 93 patients with HCV GT1a infection and RASs at positions 28, 30, 31, or 93 who were prescribed elbasvir/grazoprevir for 16 weeks and had ≥ 4 prescriptions for ribavirin; this cohort included 17 patients with a single RAS at position 28 who were excluded from the primary analysis cohort (Table 1). Of these 93 patients, 20 (21.5%) had a daily ribavirin dose of < 800 mg prescribed, 11 (11.8%) had a daily ribavirin dose of 800 mg, 12 (12.9%) had a daily ribavirin dose of 1000 mg, and 50 (53.8%) had a daily ribavirin dose of 1200 mg.
Patient demographics and characteristics (evaluable cohort).
| Patients With HCV GT1a Infection | ||
|---|---|---|
| Characteristic | Primary objective cohort(NS5A RASs at positions 30, 31, or 93)N = 76 | Secondary objective cohort(NS5A RASs at positions 28, 30, 31, or 93)N = 93 |
| Sex, n (%) | ||
| Male | 73 (96.1) | 88 (94.6) |
| Female | 3 (3.9) | 5 (5.4) |
| Age, years, mean (SD) | 62.8 (5.0) | 62.6 (5.5) |
| Race, n (%) | ||
| Asian or Pacific Islander | 1 (1.3) | 1 (1.1) |
| Black or African American | 32 (42.1) | 40 (43.0) |
| White | 40 (52.6) | 49 (52.7) |
| Missing/unknown | 3 (3.9) | 3 (3.2) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 5 (6.6) | 6 (6.5) |
| Not Hispanic or Latino | 69 (90.8) | 85 (91.4) |
| Missing/unknown | 2 (2.6) | 2 (2.2) |
| BMI, n (%) | ||
| < 30 kg/m2 | 50 (65.8) | 58 (62.4) |
| ≥ 30 kg/m2 | 26 (34.2) | 35 (37.6) |
| Baseline HCV RNA, n (%) | ||
| < 2,000,000 IU/mL | 25 (32.9) | 32 (34.4) |
| ≥ 2,000,000 IU/mL | 49 (64.5) | 59 (63.4) |
| Missing/unknown | 2 (2.6) | 2 (2.2) |
| Baseline platelet count, n (%) | ||
| < 100 × 103/µL | 3 (3.9) | 3 (3.2) |
| ≥ 100 × 103/µL | 73 (96.1) | 89 (95.7) |
| Missing/unknown | 0 (0) | 1 (1.1) |
| Cirrhosis,*n (%) | ||
| Yes | 11 (14.5) | 14 (15.1) |
| No | 65 (85.5) | 79 (84.9) |
| Previous treatment for HCV infection†, n (%) | ||
| Yes | 20 (26.3) | 26 (28.0) |
| No | 56 (73.7) | 67 (72.0) |
| HIV coinfection, n (%) | 4 (5.3) | 5 (5.4) |
| Diabetes, n (%) | 30 (39.5) | 37 (39.8) |
| Hypertension, n (%) | 57 (75.0) | 71 (76.3) |
| Dyslipidemia, n (%) | 50 (65.8) | 64 (68.8) |
| HBV coinfection, n (%) | 1 (1.3) | 1 (1.1) |
| Deyo CCI score | ||
| 0 | 23 (30.3) | 27 (29.0) |
| 1/2 | 29 (38.2) | 35 (37.6) |
| ≥3 | 24 (31.6) | 31 (33.3) |
| Health care visits 6 months before index date | ||
| ≤ 2 | 0 (0) | 0 (0) |
| 3–4 | 3 (3.9) | 4 (4.3) |
| ≥ 5 | 73 (96.1) | 89 (95.7) |
Previous treatment for HCV infection included interferon or peginterferon with or without ribavirin in combination or an interferon-containing regimen with a protease inhibitor. Patients who had previously received an all-oral direct-acting antiviral regimen were excluded.
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; GT1a, genotype 1a; HBV, hepatitis B virus; HCV, hepatitis C virus; RAS, resistance-associated substitution; SD, standard deviation.
