The A.A.E.E.H has developed this guideline for the best care of patients with hepatocellular carcinoma (HCC) from Argentina. It was done from May 2018 to March 2020. Specific clinical research questions were systematically searched. The quality of evidence and level of recommendations were organized according to GRADE. HCC surveillance is strongly recommended with abdominal ultrasound (US) every six months in the population at risk for HCC (cirrhosis, hepatitis B or hepatitis C); it is suggested to add alpha-feto protein (AFP) levels in case of inexeperienced sonographers. Imaging diagnosis in patients at risk for HCC has high specificity and tumor biopsy is not mandatory. The Barcelona Clinic Liver Cancer algorithm is strongly recommended for HCC staging and treatment-decision processes. Liver resection is strongly recommended for patients without portal hypertension and preserved liver function. Composite models are suggested for liver transplant selection criteria. Therapies for HCC with robust clinical evidence include transarterial chemoembolization (TACE) and first to second line systemic treatment options (sorafenib, lenvatinib, regorafenib, cabozantinib and ramucirumab). Immunotherapy with nivolumab and pembrolizumab has failed to show statistical benefit but the novel combination of atezolizumab plus bevacizumab has recently shown survival benefit over sorafenib in frontline.
alpha-feto protein
lens-culinaris AFP
alanine amino transferase
arterial phase hyperenhancement
aspartate amino transferase
area under the receiving operator curve
confidence interval 95%
complete response
clinically significant portal hypertension
computed axial tomography
desgamma carboxiprothrombin
disease control rate
drug-eluting beads
disease free survival
Eastern Coperative Oncology Group
fibroblast derived growth factor
hepatocellular carcinoma
hepatitis B virus
hepatitis C virus
hand-foot skin reaction
hazard ratio
immune-related adverse events
likelihood ratio
liver resection
liver stiffness measure
liver transplantation
magnetic resonance image
non-acoholic fatty liver disease
odds ratio
objective response rate
progressive disease
platelet derived growth factor
percutaneous ethanol injection
partial response
relative risk
relative risk reduction
absolute risk
randomized clinical trial
Response Evaluation Criteria for Solid Tumors
radiofrequency ablation
recurrence free survival
sensitivity
specificity
transarterial chemoembolization
transarterial radioembolización
tirosin kinase inhibitor
time to progression
ultrasound
vascular endothelial growth factor
With the advent of new diagnostic and treatment tools for hepatocelluar carcinoma (HCC) during the last decade, the Argentinian Association for the Study of Liver Diseases (A.A.E.E.H) has developed this clinical practice guideline. The aim of the A.A.E.E.H was to develop a simple clinical tool, not a rigid dogma, applicable to national general physicians, hepatologists, gastroenterologists, oncologists, radiologist, and surgeons, for the best care of patients with HCC.
2Materials and methodsThis guideline has been focused on epidemiology, surveillance, diagnosis, staging and treatment for patients with HCC. It has been carried out by a multidisciplinary board from May to December 2018 and up-dated in March 2020. Teams were invited to develop a synthesis of specific clinical research questions (Appendix 1), which were structured according to Population, Intervention/Exposure, Comparison and Outcomes (PICO). The evidence was systematically searched using Medline-Pubmed (MESH terms) and other databases (Trip-database, Lilacs) including original articles; editorial comments, personal opinions, reviews among others, were excluded.
The quality of the evidence and level of recommendations followed the Grade of Recommendation Assessment, Development and Evaluation (GRADE), including quality of evidence (High, Moderate, Low and Very Low) [1,2] using other guidelines for randomized clinical trials (RCT) [3], systematic reviews [4] and observational studies [5]. The strength of each recommendation followed a) balance between risks and benefits, b) precision of estimations (95% confidence intervals –CI), c) values and preferences of the patients and d) costs of interventions. For strong recommendations this guideline uses the term “it is recommended” and for conditional or weak ones “it is suggested”[1] (Appendix 1).
