Some studies suggest chronic HCV infection diminishes responses to the anti-HBV vaccine. We evaluated the efficacy of double versus standard dose HBV vaccination among HCV patients without cirrhosis.
Patients and Methods141 adults with untreated chronic HCV were randomized to HBV vaccination with double dose (40μg) or standard dose (20μg) at 0, 1 and 6 months; 70 healthy HCV-negative patients given standard dose served as controls. Vaccine response was defined by anti-HBs ≥10 mIU/mL.
Results128 patients (60 double, 68 standard doses) completed the study. Patients were of median age 52 years, 61% female, 60% fibrosis <2 of 4, and 76% genotype 1 with median 6-log 10 IU/mL HCV RNA. Overall seroprotection rate was 76.7% (95% CI: 65-87) in the 40μg versus 73.5% (95% CI: 63-84) in the 20μg dose HCV-positive groups (p =0.68) and 91.2% (95%CI:84-99) in HCV-negative controls (p =0.011 and 0.003, respectively). In multivariate logistic regression, vaccine dose (double vs. standard dose) was not associated with vaccine response (OR=0.63, p =0.33). Of 32 HCV-infected patients who were non-responders to 3- doses, 25 received the fourth dose of vaccine. The fourth dose seroconversion rate for the 40μg and 20μg groups were 45.5% and 21.4%, respectively.
ConclusionsIn HCV-infected patients without cirrhosis, impaired responses to HBV vaccination cannot be overcome by the use of double dose HBV vaccination, but adding a fourth dose of vaccine for non-responders may be an effective strategy. Other adjuvant measures are needed to enhance seroconversion rates in these patients.
Trial registerU 1111-1264-2343 (www.ensaiosclinicos.gov.br)
It is estimated that 1% of the world's population has chronic HCV infection [1], with 71 million chronic carriers [2] at risk of developing cirrhosis and/or hepatocellular carcinoma (HCC). Furthermore, 400,000 [3] die every year due to complications of HCV-associated liver diseases. Although direct antiviral agents (DAA) can effectively eliminate infection with HCV globally, there remain barriers to access to diagnoses and treatment [4]. Additionally, among those with chronic HCV infection, susceptibility to HBV infection is common. In a recent study of more than 4000 patients with chronic HCV infection, 50% were found to be susceptible to HBV [5]. Another large Italian study of 6628 chronically infected with HCV under DAA therapy found two-thirds were without evidence of HBV seroprotection [6]. Thus, the provision of vaccination to HCV-infected persons remains a major public health need.
In patients with chronic liver disease secondary to HCV, superinfection by HBV can cause serious complications, including acute liver failure or a greater risk of progression to hepatic fibrosis and its complications [7]. All societal guidelines for the management of chronic HCV infection recommend testing of HBV status and vaccination of those without immunity [8,9]. Using the standard vaccination schedule of 3 doses administered at 0, 1 and 6 months, protective antibody concentrations are achieved in >95% of healthy infants, children and adolescents and in >90% of healthy adults [10]. Individuals with hepatic diseases demonstrate low immunogenicity to anti-HBV and some studies, particularly in patients with hepatitis C have presented a decreased response to anti-HBV immunization. Rates of vaccine responses vary from 40-60% and 60-80% in HCV-infected patients with and without cirrhosis, respectively, given a standard course of HBV vaccination [11,12]. This contrasts with the 90-95% responses reported in healthy populations [13]. In addition to advanced fibrosis and liver cirrhosis [14,15], other co-factors such as advanced age, overweight, renal failure, smoking, and co-infection with HIV may impact and reduce sero-responsiveness [16]. These factors may have a greater impact on patients with chronic HCV due to viral-induced immune exhaustion [17].
Our study sought to evaluate the efficacy of the vaccine, Butang®, using double dose versus standard dose vaccination in patients with chronic HCV infection without cirrhosis. Secondarily, we examined whether an additional fourth dose of the HBV vaccine could further improve the achievement of seroprotective titers in non-responders to 3 doses of the HBV vaccine.
