Although hyponatremia and hepatic encephalopathy (HE) are known independent predictors of mortality, their combined effect is unknown. We investigated whether the inpatient mortality differed among patients with both hyponatremia and HE compared to those with either hyponatremia or HE alone.
Materials and MethodsIn this retrospective study, data were extracted from the National Inpatient Sample (NIS) to identify US adults (aged ≥18 years) with cirrhosis between January 1st, 2016, and December 31st, 2017. We analyzed the effects of hyponatremia, HE, or a combination of hyponatremia and HE on inpatient mortality using logistic regression.
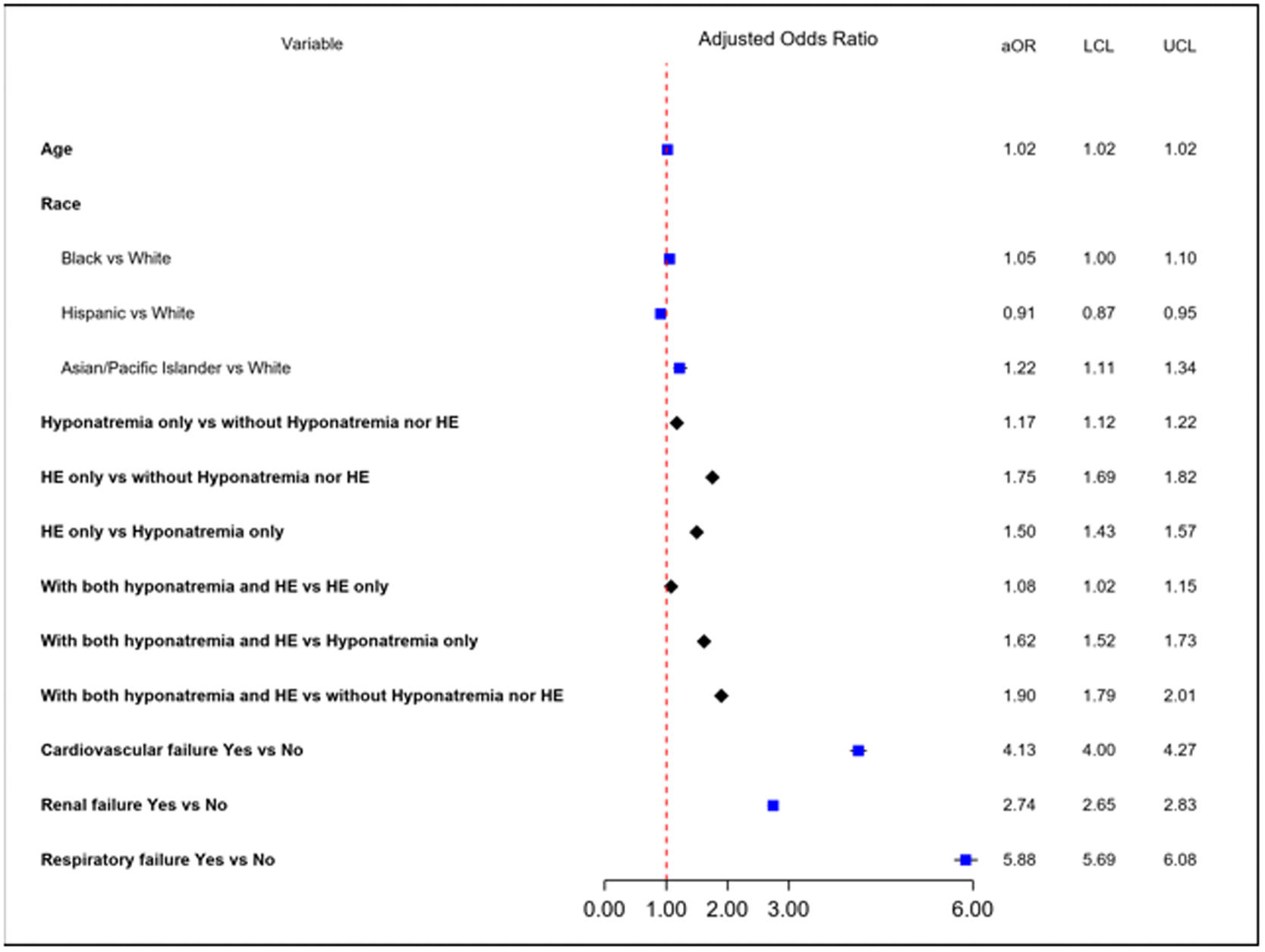
ResultsAmong 309,841 cirrhosis-related admissions, 22,870 (7%) patients died during hospitalization. Those with a combination of hyponatremia and HE had higher mortality (14%) than those with HE only (11%), hyponatremia only (9%), and neither hyponatremia nor HE (6%) (p<0.001). When compared to patients without hyponatremia or HE, patients with both hyponatremia and HE had the highest odds (adjusted odds ratio or aOR) of inpatient mortality (aOR 1.90, 95% CI: 1.79 – 2.01) followed by patients with HE only (aOR 1.75, 95% CI: 1.69 – 1.82) and patients with hyponatremia only (aOR 1.17, 95% CI: 1.12 – 1.22). Patients with HE only had 50% higher odds of inpatient mortality when compared to those with hyponatremia only (aOR: 1.50, 95% CI: 1.43 – 1.57).