The primary (n = 76) and secondary (n = 93) analysis cohorts were similar with regard to patient demographics and clinical characteristics (Table 1). In the secondary analysis cohort (n = 93), 95% of patients were male, and there were slightly more White patients compared with Black or African American patients (53% vs. 43%). Approximately 38% of the patients in this cohort had a body mass index of ≥ 30 kg/m2, 63% had baseline HCV RNA of ≥ 2,000,000 IU/mL, 15% were cirrhotic, and 72% were HCV-treatment naive. The most common comorbidities were hypertension (76%), dyslipidemia (69%), and diabetes (40%).
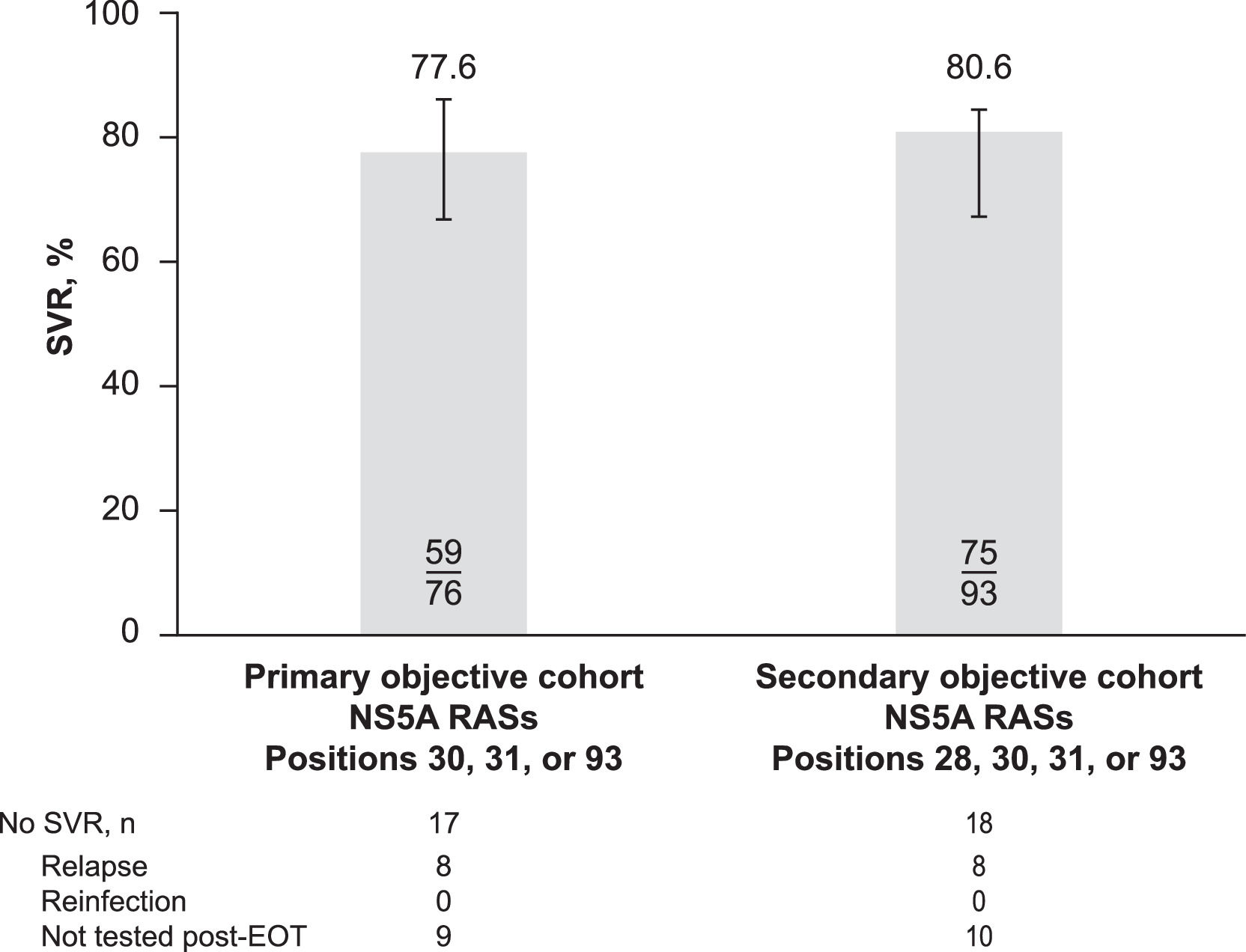
3.1Virologic outcomesSVR was achieved by 77.6% (59/76) of patients in the primary cohort and 80.6% (75/93) of those in the secondary cohort (Fig. 3). Of the 18 (19.4%) patients in the secondary cohort who failed to achieve SVR, 8 were confirmed with relapse and 10 did not have viral sequencing data at or after virologic failure to distinguish relapse from reinfection. No patient had confirmed reinfection after EOT