Lower antibody (Ab) responses after SARS-CoV-2 vaccination have been reported in liver transplant (LT) recipients and those with chronic liver diseases (CLD). The role of a booster dose in those with poor responses to initial vaccination is not well defined.
MethodsIn this prospective study, we determined antibody (Ab) response to spike protein after a booster dose in LT recipients and those with chronic liver diseases (CLD) with and without cirrhosis after they had a poor response to an initial standard regimen.
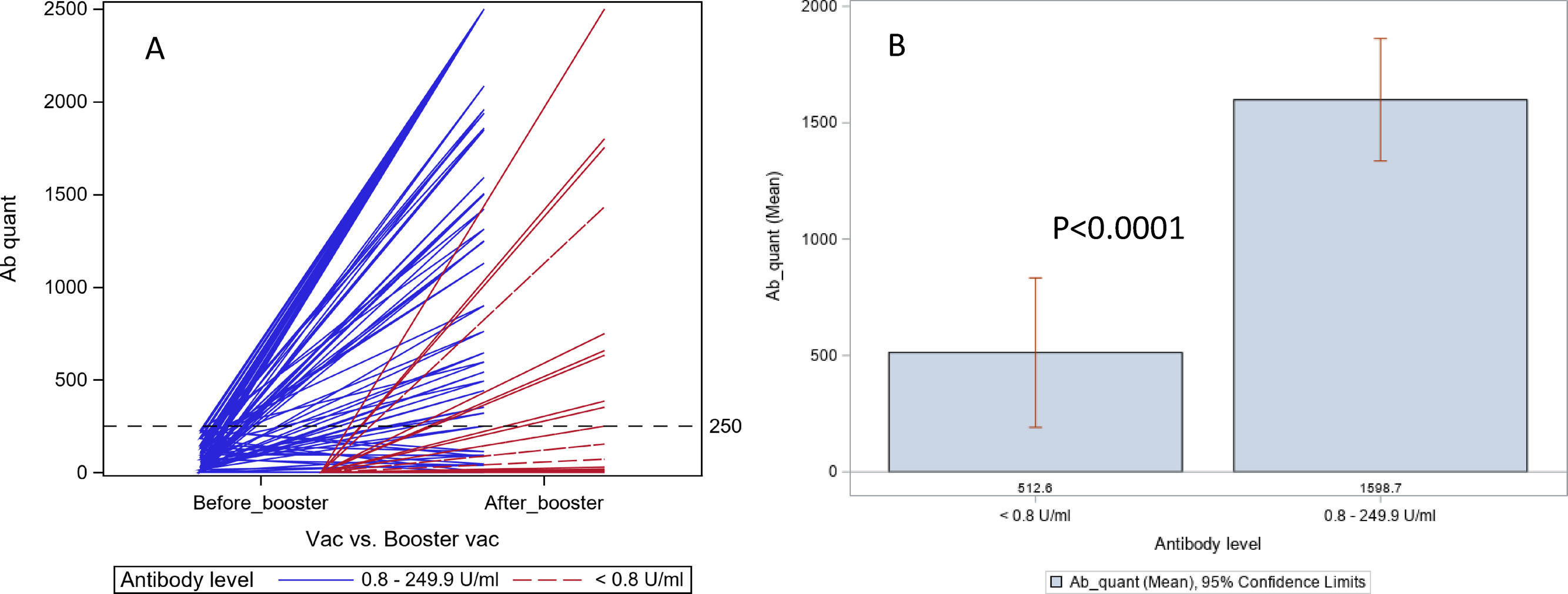
ResultsOf the 80 patients enrolled, 45 had LT, and 35 had CLD (18 with cirrhosis). A booster dose was given at a median of 138.5 days after the completion of the standard regimen. After the booster dose, 58 (73%, 31 LT, 27 CLD) had good response (≥250 U/mL), and 22 (28%, 14 LT, and 8 CLD) had poor response (7 undetectable and 15 with low Ab levels). No patient had any serious adverse events. The antibody responses were lower in those who had undetectable Ab (80 U/mL) than those who had low levels of Ab (0.80-249 U/mL) after the standard vaccination regimen (42% vs. 87%, p=0.0001). The antibody responses after homologous and heterologous booster doses were similar.