3Evidenced-based statements3.1EpidemiologyHCC is an increasing public health issue around the world, being the fifth most common cancer and the second leading cancer-related death [6,7]. In Asian countries, 60% of HCC cases are attributable to chronic hepatitis B virus (HBV) infection and vaccination with anti-HBV has dramatically reduced the incidence of HCC [8]. On the contrary, chronic hepatitis C virus (HCV) infection, followed by chronic alcohol consumption are the leading causes of HCC, with increasing HCC incidence and mortality rates in Western populations [9]. An increasing etiology of HCC during the last decade is non-alcoholic fatty liver disease (NAFLD) [10–15]. In Argentina, the HCC incidence rate is lower than 5.6 cases per 100.000 persons/year, equivalent to intermediate rates around the world [6].
This cancer originates in a cirrhotic liver or in the context of chronic liver injury in more than 90% of the cases [6,16]. In North America, HCV and alcoholic liver disease have been historically the most frequent causes of HCC but more recently, NAFLD has become a leading cause of HCC [10–12,17,18]. In Latin America, the most frequent etiology of HCC is HCV, followed by alcohol consumption [19,20]. Regional disparities have been reported, HBV in Brazil [21] and NAFLD in Chile. In Argentina, main etiologies are HCV in transplant centers and alcoholic liver disease in non-transplant centers [22,23]; whereas NAFLD has become an increasing etiology [23].
3.1.1Population at risk and HCC preventionAn estimated threshold of high risk for HCC was established in 1.5%/year [14,15]. Patients with cirrhosis of any etiology, or with chronic HBV or HCV infection are those with the highest risk of developing HCC [14,15]. Persistence of chronic infection [24–27], the degree of liver fibrosis and inflammation [28,29] and the presence of clinically significant portal hypertension (CSPH) are risk factors for HCC in HCV patients [30]. The risk of HCC decreases but it is not completely eliminated following HCV eradication, particularly in patients with advanced fibrosis (F3 or F4) [31–35] and CSPH [28,36].
Oncogenic pathways associated with HBV are independent from fibrosis grades. Presence of “e” antigen (Age), persistence of chronic inflammation, higher viral load in Age negative, pre-core mutants and the presence of advanced fibrosis have all been associated with higher risk [37]. On the contrary, asymptomatic carriers might be at lower risk, unless they present advanced fibrosis [38]. The REVEAL study cohort, including Age negative cohort, showed increasing HCC risk with higher viral loads [37]. Quantification of AgS levels might be another tool in Age- patients with lower viral load (<2000UI/ml) [39,40]. On the other hand, genotype C and pre-core mutants have been associated with higher risk of HCC [41]. More recently, values of liver stiffness measurement (LSM) have been associated with increasing Hazard Ratios (HR) of HCC, particularly with >18kPa HR 5.5 (CI 1.5;20.0) and >23kPa HR 6.6 (CI 1.8;23.8) [42]. Finally, some scoring models assessing the risk of HCC in treated patients with entecavir/tenofovir have been published, including the REACH (age, ALT levels, viral load and Age status) [43] and the PAGE score (age, gender and platelet count) [44]. A PAGE score of ≤9 points in non-cirrhotic HBV patients has a high negative predictive value for HCC [45].
The risk of HCC increases with alcohol consumption, particularly in the context of advanced fibrosis grades [46,47]. Moreover, it is a co-factor of HCC for other chronic liver diseases [48–50]. The risk of HCC in non-cirrhotic NAFLD is not clearly defined [10,12,17,23,51].
-Primary Prevention. The most effective measure is the universal vaccination of HBV [8]. Other unspecific measures include biosecurity of health procedures, sexual protective measures, avoidance of recreational drugs and alcohol consumption. Also, promotion of healthy lifestyle habits to avoid obesity and metabolic syndrome are recommended.