with elbasvir/grazoprevir plus ribavirin. Of the 8 patients with confirmed relapse, 4 had treatment-emergent NS5A substitutions. Two patients had baseline NS5A RASs at position 31 and had treatment-emergent RASs at position 30 at relapse: 1 with a Q30R RAS and 1 with Q30K and Q30T RASs. A third patient had baseline NS5A RAS at position 93 and had an L31M treatment-emergent RAS. A fourth patient had baseline RASs at positions 28 and 30 and had an M28A treatment-emergent RAS.
SVR in patients with RASs at positions 28, 30, 31, and 93 (by analysis cohort). EOT, end of treatment; RAS, resistance-associated substitution; SVR, sustained virologic response, defined as hepatitis C virus RNA below the lower limit of quantification ≥ 70 days after end of treatment.
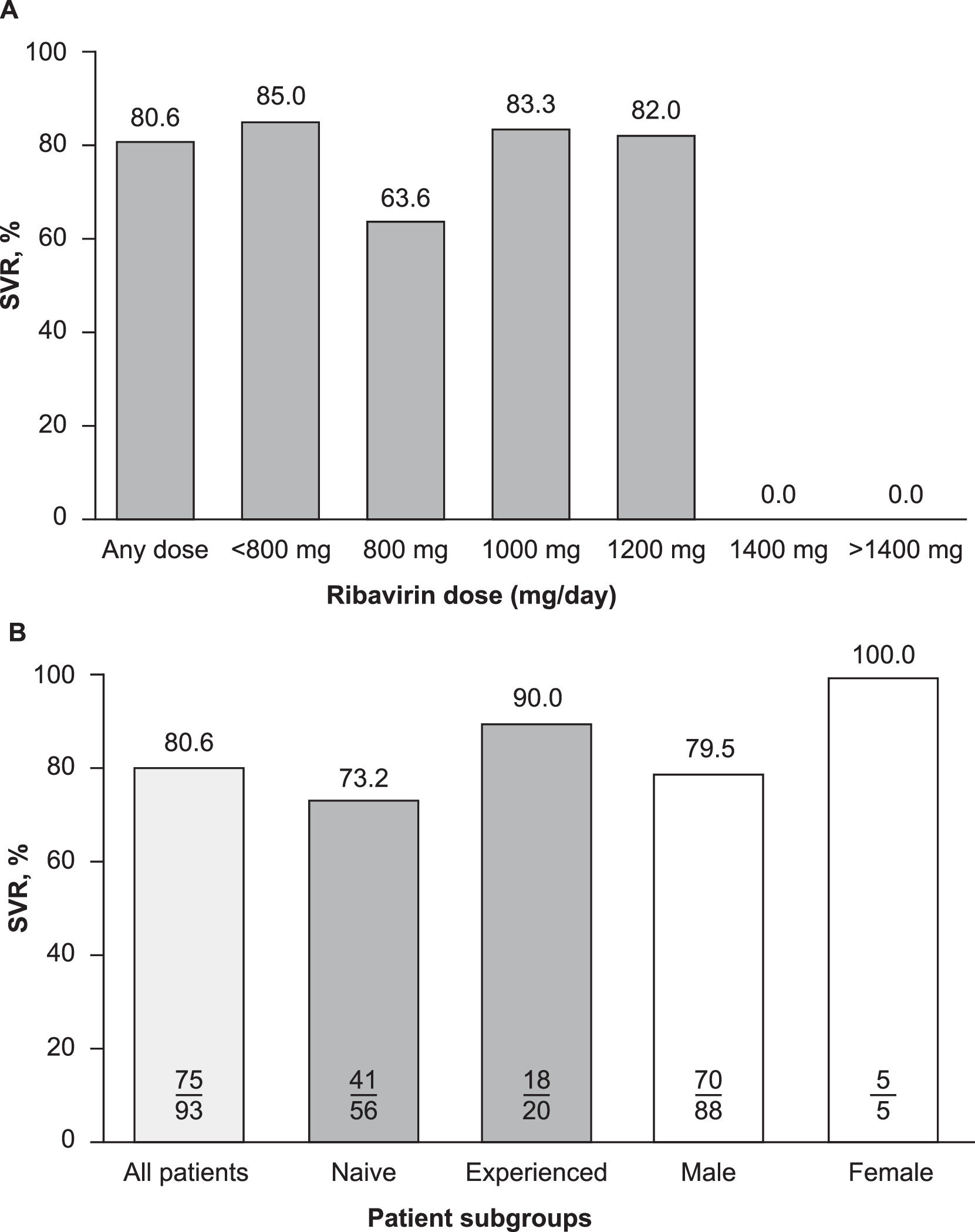
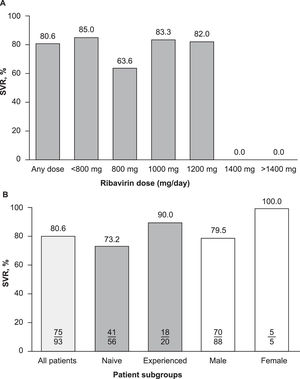
Subgroup analyses did not reveal any correlations between demographic or clinical characteristics and the effectiveness of elbasvir/grazoprevir plus ribavirin, although patient numbers in certain subgroups were small, precluding