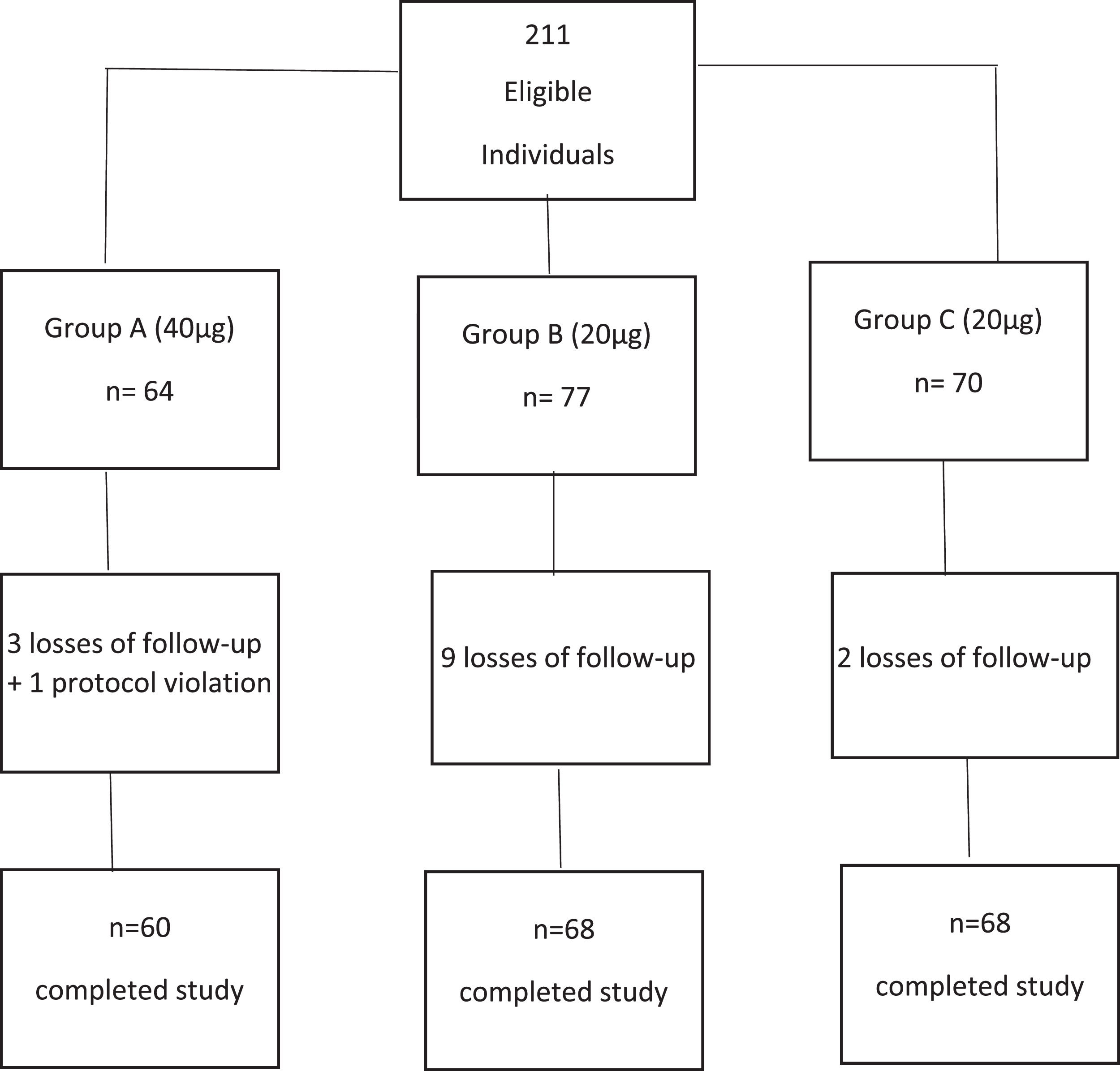
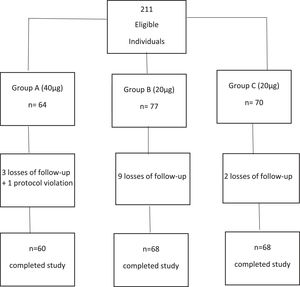
2Materials and Methods2.1Study design (Fig. 1)This is a randomized, active control trial to compare the efficacy of the recombinant HBV vaccine (Butang®; Butantã Institute, São Paulo, Brazil) 40μg (double dose) and 20μg (active control) given as three doses at 0, 1 and 6 months in patients with chronic HCV infection. Healthy non-HCV-infected adults were included as controls. The protocol was amended on June 26, 2012, to provide a 4th dose of vaccine to initial non-responders, with the dose group (20 or 40μg) maintained for this 4th dose.