-Secondary Prevention. The most effective measures are viral eradication in chronic HBV [45,52–55] and HCV [24–26,31–34], stopping alcohol consumption [48,50,56,57] and promoting healthy lifestyle habits [58,59]. Direct-acting antivirals have shown decreasing incidence of HCC in those achieving HCV eradication [31,34,35,60]. Treatment of HBV has been associated with a RRR of 51% for HCC in a metanalysis [53]. This effect was observed even in patients with cirrhosis [45], although the risk was not completely abolished [55]. The rate in which the risk of HCC decreases in patients who present treatment-related fibrosis reversion is uncertain [52,53]. Some metanalyses have shown that coffee consumption may decrease the risk of HCC in patients with chronic liver disease [61,62]. However, the low quality of the evidence does not support any strong recommendation.
-Tertiary Prevention. In HBV, viral load has been associated with higher HCC recurrence after ablative therapies or LR [63,64]; treatment with interferon alfa 2b following resection did not reduce HCC recurrence rate [65] but opposite results were observed using nucleotide/nucleoside analogs [66]. In HCV, interferon-based regimens following ablation or liver resection did not reduce HCC recurrence in two RCT [67,68] and controversial data has been reported following direct-acting antivirals [69,70]. A higher tumor progression rate was not observed in HCV patients listed for liver transplantation (LT) treated with direct-acting antivirals [71,72] (Table 1).
Quality of evidence and level of recommendations for HCC prevention, surveillance, diagnosis and staging.
| Topic | Statement | Grade of Recommendation | Quality of the evidence |
|---|---|---|---|
| Primary Prevention | It is recommended universal vaccination for HBV, to reduce alcohol consumption, avoid illicit recreational drugs, to promote biosecurity and healthy life styles. | Strong | High |
| Secondary Prevention | It is recommended to eradicate/treat HBV or HCV chronic infections, to stop alcohol abuse. | Strong | Moderate to high |
| It is uncertain to recommend coffee consumption in patients with chronic liver disease. | Conditional | Low | |
| Tertiary Prevention | It is suggested to eradicate/treat HCV chronic infection after curative therapies. | Conditional | Low |
| It is recommended to eradicate/treat HBV chronic infection after curative therapies. | Strong | Moderate | |
| Surveillance | It is recommended for patients with cirrhosis and preserved liver function irrespective of etiology. | Strong | Low |
| It is recommended for patients with decompensated cirrhosis listed for liver transplantation. | Strong | Low to very low | |
| It is recommended for patients with chronic HBV, previous family history of HCC1. | Strong | Moderate | |
| It is recommended for patients with chronic HCV and F>2. | Strong | Low | |
| It is suggested for any chronic liver disease with F>2. | Conditional | Very Low | |
| It is recommended after viral eradication of HBV or HCV in patients with F>2. | Strong | Low to moderate | |
| It is recommended to use abdominal ultrasound every 6 months; performed by expert sonographers. | Strong | Low to Moderate | |
| It is suggested to use alpha-feto protein (AFP) as additional tool for HCC surveillance (Fig. 1). | Weak | Low to very low | |
| Diagnosis | It is recommended to perform HCC diagnosis either with CT or MRI when specific hallmarks are observed in the population at risk (APHE plus washout in delay portal phase). | Strong | High |
| A tumor biopsy is not recommended for HCC diagnosis confirmation in cases with imaging hallmarks. | Strong | High | |
| It is recommended to include LIRADS in radiological reports as well as detailed descriptions. | Strong | Very low | |
| AFP or other biomarkers are not recommended for HCC diagnosis. | Strong | High | |
| PET CT is not recommended for HCC diagnosis, staging or prognosis. | Strong | Low to moderate | |
| Staging | The Barcelona Clinic Liver Cancer staging is recommended for staging and therapeutic clinical-decision-making. | Strong | High |
| It is recommended to perform clinical-decision-making in the context of multidisciplinary working teams. | Strong | Low | |
| It is not suggested to support BCLC-B sub-staging classification. | Conditional | Low to very low | |
Notes: 1 – Among patient with chronic HBV, in the absence of fibrosis grade >2 or family history, it is suggested to assess risk of HCC and need for surveillance using the PAGE-B score. A PAGE-B score ≤9 points can exclude patients for surveillance.