any meaningful interpretation. Descriptive evaluations of SVR and ribavirin dose levels were not correlated (Fig. 4A), and the rate of SVR was generally similar across all ribavirin dose levels (82%–85%). However, patients who were prescribed a daily dose of ribavirin 800 mg achieved an SVR rate of 64% (7/11). SVR rates were also numerically lower in males compared with females (80% vs. 100%) and in patients who were HCV treatment-naive compared with those who were treatment-experienced (73% vs. 90%) (Fig. 4B). Of the 17 patients with cirrhosis who were included in this study (17/98, 17.3%), 15 achieved SVR (15/17, 88%) and two did not achieve SVR.
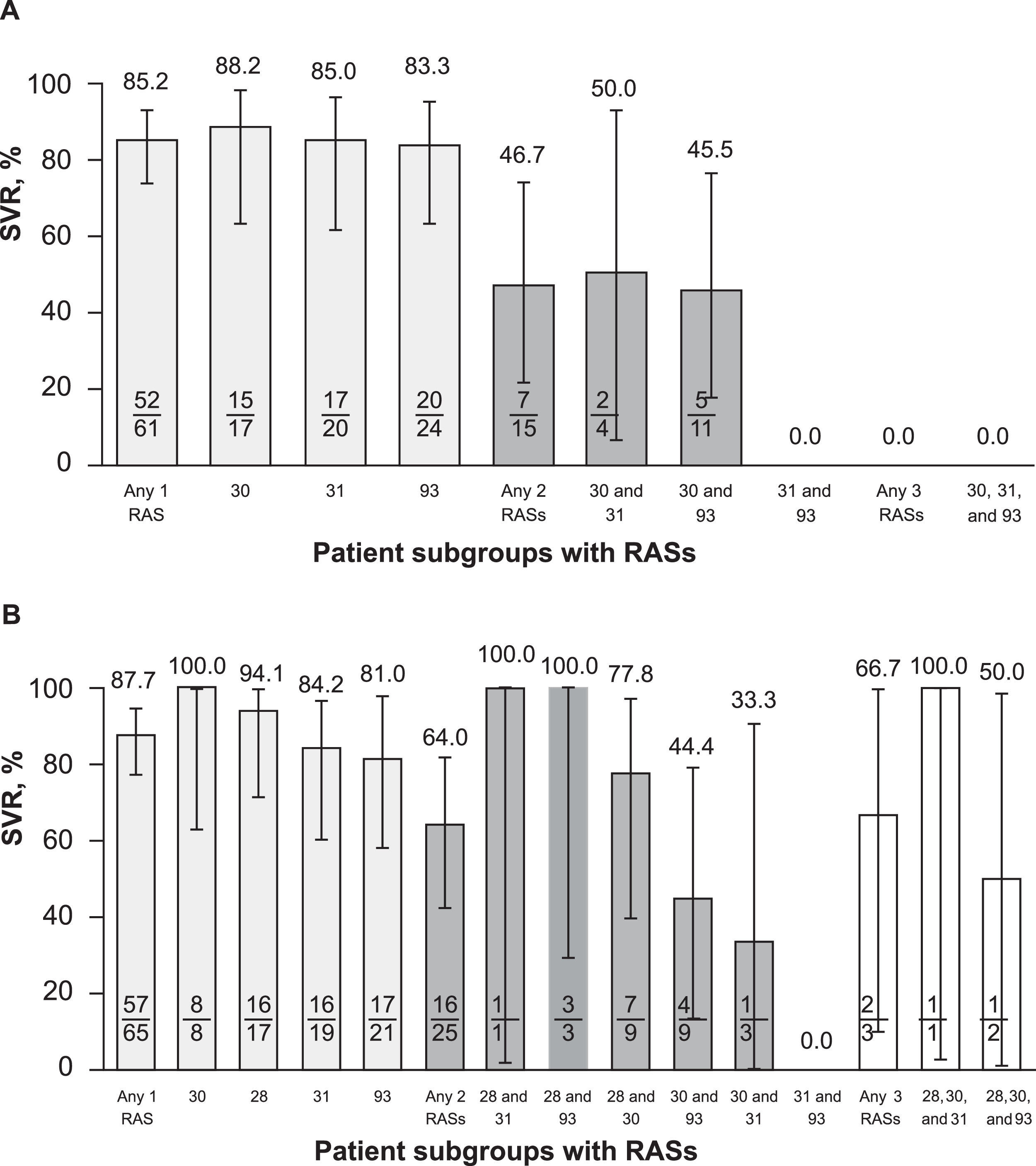
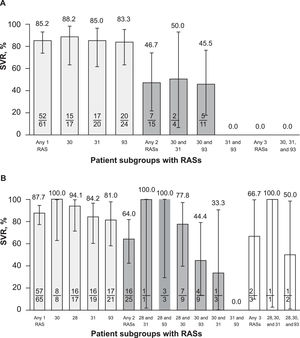
3.2Impact of RASs on SVRFor the primary objective (RAS at positions 30, 31, or 93; n = 76), SVR rates were 85% (52/61) and 47% (7/15) in patients with a single and a double RAS, respectively (Fig. 5A). Regardless of RAS position, SVR rates were similar in patients with a single RAS (88.2%, 85.0%, and 83.3% at positions 30, 31, and 93, respectively) but were ≤ 50% in patients with double RASs regardless of the positions of the 2 RASs. There was no patient with RASs at positions 30, 31, and 93.
For the secondary objective (RAS at positions 28, 30, 31, or 93; n = 93), SVR was achieved by 87.7% (57/65) of patients with any single RAS, 64.0% (16/25) in those with any double RAS, and 66.7% (2/3) in those with any triple RAS (Fig. 5B). Among patients with a single RAS, substitutions at position 93 were the most impactful, with an SVR of 81.0% (17/21) followed by substitutions at position 31 (84.2%, 16/19) and position 28 (94.1%, 16/17); position 30 was the least impactful, with an associated SVR of 100% (8/8). Among patients with double RASs, SVR rates were 100% in the 4 patients with double RASs at 28 and 31 (1/1) and at 28 and 93 (3/3) but were lower in those with double RASs at positions 30 and 93 (44.4%, 4/9) and 30 and 31 (33.3%, 1/3); 2 of 3 patients with triple RASs achieved SVR (66.7%), with a single virologic failure noted in a patient with substitutions at 28, 30, and 93.