ConclusionsWe have shown that a booster dose will enhance Ab responses in LT recipients and those with CLD who had poor responses after an initial vaccine regimen.
Poor antibody (Ab) responses to standard vaccination against SARS-CoV-2 have been observed in liver transplant (LT) recipients and those with chronic liver diseases (CLD) [1–2]. In a previous study, we had reported that 61% of LT recipients and 24% of those with CLD had poor Ab response to spike proteins after receiving a standard regimen of SARS-CoV-2 vaccine. In our study, most patients with CLD who had poor Ab responses were on immunosuppressants, and immunosuppression, including the number of immunosuppressants, was an independent predictor for lower Ab responses [1]. Other studies have corroborated the above observations in organ transplant recipients [3–5]. The Center for Disease Control and Prevention has recently recommended booster dose for immunocompromised and elderly patient population 6-8 months after the initial vaccination [6]. However, the Ab responses in those who fail to mount a good response after the initial vaccination have not been well established. In this prospective study, we assessed the antibody (Ab) responses after a booster dose with either mRNA (Pfizer/Moderna) or Johnson & Johnson (JnJ) vaccine in LT recipients and those with CLD who had low Ab levels after the initial standard regimen as recommended by the manufacturers.
2MethodsWe studied LT recipients and those with CLD (with or without cirrhosis) who had poor antibody response to SARS-CoV-2 spike protein after 2 doses of mRNA vaccines or a single dose of JnJ vaccine. We defined poor Ab response as ‘undetectable’ if Ab levels are ≤0.80 U/mL and ‘low’ if levels are between 0.80 U/mL and 249.9 U/mL. Patients who had a previous positive SARS-CoV-2 test (RT-PCR, antigen, or Ab tests) were not included.
Antibodies (predominantly IgG) against receptor binding domain to SAR-CoV-2 spike protein was assessed using the Roche electrochemiluminescence, semi-quantitative immunoassay (Elecsys® Ant-SARS-CoV2 semi-quantitative) via LabCorp. It is a commercially available assay under the Emergency Use Authorization. The laboratory had reported that the test was excellent (99.98% negative and 96.6% positive) in detecting antibodies in patients with PCR confirmed COVID-19 [7]. The Ab to spoke protein has been shown to correlate with in vitro neutralization of SARS-CoV-2 [8]. According to the manufacturer, titers ≥250 U/mL were considered positive and <0.8 U/mL were negative.
The demographic characteristics were summarized as mean and standard deviation or median for continuous variables or frequency for categorical variables. A Chi-square test was used to calculate the difference among the groups after the booster dose. All analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
2.1Ethical statementThe institutional review board approved the study. The informed consent was obtained verbally and recorded in the electronic medical records as requested by the institutional review board.
3ResultsWe studied 80 patients with a poor response after the standard dose of vaccine, of which 45 were LT recipients, 18 had cirrhosis (six decompensated), and 17 patients had CLD without cirrhosis. Among these, 47 of 80 (59%) patients received booster doses with Pfizer, 27 (34%) Moderna, and 6 (7%) JnJ vaccine; the corresponding numbers for initial vaccination was 41 (51%), 24 (30%) and 15 (19%) respectively. Patient characteristics, concomitant medications, and Ab levels are shown in Table 1. A booster dose was given at a median of 138.5 days after the completion of the standard regimen, and antibody levels after the booster dose were measured after a median of 28 days (Table 1). Of those, 19 received a heterologous vaccine (mRNA followed by JnJ or vice versa), and 61 received a homologous vaccine (mRNA followed by mRNA or JnJ followed by JnJ) (Supplementary Table 1).