Abbreviations: APHE: arterial phase hyper-enhancement; CT: computed axial tomography; F: fibrosis; MRI: magnetic resonance image.
Surveillance is the sequencing or repetitive application of a cost-effective screening tool on a population at risk for a specific health problem, aiming at reducing its mortality rate avoiding the so-called lead and length-time bias. Surveillance failure is not the same as screening failure. Whereas the first one refers to the failure of any procedure associated with the surveillance program (e.g. non adherence), screening failure refers to false-negative tests associated with each screening tool.
Among HBV patients, the evidence comes from prospective observational studies [73,74] and three RCT with different risk of bias [75–77]. In cirrhosis, the evidence is even less robust [78–84]. Some metanalyses with significant heterogeneity [85,86] and Markov modeling approaches have evaluated its cost-effectiveness [87–92]. Despite these unrobust data, it is strongly recommended to perform HCC surveillance in the population at risk (Table 1).
This panel considers that the aim of screening tool is to detect a single HCC of <3cm because it is associated with higher likelihood for curative therapies and better survival [78,93–99]. Screening tools for HCC that have been explored are abdominal ultrasound (US), CT or MRI scans and different biomarkers.
3.2.2Comparison of screening tools for HCCUS is the most cost-effective screening tool [100,101]; though it has low sensitivity (range 30–70%), it is operator dependent and a negative test does not completely rule out HCC. On the contrary, it has elevated specificity (>90%). US accuracy should be analyzed within the framework of serial repetition over time, ideally a 6-month interval [96,99]. In this context, a 34% rate of US screening failure is expected, higher when performed by inexperienced operators [98] or inconsistent repetition [97,98,102]. In Argentina the rate of “surveillance failure” has been reported to be 42% [103]; whereas “screening failure” was observed between 26–32% of the cases [103].
The use of alpha-fetoprotein (AFP) alone or in combination with abdominal US has been less effective for detection of single <3cm HCC [75,79,96,99]. There is lack of consensus on AFP thresholds with different sensitivities (Se) and specificities (Sp): AFP >20ng/ml: Se 60% Sp 90%, AFP >100ng/ml Se 31% Sp 98.8%, AFP >200 Se 22% Sp 99.4%, AFP >400 Se 17% Sp 99% [79]. The panel suggests to include AFP values at different thresholds to avoid US screening failure by inexperienced operators [98] (Fig. 1). Other biomarkers including lens-culinaris fraction of AFP (AFP-L3) and desgamma carboxiprothrombin (DCP) have not been better than AFP as screening tools either alone or in combination with US [104–106].
Surveillance and diagnostic algorithm for hepatocellular carcinoma.
Note: See additional information in Appendix 2.
Imaging HCC diagnosis can be performed with high specificity and tumor biopsy is not mandatory in the population at risk [107–112]. Triphasic scans including arterial, portal and delayed phases, either with computed axial tomography (CT) or magnetic resonance imaging (MRI) accurately perform HCC diagnosis. Specificities range from 85% to 100% for liver nodules ≥10mm showing imaging hallmarks including homogenous (non-rim) arterial phase hyperenhancement (APHE) and washout (non-peripheral) in portal phase [107–112]. Typical hallmarks may not be present in all HCCs. The presence of enhacing pseudocapsule during the late phase or increasing diameter of pre-existing nodule with APHE are additional criteria [113]. Reported sensitivities for nodules >20mm of diameter are similar with CT 92% and MRI 95%; however, MRI has superior accuracy for nodules <20mm with sensitivity of 70% and specificity of 83% (67% and 76% for CT, respectively) [114,115].