4DiscussionThis analysis of VA patients represents one of the largest observational studies in those with HCV GT1a infection and confirmed baseline NS5A RASs. The study demonstrated SVR rates of 78%–81% in patients with GT1a infection and baseline RASs prescribed elbasvir/grazoprevir plus ribavirin for 16 weeks. The RASs at positions 28, 30, 31, and 93 are relatively uncommon among patients with HCV GT1a infection. In the elbasvir/grazoprevir phase 2/3 clinical development program, 62 of 561 (11%) patients with HCV GT1a infection had baseline RASs at positions 28, 30, 31, and 93 [5]. Data from the phase 2/3 clinical development program demonstrated that SVR rates of 70% (39/56) and 98% (441/450) were achieved in patients with and without baseline RASs who received elbasvir/grazoprevir for 12 weeks; however, SVR rates were increased to 100% (6/6) among patients with baseline RASs when treatment was supplemented with ribavirin and extended to 16 weeks [5]. Thus, extending the duration of elbasvir/grazoprevir treatment and adding ribavirin increased the effectiveness in the subgroup of patients with HCV GT1a infection with baseline NS5A RASs. Thus, the relatively large cohort of patients treated with elbasvir/grazoprevir in the VA represented a unique opportunity to further assess the effectiveness of elbasvir/grazoprevir plus ribavirin for 16 weeks in a real-world population of patients with HCV GT1a infection with baseline NS5A RASs. The VA real-world effectiveness with elbasvir/grazoprevir has been summarized in an earlier observational study by Kramer and colleagues [14], which includes data from 2436 veterans with HCV GT1a, 1b, 2, 3, and 4 infection who were prescribed elbasvir/grazoprevir regimens for 12 to 16 weeks, with and without concomitant ribavirin. An SVR rate of 95.6% was achieved with consistently high rates of SVR across a diverse cohort of patients, including those with diabetes, depression, HIV coinfection, and a history of drug or alcohol abuse [14]. This subgroup of patients with baseline NS5A RASs, representing approximately 12% of treatment-naive patients with HCV GT1 infection in the United States, represents a hard-to-treat patient subgroup that requires an intensified elbasvir/grazoprevir regimen compared with patients with GT1a infection and no baseline RASs. Distinguishing between patients with HCV GT1a infection with and without NS5A resistance requires testing for baseline NS5A RASs prior to initiation of treatment with elbasvir/grazoprevir. In the present study, SVR rates were 78% in patients with RASs at positions 30, 31, or 93, and 81% in patients with NS5A RASs at positions 28, 30, 31, or 93. In the primary analysis, SVR rates were 85% in patients with any single RAS (any amino acid position) and 47% in patients with any double RAS. In the secondary analysis, SVR rates also declined with an increasing number of RASs; SVR rates were 88%, 64%, and 67% in patients with any single, double, or triple RAS, respectively.
The impact of baseline NS5A RASs on treatment outcomes with elbasvir/grazoprevir was unknown at the time of the elbasvir/grazoprevir phase 2/3 clinical program, and therefore, clinical trials were not designed to prospectively evaluate the efficacy of elbasvir/grazoprevir in the subgroup with baseline NS5A RASs. It was only during post hoc analysis of the clinical trial data that the impact of baseline RASs on SVR became apparent. Therefore, prospective evaluation of elbasvir/grazoprevir plus ribavirin for 16 weeks in patients with HCV GT1a infection and baseline RASs is lacking. The results from the present observational study may bridge an important gap in the understanding of the clinical utility of elbasvir/grazoprevir and, for the first time, may confirm the clinical effectiveness of an intensified elbasvir/grazoprevir plus ribavirin regimen for patients with GT1a infection and baseline RASs. These data also support the requirement for baseline NS5A resistance testing in patients with HCV GT1a infection prior to initiating treatment with elbasvir/grazoprevir. In Europe, the requirement for baseline resistance testing may be replaced by the use of baseline viral load (≤ 800,000 IU/mL vs. > 800,000 IU/mL) as a method for stratifying GT1a patients to a standard 12-week regimen of elbasvir/grazoprevir or a 16-week regimen of elbasvir/grazoprevir plus ribavirin [19]. A recent analysis has shown that baseline viral load does not perform well in identifying patients who require the 16-week regimen and can result in significant overtreatment in some patients [20]. This study confirmed that baseline resistance testing more accurately delineates patients with GT1a infection who can be treated for 12 weeks from those who require a 16-week regimen plus ribavirin [20].