ConclusionsIn this nationwide study, the presence of both hyponatremia and HE was associated with higher inpatient mortality than either hyponatremia or HE alone.
Liver disease accounts for nearly 2 million deaths per year worldwide, and there has been an increase in the age-standardized prevalence of decompensated cirrhosis between 1990 and 2017 [1]. The morbidity and mortality associated with cirrhosis is known to increase significantly once hepatic decompensation occurs, and 1-year mortality may reach over 50% depending on the type, the severity and the cause of hepatic decompensation [2–6]. Overt hepatic encephalopathy (HE) and hyponatremia are both associated with very high short-term mortality [4–12]. Compared to cirrhotic patients without HE, those with overt HE have shorter survival and a two-fold greater risk of 1-year mortality [4-5,13]. Additionally, HE has become one of the primary causes of hospitalization and readmission [7,8]. Hyponatremia, another complication of decompensated cirrhosis, is prevalent in more than half (57%) of hospitalized cirrhotic patients and is an independent predictor of mortality, including those who present with acute on chronic liver failure (ACLF) [10–13].
Hyponatremia and HE are both interconnected and can occur together in hospitalized patients with cirrhosis [10,14–17]. Hyponatremia may affect the osmotic balance of astrocytes and may result in astrocyte swelling, compounding the glutamine induced astrocyte swelling and dysfunction induced by hyperammonemia. Furthermore, the depletion of organic osmolytes such as myoinositol in the presence of hyponatremia predisposes brain cells to cerebral edema. Hyponatremia in this setting is a second osmotic hit to the astrocytes, exacerbating intracellular edema and thereby precipitating or worsening overt HE [15–17]. While hyponatremia and HE are known independent predictors of mortality, their combined effect on mortality remains unknown. This study determined whether there was a difference in inpatient mortality in those with hyponatremia and HE compared to those with either hyponatremia or HE alone using a large administrative database.
2Materials and Methods2.1Data acquisitionThis was a retrospective study where data were extracted from the National Inpatient Sample (NIS) from 2016 to 2017. The NIS is the largest publicly available administrative database developed by the Agency for Healthcare Research and Quality (AHRQ) as part of the Healthcare cost and utilization project (HCUP) [18]. It represents approximately 20% stratified sample of discharges from all community hospitals but excludes long-term acute care and short-term rehabilitation hospitals. It contains information on more than 7 million hospital discharges annually, including information about primary and secondary diagnoses during each hospital stay, procedures, patients' demographics, severity and comorbidity measures, hospital characteristics, payment source, mortality, total charges, and length of stay. Beginning from October 1st, 2015, all hospital administrative data across the United States adopted ICD 10 codes for disease classification as well as for procedures. The calendar year for 2016 and 2017 included in this study uses ICD 10 CM/PCS codes.
2.2Study population and variablesData of adults (aged ≥18 years) with cirrhosis between January 1st, 2016, and December 31st, 2017, were collected after excluding those with hepatocellular carcinoma and acute liver failure. Patients with cirrhosis were identified by primary or secondary ICD codes for cirrhosis and/or by the presence of any of the associated complications, including esophageal varices, bleeding esophageal varices, ascites, HE, spontaneous bacterial peritonitis (SBP), portal hypertension, hepato-pulmonary syndrome, and hepatorenal syndrome. The ICD 10 codes used for the diagnosis of cirrhosis are shown (Supplementary Table 1).