Liver nodules <10mm are not specific for HCC even showing hallmarks in MRI or CT scans. APHE is more frequently observed in these tiny lesions, whereas washout may not be present in nodules between 10 and 20mm of diameter [115]. In this regard, MRI with hepatobiliary contrast agents (Gadoxetic acid) may be useful to rule out malignancy, but it may decrease HCC specificity [107,108,112,116] (Fig. 1).
Radiological reports should be described in detail and include the LI-RADS system (liver imaging reporting and data system) [108,117]. LI-RADS is not applicable to patients without cirrhosis, without chronic HBV or HCV, or with vascular liver disorders (e.g. Budd Chiari syndrome, portal vein thrombosis, cardiac congestion and diffuse nodular regenerative hyperplasia) [108,118]. LI-RADS categorizes liver nodules in definitely benign (LI-RADS 1), probably benign (LI-RADS 2), with intermediate probability of HCC (LI-RADS 3), probable HCC (LI-RADS 4), definitely HCC (LI-RADS 5), probably or definitely malignant but unspecific for HCC (LI-RADS M) and definite HCC with tumor in vein (LI-RADS-TIV) [130–133]. Although LI-RADS has been validated in retrospective cohort studies [119], it has not been validated in RCT (Table 1).
3.3.2Pathological HCC diagnosisPathological diagnosis of HCC should be reserved for unconfirmed imaging diagnosis through fine needle biopsy, core needle biopsy or explant pathological analysis (Fig. 1). Cytological criteria for probable HCC include the presence of moderate to high cellularity, polygonal cells with dense cytoplasm, prominent nucleolus and surrounding sinusoidal cells, absence of biliary ducts cells, among other features. Guided core needle biopsy should be cautiously done for subcapsular liver lesions due to the risk of bleeding or tumor seeding. Histological features of HCC include cells grouped in trabeculae of 3 or more cells, cytological atypia, vascular invasion and loss of reticulin trama. Nuclear grading and histological classification should be assessed according to Edmondson and Steiner and WHO 2010 criteria, respectively. There is no patognomonic immunohistochemistry but the presence of at least 2 out of 3 signs, Glypican 3 or Heat Shock Protein 70 or Glutamine Sintetase, has 60% sensitivity and 100% specificity for HCC [120]. Molecular evaluation of HCC may be used for research purposes.
3.3.3Biomarkers for HCC diagnosis and prognostic purposesSerum AFP has been extensively studied as HCC biomarker. Although a threshold of 200ng/ml was shown to be the best cut-off [121], most of the patients show AFP values <20ng/ml at HCC diagnosis [80,104,122]. Also, AFP is not completely specific for HCC. For this reason, AFP is not considered a requisite for HCC diagnosis. Nevertheless, AFP has been associated with recurrence risk in patients receiving liver resection (LR) [123,124] or radiofrequency ablation (RFA) [125–127], with tumor progression following transarterial chemoembolization (TACE) [128–131] and as a prognostic marker with systemic therapies [132–134]. Extensive evidence has been published regarding AFP values as a selection tool for LT [135–138]. Other biomarkers have not been included in the daily practice except in Asian countries [104–106] (Table 1).
3.4StagingDifferent staging algorithms have been proposed including the Okuda system [139], the GRETCH score [140], the CLIP (Cancer of the Liver Italian Program) [141], CUPI [142], JIS (Japan Integrated System) [143], HKLC (Hong-Kong Liver Cancer) [144] and the BCLC staging (Barcelona-Clinic Liver Cancer) [145].