2.2Study populationInclusion criteria for all participants were age ≥18 years old, negative for all HBV markers (HBsAg, anti-HBs and anti-HBc) and no prior history of vaccination for B hepatitis. For hepatitis C-infected participants, additional inclusion criteria were the presence of HCV RNA, naïve to HCV treatment and an absence of cirrhosis as documented by liver biopsy. Note that transient elastography was not available as a noninvasive method to assess hepatic fibrosis in our facility. The healthy controls were anti-HCV negative, negative for all HBV markers and anti-HIV negative.
All study participants were recruited from the outpatient clinic at the Division of Gastroenterology and Hepatology of the University Sao Paulo School of Medicine, São Paulo (Brazil). Healthy controls were recruited among the relatives of HCV-positive patients and who were without known liver disease and were negative for HCV and HBV serologic markers.
During the study period, no participant received interferon, immunosuppressive agents, antiviral drugs, or other vaccines. DAA therapy was not given during the entire period of the study.
2.3Serologic and virologic testingBlood samples were tested at a central laboratory and included complete blood count, prothrombin activity time, albumin, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) as well as serological tests (Architect immunoassays for HBsAg, anti-HBc, anti-HCV, Abbott Laboratories, Diagnostics Division, Ireland) according to the instructions of the manufacturer.
For the quantification of anti-HBs, the Architect Anti-HBs assay (Abbott Ireland Diagnostics Division) was used, whose reported global sensitivity and specificity corresponded to 97.54% and 99.67% with a confidence interval of 95% for both and a range of 0.0 to 1000 mIU/mL [18].
Anti-HCV positive individuals were tested for HCV-RNA using Abbott Real Time HCV assay, whose lower limits of quantification was 12 IU/mL [19].
2.4VaccineThe recombinant vaccine used was Butang®, a Brazilian vaccine produced by Butantã Institute (IB), using Hansenulla polymorpha yeast cells. The concentration of the antigen HBs (HBsAg) is equivalent to 25ugr/dose (in 1 ml) and the adjuvant used is aluminum hydroxide up to 1.25 mg (in aluminum) and thimerosal 0.05 mg. In pre-licensure studies, Butang® showed low reactogenicity and good immunogenicity in adults compared to the reference vaccine (Engerix B, Glaxo Smith Kline) [20,21].
2.5Randomization and blindingPatients with chronic hepatitis C were randomly assigned 1:1 to 40μg (group A) and 20μg (group B) of the HBV vaccine and healthy controls (group C) received 20μg HBV vaccine (Fig. 1). All participants received the vaccines at 0, 1 and 6 months. Randomization was performed using the sealed envelope system. To account for potential early dropouts, an additional 10% per group was added to each group before assigning to the sealed envelopes – for a total of 77 for group A and 77 for group B. Once patients consented, a randomly selected sealed opaque envelope was opened to assign group allocation. The study was only blinded to patients.
2.6Statistical analysis2.6.1Sample sizeTo calculate the sample size obtained in a simple random form, we used the following formula: N= Z2*[P *(1-P)] / D2, where Z= value of the normal standard distribution corresponding to the level of the desired trust (Z=1.96 to 95% confidence level), P= expected prevalence - 0.7% or 0.007, D= acceptable mistakes in the estimation (half range of the IC – precision measure)- 0.02. The sample size was based on an estimate of the proportion/prevalence with a specified level of confidence and precision. This yielded an N (by group) of 67 + 5% loss prediction= 70 cases by group [22,23].