We collected patients' characteristics, including age, sex, and race. Since race/ethnicity was not available for Georgia, Illinois, Kentucky, Maine, Minnesota, Nebraska, Nevada, Ohio, Oregon, Washington, and West Virginia, a dummy variable was created for missing data in the models to prevent the observation from being dropped. Hospital characteristics, including hospital region, bed size, academic status, and healthcare payers, were collected (Supplementary Table 2). All complications, including bleeding esophageal varices, ascites, HE, SBP, hepato-pulmonary syndrome, hepatorenal syndrome, and major organ failures, were collected. The Elixhauser comorbidity index was calculated by using the tool provided by HCUP after excluding liver disease [19,20].
2.3The outcome of interestThe primary outcome of our study was to compare inpatient mortality in those with both hyponatremia and HE to those with hyponatremia or HE only.
2.4Statistical analysisWe divided patients with cirrhosis into four groups: 1) those without hyponatremia or HE, 2) those with both hyponatremia and HE, 3) with HE only, and 4) with hyponatremia only. Descriptive statistics for patient characteristics are provided as means and standard deviations (SDs) or median and interquartile range (IQR) as appropriate for continuous variables and frequencies for categorical variables. Patient characteristics were compared among four groups using the Chi-Square test for categorical variables and one-way ANOVA for continuous variables; normality was checked for all the continuous variables, nonparametric Kruskal-Wallis test was used when data were not normally distributed. We made a pairwise comparison between different groups using Bonferroni-adjusted multiple Chi-Square tests for categorical variables and ANOVA or nonparametric Wilcoxon Pairwise Two-Sided Multiple Comparison Analysis with DSCF (Dwass, Steel, Critchlow-Fligner) Method as appropriate for continuous variables.
Logistic regression analysis was performed to evaluate the effect of groups (neither hyponatremia nor HE, HE only, hyponatremia only and hyponatremia plus HE) and other risk factors, which included demographics, comorbidities, etiology of liver disease, and complications secondary to cirrhosis, on inpatient mortality. We started with univariate analysis, followed by multivariable analysis using a forward model selection approach. The final model was selected by balancing goodness-of-fit (e.g., Bayesian information criteria). Collinearity was checked; if two variables were highly correlated, one was included in the model each time. The final model retained variables with p values of 0.05 or less. Estimations of adjusted odds ratios (aOR) and 95% confidence intervals (CI) are reported. All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA).