Patient characteristics, comorbidities and antibody response to booster dose for COVID-19 vaccination.
AA: African American; Ab: antibody; AIH: autoimmune hepatitis; BMI: body mass index; b/t: between CC: compensated cirrhosis; DC: decompensated cirrhosis; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; SD: standard deviation. *Others: Hispanic and Asians. **Other liver disease: hepatocellular carcinoma, hemochromatosis, portal hypertension, porto-pulmonary hypertension. ***Other medications: cyclosporine, mycophenolic acid, sirolimus and TK Inhibitor.
Of the 80 patients who had a booster dose, 7 (9%) remained with undetectable (<0.80 U/mL) Ab levels, 15 (19%) had low Ab levels (0.80-249 U/mL) and 58 (73 %) had Ab levels above 250U/mL (Fig. 1, Table 1).
In 26 patients who had undetectable (<0.80 U/mL) Ab titer before the booster dose, 11(42%) had good (≥250 U/mL) response, 10 had low Ab titers, and 5 remained to have undetectable Ab titers. In 54 patients who had low Ab titers before booster dose, 47 (87%) had a positive response, 5 had low Ab titers, and 2 remained to have undetectable Ab titers. The difference in response after booster dose between the undetectable and low Ab group was highly significant (42% vs. 87%, p=0.0001).
Liver transplant recipients: Of the 45 patients with LT, Ab titers were undetectable in 16 (36%) and low in 29 (64%) patients (Table 1). After a booster dose, 31 (69%) patients had positive Ab titers (≥ 250 U/mL) and 14 (31%) continued to have low (n=10) or undetectable (n=4) Ab levels. Of the 10 patients with low Ab titers, 4 were on tacrolimus alone, 2 were on tacrolimus plus mycophenolate, 1 was on tacrolimus plus prednisone, 1 was on mycophenolate and prednisone, and 2 were on all three immunosuppressive medications. Of the 4 patients with undetectable titers, 2 were on tacrolimus plus mycophenolate, 1 was on tacrolimus plus prednisone, and 1 was on tacrolimus alone.
Chronic liver diseases: Thirteen of 18 cirrhotic (6 with decompensated cirrhosis) patients had low Ab titers, and 5 had undetectable Ab levels before the booster dose. The majority of the patients (n=15, 83%) had a good response after the booster dose. Of the 3 patients with poor responses, 2 had decompensated cirrhosis (Supplementary Table 2).
Among the 17 patients with CLD without cirrhosis, 12 had low Ab levels, and 5 had undetectable Ab. After the booster vaccination, 12 (71%) patients had a good response, and 5 (29%) patients had a poor response (2 undetectable and 3 low Ab levels). Both patients with undetectable Ab had autoimmune hepatitis and were on prednisone (n=1) or prednisone plus mycophenolate (n=1). Of the 3 patients with poor response, 1 had autoimmune hepatitis (on prednisone plus mycophenolate), 1 was elderly (84 years) with chronic hepatitis C, and 1 had non-alcoholic fatty liver disease.
There were no significant differences in good Ab responses (71% vs. 77%. p=0.91) between the immunosuppressed (defined as liver transplant or at least one immunosuppressant medicine, n=58) and the immunocompetent patients (n=22) after the booster dose.
Type of vaccine: Of the 80 patients, 61 received a homologous vaccine, and 19 received a heterologous vaccine. There was no significant difference (p=0.67) in good Ab responses between homologous (45/61, 74%) or heterologous booster (13/19, 68%) vaccine groups. We did not compare the order effect within the heterologous booster vaccines subgroup because of the small sample size. Three of 6 patients who received the JnJ vaccine, 19 of 27 who received Moderna, and 36 of 47 who received Pfizer booster dose had a good response (p=0.26).
No serious adverse events were reported after the booster dose. The most common side effects (≥5%) after the booster dose were local pain at the injection site (43%) and fatigue (11%).