2.6.2Analytic approachThe primary endpoint was anti-HBs ≥10 mIU/mL assessed one month after completing the vaccine series, interpreted as evidence of seroprotection.
Intention-to-treat analysis (ITT) included all participants, treating those dropping out or breaking protocol as treatment failures. Regardless of adherence to the vaccination and follow-up schedule, participants remained in their assigned dose regimen groups.
Per protocol analysis (PP) included only participants who completed all the steps of the study, with the necessary adherence to the prescribed procedures and follow-up. Participants dropping out or breaking protocol were excluded from this analysis.
Characteristics of participants were summarized using medians and percentages and compared by dose regimen (standard or double dose) using the Wilcoxon rank sum and Fisher's exact tests. Intention-to-treat and per protocol seroconversion rates and 95% confidence intervals (CI) were calculated by dose regimen and compared using Fisher's exact test. Among patients with chronic HCV infection, the association of anti-HBs seroconversion with dose regimen and clinical characteristics was assessed using logistic regression. Odds ratios with 95% CI were estimated. The primary explanatory variable of interest was the dose regimen (standard or double). The final multivariable model was adjusted for potential confounders, including age at first dose, sex, ethnicity, HCV genotype, presence of diabetes mellitus, obesity/overweight, tobacco and alcohol use.
2.7Ethical statementWritten informed consent was obtained from each patient included prior to any study procedures and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethics Committee for the Analysis of CAPPesq Research Projects of the Clinical Board of Hospital das Clínicas and of the Faculty of Medicine of the University of São Paulo (APPROVAL NUMBER/ID: 0389/09).
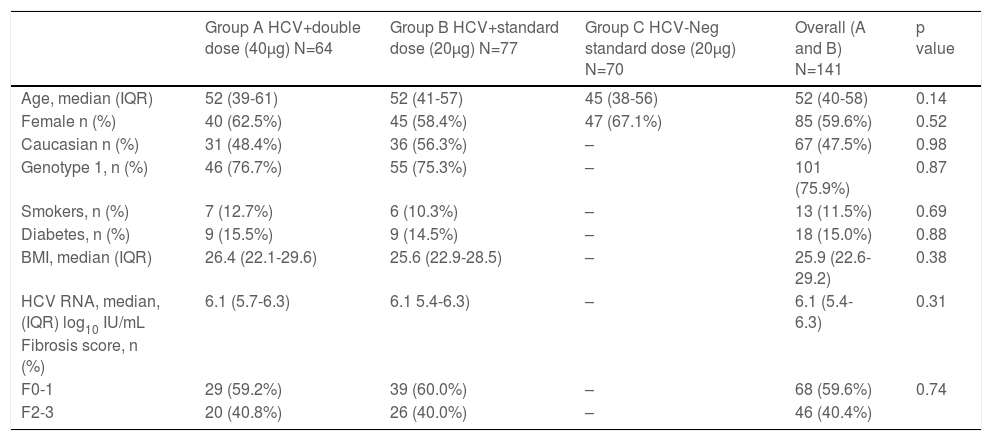
3Results3.1Study populationA total of 141 participants were randomized to double dose, group A (N=64) or standard dose, group B (N=77) and formed the intention-to-treat analysis. A total of 128 completed all three doses of vaccination and follow-up testing (per protocol analysis). Also, 70 healthy controls were recruited, with 68 completing vaccination and follow-up visits (Figure 1). In the intention-to-treat population, HCV-positive participants had a median age of 52 years old, 60% were female, and 48% were Caucasian, 40% F2-3 fibrosis, and 76% genotype 1 with a median 6.1-log10 HCV-RNA (Table 1). Controls were younger (median 45 years old) and had a slightly higher proportion of females (67%).