2.5Ethical statementThe study used a deidentified database and was exempt from IR approval and the informed consent process. Consent to participate: not applicable since the deidentified database was used.
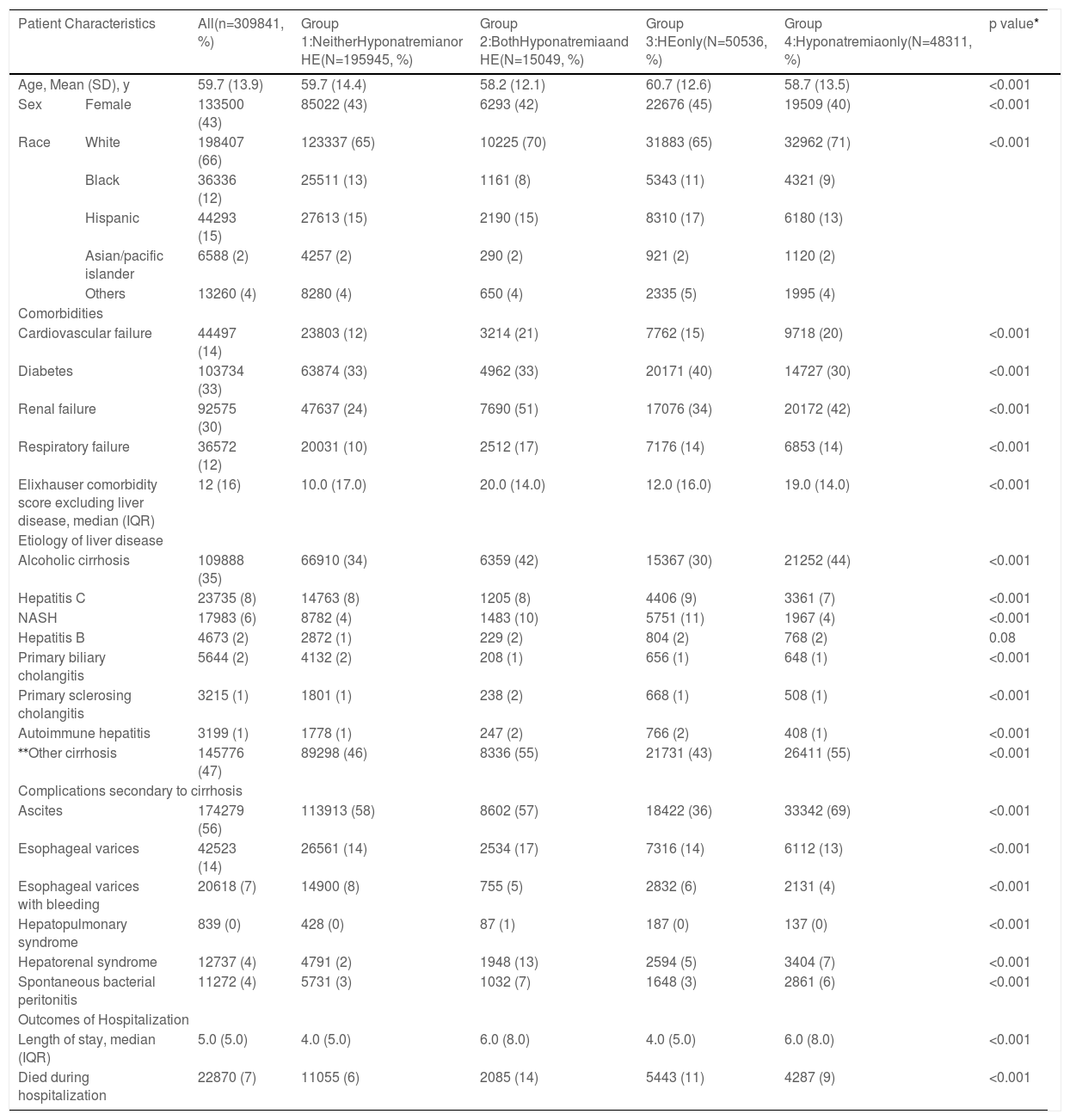
3Results3.1Characteristics of the study cohortAmong 309,841 cirrhosis-related hospitalizations from 2016 to 2017, 195,945 (63.2%) had neither hyponatremia nor HE; 50,536 (16.3%) had HE only, 48,311 (15.6%) had hyponatremia only, and 15,049 (4.9%) had both hyponatremia and HE (Table 1). The mean (SD) age was 59.7 (13.9) years, and 133,500 (43%) were female. The majority of patients were white, followed by Hispanics and blacks (Table 1). In all groups, the majority of the payers were Medicare (145,686, 47%), located in an urban teaching setting (217,176, 70%), and were treated in a large hospital bed size (170,782, 55%) (Supplementary Table 2).
Clinical characteristics of the study cohort at the time of admission.
| Patient Characteristics | All(n=309841, %) | Group 1:NeitherHyponatremianor HE(N=195945, %) | Group 2:BothHyponatremiaand HE(N=15049, %) | Group 3:HEonly(N=50536, %) | Group 4:Hyponatremiaonly(N=48311, %) | p value* | |
|---|---|---|---|---|---|---|---|
| Age, Mean (SD), y | 59.7 (13.9) | 59.7 (14.4) | 58.2 (12.1) | 60.7 (12.6) | 58.7 (13.5) | <0.001 | |
| Sex | Female | 133500 (43) | 85022 (43) | 6293 (42) | 22676 (45) | 19509 (40) | <0.001 |
| Race | White | 198407 (66) | 123337 (65) | 10225 (70) | 31883 (65) | 32962 (71) | <0.001 |
| Black | 36336 (12) | 25511 (13) | 1161 (8) | 5343 (11) | 4321 (9) | ||
| Hispanic | 44293 (15) | 27613 (15) | 2190 (15) | 8310 (17) | 6180 (13) | ||
| Asian/pacific islander | 6588 (2) | 4257 (2) | 290 (2) | 921 (2) | 1120 (2) | ||
| Others | 13260 (4) | 8280 (4) | 650 (4) | 2335 (5) | 1995 (4) | ||
| Comorbidities | |||||||
| Cardiovascular failure | 44497 (14) | 23803 (12) | 3214 (21) | 7762 (15) | 9718 (20) | <0.001 | |
| Diabetes | 103734 (33) | 63874 (33) | 4962 (33) | 20171 (40) | 14727 (30) | <0.001 | |
| Renal failure | 92575 (30) | 47637 (24) | 7690 (51) | 17076 (34) | 20172 (42) | <0.001 | |
| Respiratory failure | 36572 (12) | 20031 (10) | 2512 (17) | 7176 (14) | 6853 (14) | <0.001 | |
| Elixhauser comorbidity score excluding liver disease, median (IQR) | 12 (16) | 10.0 (17.0) | 20.0 (14.0) | 12.0 (16.0) | 19.0 (14.0) | <0.001 | |
| Etiology of liver disease | |||||||
| Alcoholic cirrhosis | 109888 (35) | 66910 (34) | 6359 (42) | 15367 (30) | 21252 (44) | <0.001 | |
| Hepatitis C | 23735 (8) | 14763 (8) | 1205 (8) | 4406 (9) | 3361 (7) | <0.001 | |
| NASH | 17983 (6) | 8782 (4) | 1483 (10) | 5751 (11) | 1967 (4) | <0.001 | |
| Hepatitis B | 4673 (2) | 2872 (1) | 229 (2) | 804 (2) | 768 (2) | 0.08 | |
| Primary biliary cholangitis | 5644 (2) | 4132 (2) | 208 (1) | 656 (1) | 648 (1) | <0.001 | |
| Primary sclerosing cholangitis | 3215 (1) | 1801 (1) | 238 (2) | 668 (1) | 508 (1) | <0.001 | |