The BCLC was originally proposed according to the best available evidence, was externally validated in cohort studies [146–148] and adopted in international guidelines (Fig. 2). It includes five different stages according to baseline prognostic variables and the best evidenced-based therapeutic approach. Very early HCC or BCLC stage 0 includes patients with Eastern Cooperative Oncology Group (ECOG) 0, preserved liver function and single HCC <20mm and the best approach is RFA or LR [149–152]. Early HCC or BCLC-A corresponds to patients with preserved liver function, ECOG 0-1 and tumors including (a) single lesion without limit on diameter and (b) up-to 3 nodules less or equal than 30mm. Either CSPH or total bilirubin level >1mg/dl are prognostic variables that preclude LR, whereas these patients are candidates for LT if remaining within transplant criteria [154,155]. Intermediate BCLC-B stage are those patients with ECOG 0-1, preserved liver function with or without evidence of portal hypertension and multinodular tumors or those beyond BCLC-A stage. TACE is the treatment of choice [156–158], whereas in particular cases TARE might be indicated. Advanced HCC or BCLC-C stage includes patients with preserved liver function, with or wihout evidence of portal hypertension, ECOG 0–2 and tumor progressing or not responding after 2 TACEs or those with macrovascular invasion or extrahepatic spread. First and second-line sequencing therapy has achieved a median survival above 26 months in this group of patients [158]. Finally, BCLC-D stage includes patients with unpreserved liver function with any tumor burden or patients with ECOG 2–3; in these patients best supportive care is the treatment of choice. In this latter group, LT is the treatment of choice only if tumor burden is within transplant criteria or other co-morbidities do not exclude them for LT. Preserved liver function should be addressed including biochemical (total bilirubin, prothrombin time, serum creatinine and albumin) and clinical assessment (presence of CSPH including ascites and its complications, hepatic encephalopathy among other complications associated with portal hypertension). Scoring models like Child–Pugh or MELD score may be additional tools [159].
Staging for hepatocellular carcinoma according to the Barcelona Clinic Liver Cancer (BCLC).
Note: This figure is adopted from its original and latest version from the BCLC. Some changes were done, particularly for liver transplant candidates regarding BCLC-D or BCLC-B (if these patients are selected through composite models or specific UCSF downstaging protocol+AFP <100ng/ml). Preserved liver is equivalent to Child Pugh A or absence of any of the following decompensation events: jaundice, ascites, hepatic encephalopathy, hepatorenal syndrome, variceal bleeding. ECOG: Eastern Cooperative Oncology Group (performance status).
It is important to underline that the BCLC is not a rigid algorithm and some patients may be treated with subsequent recommended therapy (“treatment stage-migration”) [160]. Moreover, dynamic changes over time on liver function before or after each treatment should be considered. Finally, it includes the feasibility of each treatment. Other sub-staging algorithms for BCLC-B have been proposed (score ITALICA, HKLC) [144,161,162] or those avoiding ascites or hepatic encephalopathy grades (ALBI grade score) [163]. Adherence to the BCLC algorithm has been reported between 40% and 60% around the world [164–169] and 53% in Argentina [103].
3.5Locoregional treatments for HCC3.5.1Locoregional-ablative therapiesThe most frequently used locoregional ablative therapies include RFA, PEI and microwave ablation (MWA), either percutaneously or laparoscopicaly [127,170,171]. PEI consists of the injection of ethanol solution generating tumor coagulative necrosis [151,172,173]. RFA and MWA generate thermal tissue damage through radiofrequency or microwave mechanisms; the effect of MWA does not decrease when applied near blood vessels (as it happens with RFA). PEI's main limitation is tumor diameter and fibrosis septa and may be an alternative when RFA is unfeasible (e.g. subcapsular or peri-biliary or vascular lesions) [174]. RFA is more effective than PEI for local tumor control, HCC recurrence [173] and overall survival [151] with similar hospital stay and fewer adverse events [172]. In a metanalysis with moderate quality, RFA and MWA presented similar overall survival, recurrence rates and local tumor control but MWA presented fewer adverse events [175]. Other ablative techniques such as cryoablation [176], irreversible electroporation (IRE) [177] and stereotaxic body radiation therapy (SBRT) have been developed but evidence-based supporting the superiority over RFA is lacking [178–180]. The only RCT comparing the superiority of cryoablation over RFA was negative [178]. Table 2 shows level of recommendations and quality of the evidence.