Our analysis has several strengths. To our knowledge, it is one of the largest real-world observational studies of treatment effectiveness for patients with HCV GT1a infection and baseline NS5A RAS. In addition, this study used a hybrid methodological approach of extracting clinical and administrative data from the CDW, which was then confirmed through a structured manual review of EHRs. Manual abstraction of health records was used to confirm the presence of specific baseline NS5A RAS at the amino acid positions of interest, selected laboratory results, and outcomes of interest since these data may not be consistently recorded in the CDW administrative databases. This approach ensured a high validity and completeness of the study variables and outcomes of interest, including NS5A resistance data and the presence of treatment-emergent resistance.
Our study has several limitations. Underreporting and/or misreporting of data are possible sources of bias with retrospective observational studies; however, any such bias would be nondifferential and, therefore, unlikely to markedly influence the final results. We also used clinical definitions that have been previously validated in other VA studies. It is also possible that some VA patients sought care or treatment outside the VA system, which resulted in missing data. However, only one patient met the inclusion criteria for this analysis but did not have information available to determine SVR; this patient was therefore excluded from the analysis, suggesting a minimal impact on the study outcomes observed. In addition, baseline NS5A RAS testing may not be routinely performed as part of clinical care for patients with HCV GT1a infection. This study was also subject to the known limitations of secondary database studies with EHR review. Cross-validation between CDW and EHR was performed when applicable to minimize the impact of erroneous data. Most patients in our study were men, so the generalizability of these VA data to other populations is also a limitation.
5ConclusionsIn conclusion, our study extends the understanding of the effectiveness of elbasvir/grazoprevir treatment for patients with HCV GT1a infection and baseline NS5A RASs. The SVR rates of 78%–81% in our study show some improvement over the integrated data from the phase 2/3 clinical trial program, which indicated an SVR rate of ∼70% in patients with GT1a infection and baseline RASs receiving elbasvir/grazoprevir for 12 weeks without ribavirin. The use of chart abstraction to complement data from the CDW allowed a detailed assessment of NS5A RAS testing and permitted a full evaluation of the correlation between baseline NS5A RASs and treatment outcomes in this hard-to-treat patient subgroup. This analysis highlights the opportunities in leveraging real-world data to further understand treatment outcomes in smaller discrete subgroups of patients with HCV infection who are unable to be thoroughly evaluated in clinical trials.
FundingThis study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author contributionsConception, design, or planning of the study: BAH, TCM, DDH, FK, MR, JRK; acquisition of the data: KRC, JRK; analysis of the data: BAH, DDH, JRK; interpretation of the results: BAH, TCM, DDH, FK, MR, JRK; drafting of the manuscript: BAH, DDH, KRC; critically reviewing or revising the manuscript for important intellectual content: all authors.
Data availability statementAll aggregated analyses tables generated during this study are included in this published article. De-identified patient level datasets generated and/or analyzed in the study are not available owing to VA restrictions preventing the release of patient-level data.
DisclaimerThe opinions and assertions contained herein are the sole views of the authors and are not to be construed as official or as reflecting the views of the US Department of Veterans Affairs or the US government.
The authors thank the participants, their families, investigators, and site personnel who participated in this study. This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The work is also supported in part by the US Department of Veterans Affairs Center for Innovations in Quality, Effectiveness, and Safety (CIN 13–413) at Michael E. DeBakey VA Medical Center, Houston, TX, USA, where Drs. Kramer and Kanwal are research scientists. Medical writing and editorial assistance were provided by Tim Ibbotson, PhD, of ApotheCom (Yardley, PA, USA) and funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.