| Autoimmune hepatitis | 3199 (1) | 1778 (1) | 247 (2) | 766 (2) | 408 (1) | <0.001 | |
| ⁎⁎Other cirrhosis | 145776 (47) | 89298 (46) | 8336 (55) | 21731 (43) | 26411 (55) | <0.001 | |
| Complications secondary to cirrhosis | |||||||
| Ascites | 174279 (56) | 113913 (58) | 8602 (57) | 18422 (36) | 33342 (69) | <0.001 | |
| Esophageal varices | 42523 (14) | 26561 (14) | 2534 (17) | 7316 (14) | 6112 (13) | <0.001 | |
| Esophageal varices with bleeding | 20618 (7) | 14900 (8) | 755 (5) | 2832 (6) | 2131 (4) | <0.001 | |
| Hepatopulmonary syndrome | 839 (0) | 428 (0) | 87 (1) | 187 (0) | 137 (0) | <0.001 | |
| Hepatorenal syndrome | 12737 (4) | 4791 (2) | 1948 (13) | 2594 (5) | 3404 (7) | <0.001 | |
| Spontaneous bacterial peritonitis | 11272 (4) | 5731 (3) | 1032 (7) | 1648 (3) | 2861 (6) | <0.001 | |
| Outcomes of Hospitalization | |||||||
| Length of stay, median (IQR) | 5.0 (5.0) | 4.0 (5.0) | 6.0 (8.0) | 4.0 (5.0) | 6.0 (8.0) | <0.001 | |
| Died during hospitalization | 22870 (7) | 11055 (6) | 2085 (14) | 5443 (11) | 4287 (9) | <0.001 | |
HE: hepatic encephalopathy; IQR: interquartile range; NASH: nonalcoholic steatohepatitis; SD: standard deviation.
The most common etiology of liver disease was alcoholic liver disease (35%), followed by hepatitis C (8%) and nonalcoholic steatohepatitis (NASH) (6%). In terms of cirrhosis-related complications, ascites was the most common (174,279, 56%). Diabetes mellitus (DM) was present in one-third of the study cohort. Among organ failures, renal failure was seen in 30%, cardiovascular failure was seen in 14%, and respiratory failure was seen in 12% of patients (Table 1). The overall prevalence of ascites was 56% and hepatorenal syndrome (HRS) was 4%; the distribution among the groups is shown in Table 1.
There were many differences among the groups, as shown in Table 1. While DM was more common in those with HE, renal failure and cardiovascular failure were more common in hyponatremia patients. Half of the patients with hyponatremia and HE had renal failure. NIS datasets do not differentiate between HRS type 1 or type 2. However, it is reasonable to assume that most of these patients had HRS-AKI (by the revised definition). HRS was more common in those with hyponatremia and HE (13%) compared to only 2% in those without HE or hyponatremia (Table 1). HRS was also twice as common (13%) in those with HE (5%) and hyponatremia (7%) compared to those with either HE or hyponatremia only. The median Elixhauser comorbidity scores were higher in hyponatremia groups.
In the overall study cohort, the median (IQR) length of stay was 5 ± 5 days. The median (IQR) length of stay in the hyponatremia plus HE group was 6 ± 8 days, similar to the group with hyponatremia only but longer than the HE only group (4 ± 5) days. Only 36.7% with both HE and hyponatremia were discharged home, in contrast to 56.6% without HE or hyponatremia (Supplementary Table 3).