Baseline Characteristics of the Study Groups
Values expressed as number (percentage) or range (median)
BMI: body mass index
HCV-RNA: hepatitis C RNA level
IQR: interquartile range (Q3-Q1)
Applied tests: Chi-Square for the proportions and Mann-Whitney for the continuous variables
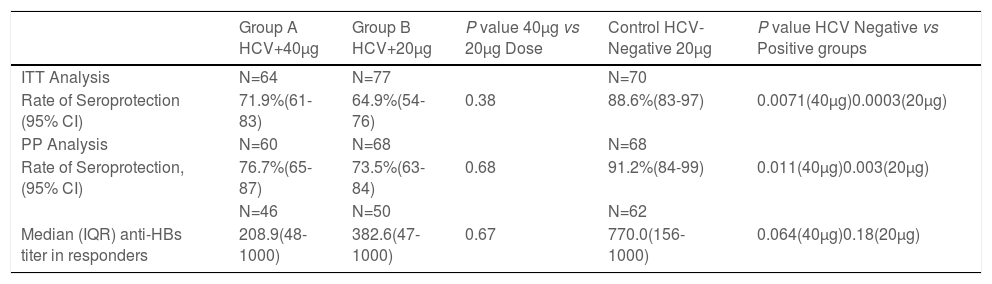
In the ITT analysis, the anti-HBs seroconversion rates (anti-HBs ≥10mUI/ml) were 71.9% (95% CI 61-83) for HCV-infected participants receiving a double dose, 64.9% (95% CI 54-76) for HCV-infected participants receiving standard dose and 88.6% (95% CI 83-97) in healthy controls (Table 2). Seroprotection rates were not significantly different between HCV-infected patients receiving double versus standard dose vaccination (p=0.38). Seroprotection rates for HCV-positive participants were significantly lower than healthy controls in both the double dose group (p= 0.0071) and standard dose group (p= 0.0003) (Table 2). None of the HCV-infected individuals or healthy controls became infected with HBV during the study period, as reflected by anti-HBc negativity.
HBV Seroprotection Rates: Intention to Treat and Per Protocol Results
ITT: intent to treat; PP: per protocol
Seroprotection defined by anti-HBs≥10 mIU/mL
IQR: interquartile range (Q3-Q1)
In the PP analysis, the seroconversion rates were 76.7% (95% CI 65-87) and 73.5% (95% CI 63-84) for HCV-infected participants receiving double doses versus standard-dose vaccine (p=0.68) (Table 2). The HCV-negative group had a significantly higher rate of seroconversion (91.2.%, 95% CI 84-99) when compared to the double dose group and the standard dose group.
3.3Efficacy of 4th dose of vaccine in initial non-respondersOf 32 HCV-infected patients who did not respond to the primary vaccination strategy (double dose versus single dose), 25 received a fourth dose of vaccine. Among them, eight seroconverted (32.0%; 95% CI 13.7-50.3) and seroconversion rates for the 40μg and 20μg groups were 45.5% (95% CI 16.0-74.9) and 21.4% (95% CI 0.0-42.9) respectively (p=0.19). Median anti-HBs titer for those eight who seroconverted after the fourth dose was 81 mIU/mL [IQR 53-94].
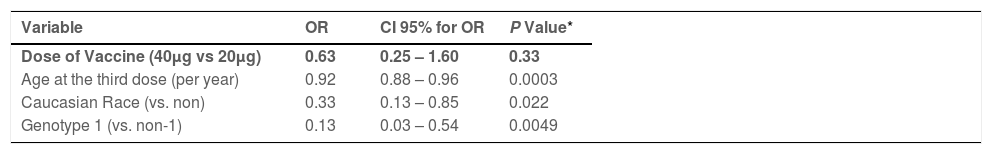
3.4Predictors of response to HBV vaccineThe variables were selected by Univariate Logistic Regression analysis (Table 1 -Supplement Material). In the Multivariate Logistic Regression analysis, the vaccine dose (double dose versus standard dose) was not associated with anti-HBs seroconversion (OR=0.63, p=0.33). Only advanced age (OR=0.92, p=0.0003), caucasian ethnicity (OR=0.33 p=0.022) and HCV genotype 1 (OR=0.13, p=0.0049) were associated with lower a probability of anti-HBs response in HCV-infected patients (Table 3). A significantly lower response in those >40 years of age (vs. <40 years) in HCV-infected patients but not in controls (Table 2-Supplement Material). Overall, comparing age and vaccine response by treatment groups, we found a statistical significance of less than 0.001 (p≤0.001) and we present the multiple comparisons in Fig. 1 -Supplement Material.