4DiscussionOur study found that a booster dose of the SARS-CoV-2 vaccine increased Ab levels significantly in 69% of LT recipients and 77% with chronic liver disease, irrespective of the presence of cirrhosis, who had relatively low Ab levels after an initial standard vaccine regimen. Our findings in LT recipients confirm the observations made by other investigators in their recent studies [9,10]. Additionally, a randomized placebo-controlled trial in organ-transplant recipients confirmed that the 3rd dose of mRNA vaccine increased spike protein Ab levels above 100 U/ml in 55% patients compared to 18% who received placebo [11]. Unlike our study, the above study included patients irrespective of their pre-booster Ab levels, but showed that the increase in Ab levels was not a random event. We also found that those who had undetectable Ab levels after the initial standard regimen were less likely (42% vs. 87%, p=0.0001) to have a good response after the booster dose compared to those who had low Ab levels. The antibody responses were similar after a homologous and heterologous booster dose.
To our knowledge, there has been no previous study examining Ab responses to booster dose in non-transplant recipients with CLD (including those with cirrhosis). Although our sample size with cirrhosis was small, the majority responded to the booster dose regardless of the presence of cirrhosis. Indeed, of 5 patients with CLD (without cirrhosis) who had poor Ab response after booster dose, 3 were on immunosuppression for autoimmune hepatitis.
In our study, there was no significant difference between homologous or heterologous booster vaccines on the Ab responses. However, the small number of patients (n=19) who had heterologous booster doses did not permit us to make any firm conclusions (supplementary table 1). The question regarding the preferred type of booster dose, viral-vector, inactivated or mRNA vaccine, is currently being addressed in healthy population in many studies [12–15]. However, there are emerging data to support that heterologous vaccines produce better immune responses than homologous vaccine regimens [13,14]. In volunteers, booster doses of mRNA vaccine produced better antibody responses, but adenoviral vector vaccine produced better T-cell responses [15]. One study, that compared booster dose (3rd dose) with inactivated vaccine or mRNA vaccine, showed a higher antibody response in those who received a booster dose of mRNA vaccine irrespective of the type of initial vaccine [13]. A small study has also shown that antibody responses are better in those who received 2nd dose of mRNA vaccine among those who received 1st dose of adeno viral vector vaccine [16]. However, there is a paucity of similar data in immunocompromised, and particularly liver transplant recipients and those with chronic liver diseases, particularly with booster dose with adenoviral vaccine [17–19]. Despite the absence of any supporting data, it may be reasonable to monitor antibody responses after the booster dose and provide prophylactic neutralizing monoclonal antibody infusions or an additional booster dose in those with suboptimal responses [20]. In countries where only viral vector or inactivated vaccine is available, it will be prudent to give a booster dose with the available vaccine. The CDC or vaccine manufacturers do not recommend giving booster doses based on SAR-CoV-2 spike protein Ab levels, and neither do they make any specific recommendation regarding the type of vaccine for boosting the humoral responses.
There are few limitations to our study. There is no unified definition for undetectable Ab which is a limitation when interpreting various studies. In a previous study, we had defined groups as < 0.4 (undetectable), 0.4 – 249 (suboptimal), and ≥250 (optimal). Since then, LabCorp has defined less than 0.80 U/mL as negative, and for that reason we used <0.80 U/ml as undetectable. Our sample size was also small mainly because CDC recommended universal booster dose as our study was in progress.
5ConclusionsIn summary, as a proof of concept, we have shown that booster dose will enhance Ab responses in those who had poor responses after conventional vaccine regimen, and this should be explored further in those with chronic liver diseases and immunocompromised subjects.
Author contributionsPJT contributed to the conception and design, analysis, interpretation of the data, and the critical revision for important intellectual content. MC and IN collected the data and did the analysis. FL did the statistical analysis. All authors approved the final version and agreed to be accountable for all aspects of the work.
Data availabilityDeidentified data available at request.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interestNone.