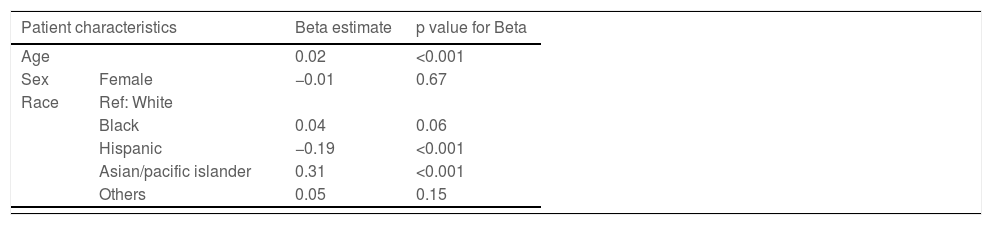
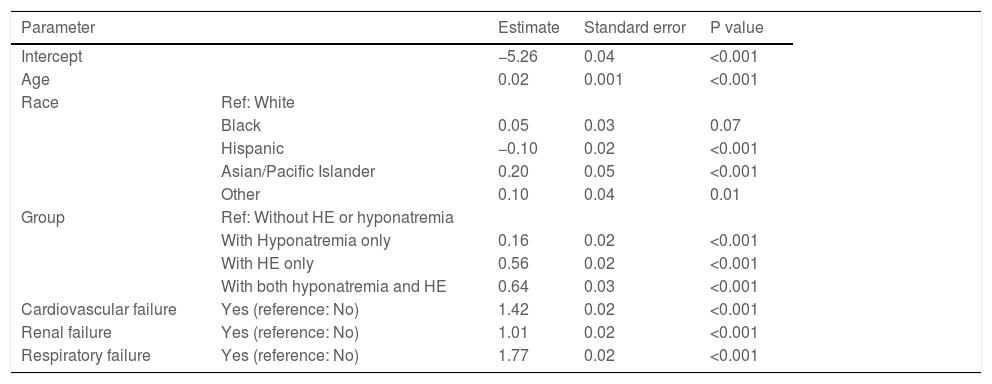
3.2Inpatient mortality and risk factorsOverall, 22,870 (7%) patients died during hospitalization. Among the four groups, a combination of hyponatremia and HE was associated with higher mortality (14%) than those with HE only (11%), hyponatremia only (9%), and neither hyponatremia nor HE (6%) (p<0.001). The univariate analysis showed that many variables were significantly associated with inpatient mortality (Table 2). However, on multivariable analysis, age, race, HE only, hyponatremia only, hyponatremia plus HE, and organ failure (cardiovascular, renal, or respiratory) were significantly associated with inpatient mortality (Table 3).
Univariate analysis: Individual association of risk factors with inpatient mortality.
| Patient characteristics | Beta estimate | p value for Beta | |
|---|---|---|---|
| Age | 0.02 | <0.001 | |
| Sex | Female | −0.01 | 0.67 |
| Race | Ref: White | ||
| Black | 0.04 | 0.06 | |
| Hispanic | −0.19 | <0.001 | |
| Asian/pacific islander | 0.31 | <0.001 | |
| Others | 0.05 | 0.15 | |
| Comorbidities | |||
|---|---|---|---|
| Cardiovascular failure | 2.25 | <0.001 | |
| Diabetes | −0.26 | <0.001 | |
| Renal failure | 1.72 | <0.001 | |
| Respiratory failure | 2.48 | <0.001 | |
| Elixhauser comorbidity score excluding liver disease | 0.07 | <0.001 | |
| Etiology of liver disease | |||
| Alcoholic cirrhosis | −0.34 | <0.001 | |
| Hepatitis C | −0.39 | <0.001 | |
| NASH | −0.50 | <0.001 | |
| Hepatitis B | −0.03 | 0.58 | |
| Primary biliary cholangitis | −0.80 | <0.001 | |
| Primary sclerosing cholangitis | 0.48 | <0.001 | |
| Autoimmune hepatitis | −0.45 | <0.001 | |
| Cirrhosis without alcohol | −1.37 | <0.001 | |
| Chronic hepatic failure with coma | 1.72 | <0.001 | |
| Other cirrhosis | −0.31 | <0.001 | |
| Hemochromatosis | −0.05 | 0.62 | |
| Unspecified biliary cirrhosis | −0.91 | <0.001 | |
| Other cirrhosis of liver | −0.52 | <0.001 | |
| Complications secondary to cirrhosis | |||
| Ascites | 0.29 | <0.001 | |
| Esophageal varices | −0.60 | <0.001 | |
| Esophageal varices with bleeding | −0.07 | 0.01 | |
| Hepatic Encephalopathy | 0.66 | <0.001 | |
| Hepatopulmonary syndrome | 0.36 | 0.001 | |
| Hepatorenal syndrome | 1.56 | <0.001 | |
| Hyponatremia | 0.44 | <0.001 | |
| Spontaneous bacterial peritonitis | 0.85 | <0.001 | |
NASH: nonalcoholic steatohepatitis
Multivariable analysis: Risk factors associated with inpatient mortality.