Multivariate Analysis, Factors Independently Associated to Achievement of Seroprotection with Vaccination Among HCV-Infected Participants
| Variable | OR | CI 95% for OR | P Value* |
|---|---|---|---|
| Dose of Vaccine (40μg vs 20μg) | 0.63 | 0.25 – 1.60 | 0.33 |
| Age at the third dose (per year) | 0.92 | 0.88 – 0.96 | 0.0003 |
| Caucasian Race (vs. non) | 0.33 | 0.13 – 0.85 | 0.022 |
| Genotype 1 (vs. non-1) | 0.13 | 0.03 – 0.54 | 0.0049 |
Variables evaluated in the model but not associated to vaccine response:
sex, BMI, smoking, diabetes, HCV-RNA titer, ALT level and fibrosis severity (F0-1 versus F2-3)
OR= odds ratio; CI= confidence interval
No serious adverse events were found during the vaccination period. The majority of symptoms, local or general, were mild in intensity. The most common adverse events were mild pain at the site of injection.
4DiscussionLiver diseases due to chronic hepatitis B and C virus infections are important global causes of liver-related morbidity and mortality [24]. Coinfection of HBV and HCV varies in prevalence, reflecting the endemicity of the viruses in different countries and risk profiles favoring transmission of the virus [25]. The precise number of HBV-HCV co-infected patients (HBV/HCV) is still unknown, but prevention of dual infection among those with chronic HCV relies upon vaccination against HBV. All the societal guidelines for the management of chronic HCV infection, as well as the World Health Organization guidance on viral hepatitis elimination [2,8,9,26], emphasize the importance of testing for HBV status amongst those with chronic HCV infection and undertaking vaccination in those at risk. Yet, the success of HBV vaccination in those with HCV is suboptimal, [27,28] even in those without cirrhosis, and alternative strategies aimed at improving seroprotection rates are needed. Double-dose vaccination is one strategy suggested to enhance seroprotection rates, but in our study, this approach did not increase the rates of seroprotection among HCV-infected non-cirrhotic patients. Interestingly, a fourth dose seemed to offer greater rates of seroconversion, though this finding needs to be validated in additional studies.
Prior studies of HBV vaccination in patients with chronic HCV infection were limited by the heterogeneity of the populations, specifically by the inclusion of patients with cirrhosis, a well-established risk factor for poor response to vaccines (HBV as well as others) [22,29,30]. Our study includes only patients without cirrhosis, yet, we confirm that chronic HCV infection per se can decrease the humoral response of hepatitis B vaccination [17,31,32]. Previous studies using Engerix-B at standard doses reported seroprotection rates among non-cirrhotics were 50.8% to 83% [33].
Recently, a new 2-dose vaccine with a novel adjuvant (Heplisav-B®) administered at 0 and 1 months was approved in the U.S., with a higher rate of seroprotective levels of anti-HBs achieved when compared to the standard 3-dose Engerix® vaccine [34], especially in the elderly, obese, diabetics and smokers. In the Constant study, not only consistently higher antibody concentrations were found with PreHevbrio vaccine (10μg/3 doses) after 2 and 3 doses vs. Engerix B (20μg/3 doses) in adults aged 18 to 45 years old, but also demonstrated non-inferiority in seroprotection rates after four weeks from the last dose, in the same age group [35]. Therefore, as stated before in our study, the Constant study emphasizes the importance of the prevention of hepatitis B in healthy young adults.
Our study uses a vaccine unique to Brazil, Butang®, that showed immunogenicity rates comparable to Engerix-B in pre-licensure studies [20,21]. We confirm the vaccine's high efficacy in non-HCV patients with a 92% seroprotection rate per protocol analysis. Yet, the vaccine response rate in non-cirrhotic patients with HCV was significantly lower, despite double dosing. Interestingly, another study using a double dose plus an accelerated immunization strategy (40μg monthly for three months) obtained 72% of seroprotection in patients with chronic liver disease compared to 92% of healthy controls [22]. Thus, collectively there appears to be little benefit from double dose, either using the standard vaccination schedule or with an accelerated approach. Interestingly, our data demonstrated that 32% (8 out of 25) of those receiving the 4th dose had seroprotective titers that were significantly increased, 81 mIU/mL (IQR 53-94). Despite the fact that the number of reconvened for the 4th additional dose corresponds to 25 patients among the 32 non-responders HCV, we would highlight the agreement that this strategy can be beneficial from assessing anti-HBs responses more uniformly after the 3rd dose, with an additional dose of the vaccine offered for those with suboptimal responses.