| Parameter | Estimate | Standard error | P value | |
|---|---|---|---|---|
| Intercept | −5.26 | 0.04 | <0.001 | |
| Age | 0.02 | 0.001 | <0.001 | |
| Race | Ref: White | |||
| Black | 0.05 | 0.03 | 0.07 | |
| Hispanic | −0.10 | 0.02 | <0.001 | |
| Asian/Pacific Islander | 0.20 | 0.05 | <0.001 | |
| Other | 0.10 | 0.04 | 0.01 | |
| Group | Ref: Without HE or hyponatremia | |||
| With Hyponatremia only | 0.16 | 0.02 | <0.001 | |
| With HE only | 0.56 | 0.02 | <0.001 | |
| With both hyponatremia and HE | 0.64 | 0.03 | <0.001 | |
| Cardiovascular failure | Yes (reference: No) | 1.42 | 0.02 | <0.001 |
| Renal failure | Yes (reference: No) | 1.01 | 0.02 | <0.001 |
| Respiratory failure | Yes (reference: No) | 1.77 | 0.02 | <0.001 |
HE: Hepatic Encephalopathy.
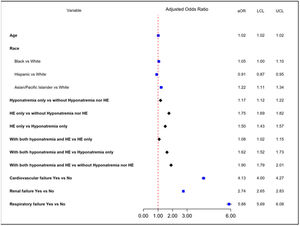
When compared to patients without hyponatremia or HE, patients with both hyponatremia and HE had the highest odds (adjusted odds ratio or aOR) of inpatient mortality (aOR 1.90, 95% CI: 1.79-2.01) followed by patients with HE only (aOR 1.75, 95% CI: 1.69-1.82) and patients with hyponatremia only (aOR 1.17, 95% CI: 1.12-1.22). When compared to patients with hyponatremia only, the aOR for those with both hyponatremia and HE was 1.62 (95% CI: 1.52-1.73). Patients with HE only had 50% higher odds of inpatient mortality when compared to those with hyponatremia only (aOR: 1.50, 95% CI: 1.43-1.57) (Fig. 1).
As expected, organ failures were associated with very high inpatient mortality. This effect was most predominant in those with respiratory failure with almost 6 times higher odds (aOR 5.88, 95% CI: 5.69-6.08), followed by cardiovascular failure (aOR 4.13, 95% CI: 4.00 – 4.27) and renal failure (aOR 2.74, 95% CI: 2.65-2.83).
3.3Impact of ascites or hepatorenal syndrome (HRS)Since over diuresis is an iatrogenic cause of hyponatremia, we stratified our patient population based on the presence or absence (most likely controlled ascites) of ascites. The impact of the combination of HE and hyponatremia on mortality among ascites and non-ascites patients was similar (Supplementary Tables 4A, 4B and 4C).
HRS is associated with very high mortality and this may mask the effect of HE on mortality. We, therefore, analyzed the data in those without HRS (prevalence 4%), and the conclusions remained unchanged (Supplementary Table 5A). We also analyzed the data, including HRS in the multivariable analysis and the conclusions remained the same (Supplementary Table 5B).
4DiscussionIn this nationwide study, we have shown that the presence of both hyponatremia and HE is associated with higher inpatient mortality when compared to those with HE or hyponatremia only. Although hyponatremia and HE are both well-known complications of patients with advanced cirrhosis with potential interactions, a specific analysis of the combined effects of hyponatremia and HE on inpatient mortality has not been previously reported.
Interaction between HE and hyponatremia has been previously reported [21]. An observational study in 997 patients assessed the association between serum sodium (sNa) levels and the prevalence of cirrhosis-related major complications. Authors reported that more than a third (38%) of patients with sNa ≤130 mEq/L experienced an episode of HE within the previous month, compared to a fourth (24%) with sNa between 131-135 mEq/L and only 15% of patients with normal sNa levels [10]. In another study, patients with a serum sodium ≤130 mEq/L, were found to have a significantly higher incidence of HE (odds ratio (OR) 3.40; CI 2.35-4.92), hepatorenal syndrome (OR 3.45; CI 2.04-5.82), and spontaneous bacterial peritonitis (OR 2.36; CI 1.41-3.93) [10,21]. The risk of HE appears to be linear, with an increase in the hazard rate of HE development by 8% for every mEq/L decrease in sNa (95% CI: 6–10%) [22]. While a higher prevalence of HE in patients with low sNa levels could be explained by more severe liver failure, there is a potential pathophysiological link between these two complications, as discussed earlier [10]. There are many potential explanations for HE in the presence of hyponatremia, as explained in our introduction [10,15-17].
In our study, HE had a higher impact on inpatient mortality as compared to those with hyponatremia only. Both HE and hyponatremia are indicators of very advanced portal hypertension. In our study, we were not able to stratify patients based on serum sodium levels since this information was not available in the NIS datasets, which is a major limitation of our study. It is possible that there could be a differential effect of severity of hyponatremia on inpatient mortality. With better outpatient management of portal hypertension, HE has become the leading cause of hospitalization and readmissions [7,8]. Despite receiving standard care, patients with a previous episode of overt HE have a 42% chance of recurrence at one year, and those with recurrent overt HE have a 46% chance of another episode within six months [23]. A previous North American study of 1560 hospitalized patients reported a significant association between HE severity and 30-day mortality independent of other extrahepatic organ failures or Model For End-Stage Liver Disease (MELD) score [24]. Based on the independent effect of moderate to severe HE on 30-day mortality, various ACLF predictive models have included moderate to severe HE as one of the organ failures. However, it is currently not included as a criterion for organ allocation [4,7,24]. Perhaps this should be addressed in a subset of patients with HE who are refractory to medical treatment and have disproportionately low MELDNa scores. Although we cannot make any firm conclusions on the pathophysiological interactions between hyponatremia and HE, our observations suggest that the presence of both hyponatremia and HE is a poor prognostic indicator, and these patients should be managed more aggressively, including expedited liver transplantation, to improve their prognosis.
There are many limitations to our study. In many patients, hyponatremia could be iatrogenic induced by over diuresis. The data on medical treatment were not available on NIS datasets. Although NIS datasets record the main reasons for hospitalization, the datasets do not have the granularity to determine the primary reason for hospitalization. However, when patients were stratified by the presence or absence of ascites, the conclusions remained the same. It is true that this does not mitigate the lack of data on diuretics use. Additionally, HRS is associated with very high inpatient mortality and the presence of HRS may mask the independent impact of HE on inpatient mortality, but when the data were analyzed in those without HRS, the conclusions remained unchanged. This was further confirmed on multivariable analysis, where HRS was included as a risk factor. Another limitation is the lack of data on immediate post-discharge mortality since it is possible that many patients were transferred to nursing homes or hospice care from the hospital, skewing our observations. Since only 36.7% with both HE and hyponatremia were discharged home in contrast to 56.6% without HE or hyponatremia, it is more than likely that we underestimated (not overestimated) the impact of HE and hyponatremia on mortality. The data collected by NIS is based on hospitalization rather than per individual patient; therefore, patients could be counted more than once. Since there are no patient identifiers, a longitudinal analysis is not possible.
Additionally, we could not assess the severity of HE or hyponatremia, so further risk stratification could not be performed. Furthermore, while administrative data codes have been used in outcomes research, validation studies using ICD-10 codes are still lacking in the United States. Despite these limitations, we believe that our observations are clinically meaningful and should alert the clinicians that the presence of both hyponatremia and HE is a poor prognostic indicator. Prospective studies should risk-stratify patients with HE further based on the severity of hyponatremia.
5ConclusionsIn this nationwide study, the presence of both hyponatremia and HE was associated with higher inpatient mortality than either hyponatremia or HE alone.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributionsConceptualization: PJT; Data curation: TZ; Formal analysis: TZ, PJT; Funding acquisition, N/A; Investigation, &Methodology: PJT; Project administration, Resources & Software: PJT; Supervision, Validation &, Visualization: PJT & TZ; Writing – original draft: MC; Writing – review & editing: PJT.
Availability of data and materialPublicly available national database from NIS