Mechanisms of diminished response to anti-HBV vaccines among non-cirrhotic HCV patients are unclear. Moorman et al. showed that non-response to the anti-HBV vaccine was associated with high levels of programmed death-1 PD-1 receptor, which mitigated T-cell stimulation and induced T-cell depletion [11]. The authors raise the possibility that blocking this negative signaling pathway might improve success rates of immunization in the setting of chronic viral infection. Shi et al. [31] analyzed the natural killer cell lectin-like receptor subfamily G member 1 (KLRG1) in chronic HCV-infected patients and showed KLRG1 was overexpressed on CD4+ T cells and associated with suppressed T cells proliferation and IL-2 secretion in chronic HCV infected which are HBV vaccine non-responders compared with HBV vaccine responders. Another study, using Engerix-B vaccine, demonstrated that activated B cells in chronic HCV-infected people produce immunoglobulins incapable of recognizing and destroying HBV, supposedly via upregulation of tumor necrosis factor (TNF) and Apo-L-related leukocyte-expressed ligand-1 (TALL-1) and inhibition of suppressor of cytokine signaling-1 (SOCS-1) [32]. Collectively, these studies lead to the obvious clinical question of whether HBV vaccination in patients with chronic HCV infection should be delayed or repeated after HCV cure.
Age and comorbidities are well-recognized factors in vaccine responsiveness. In our study, using a logistic regression model, age was the factor most strongly associated with anti-HBV seroconversion rates. BMI, smoking and diabetes were not predictive of vaccine responsiveness in our study, though the study was underpowered to evaluate the interactions between age and these comorbidities. Unexpectedly we found HCV genotype to be associated with vaccine response, though two previous studies found that HCV-infected patients and infected with genotype 1 had a poorer anti-HBV vaccine response [33]. The underlying reason for this association remains to be elucidated.
The study has some limitations. First, the vaccine used is unique to Brazil and without prior testing that other to non-Brazilian populations. However, this vaccine was previously compared to Engerix B in healthy controls with equivalent results [20,21], and we included a healthy control population for comparison. Second, not all participants returned for follow-up titers, but to address this limitation, we present both ITT and PP results. Finally, the amendment to the protocol to include the 4th dose was insufficiently powered to assess for efficacy compared to the standard three-dose vaccine schedule.
5ConclusionsIn summary, in this randomized study comparing double versus standard dose vaccination for HBV in patients with chronic HCV infection, seroprotection rates were not shown to be significantly different. Thus, this strategy is not recommended. This study highlights the importance of vaccination at a younger age, ideally prior to infection with HCV and/or the onset of comorbidities that influence vaccine responses. Evaluation of vaccine responsiveness after HCV cure as well as evaluation of new third-generation vaccines such as Hepagene™ containing pre-S1 and pre-S2 proteins and Sci-B-Vac™ [36,37], vaccines with adjuvants [38] such as HEPLISAV-B and/or additional doses [39] should be considered.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author ContributionsRoseane P. Medeiros: acquisition of data; analysis and interpretation of data; drafting of the manuscripts; study supervision; Norah A. Terrault: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Daniel F. Mazo: critical revision of the manuscript for important intellectual content; Claudia P. Oliveira: critical revision of the manuscript for important intellectual content; Jennifer Dodge: statistical analysis; Patricia M. Zitelli: administrative, technical, or material support; Marta H. Lopes: administrative, technical, or material support; Flair J. Carrilho: administrative, technical, or material support; Mário G. Pessoa: study concept and design, analysis and interpretation of data; critical revision of the manuscript for important intellectual content; study supervision