Quality of evidence and level of recommendations for HCC locoregional therapies, liver resection and liver transplantation.
| Topic | Statement | Grade of Recommendation | Quality of the evidence |
|---|---|---|---|
| RFA, PEI and others | Either liver resection or RFA are recommended for patients with single tumor <2cm. | Strong | High |
| If LR or RFA are not feasible, PEI or MWA (if costs are comparable) are suggested to be second options. | Conditional | Moderate | |
| Cryoablation is not recommended as first line option. | Strong | Moderate | |
| SBRT or IRE are not recommended as first line options but are uncertain as second options. | Conditional | Low to very low | |
| Liver resection | LR is recommended for single tumors, irrespective of diameter, absence of CSPH, preserved liver function | Strong | High |
| RFA is recommended as an alternative option to LR, specifically for patients with up-to 3 tumors less than 3cm each in patients with comorbidities. | Strong | High | |
| Liver transplantation | LT is recommended for BCLC-A patients or with decompensated cirrhosis (BCLC-D). | Strong | High |
| Composite criteria (either AFP model or Metroticket 2.0) are suggested to optimize LT candidates selection. | Conditional | Moderate | |
| AFP >1000ng/ml is recommended as an exclusion independent criteria. | Strong | Moderate | |
| Bridging therapies in the waitlist period are recommended considering tumor burden, AFP >400ng/ml and expected waitlist times. | Conditional | Low to moderate | |
| It is suggested to individualize the benefits and risks of downstaging; UCSF plus AFP <100ng/ml is suggested to identify ideal candidates. | Conditional | Low to moderate | |
| “All-comers” are not recommended for downstaging. | Strong | Low to moderate | |
| There is uncertain quality of evidence to recommend any particular locoregional modality for bridging or downstaging procedures. | Conditional | Low | |
| Adjuvant systemic therapies | Sorafenib is not recommended for reducing the risk of HCC recurrence after RFA or LR. | Strong | High |
| There is no sufficient evidence to recommend adjuvant systemic therapies prior or after LT to avoid HCC recurrence. | Conditional | Very Low | |
| Other | It is suggested to use MELD score <10 to select non-ideal LR candidates (with CSPH). | Conditional | Low to very low |
| It is uncertain to suggest or recommend multimodal combination therapies for BCLC-A or B patients. | Conditional | Low to very low | |
| It is suggested to follow BCLC algorithm for HCC recurrence therapies after RFA or PEI or LR or other locoregional therapies. | Conditional | Low to very low | |
| There is insufficient evidence to recommend any specific therapy for HCC recurrence after LT. | Conditional | Very low | |
Abbreviations: BCLC: Barcelona Clinic Liver Cancer; CSPH: clinically significant portal hypertension; IRE: irreversible electroporation; LR: liver resection; LT: liver transplantation; MELD: model for end-stage liver disease; PEI: percutaneous ethanol injection; RFA: radiofrequency ablation; SBRT: stereotaxic body radiation therapy.
The selection of appropriate candidates for LR is mandatory. Presence of extrahepatic tumor, main portal trunk invasion or unpreserved liver function are absolute contraindications [181–183]. Patients with CSPH have a higher risk of liver decompensation [153,154,184–186], higher postoperative morbi-mortality and lower 5-year overall survival [HR 1.48 (CI 1.11;1.98)] [154,187,188], even if “minor resections” are done [187]. Not clinically evident CSPH (gastroesophageal varices, ascites, portosystemic shunts) should be assessed through manometry [153,184,185,189] or surrogate markers (splenomegaly >120mm with low platelet count <100,000mm–3) [124,186]. LSM may have a role as a surrogate of CSPH, a threshold of >20kPa has been proposed [190]. Finally, a minimum remnant liver volume of 40% is important to avoid post-operative liver decompensation and can be estimated through CT volumetric assessment or other metabolic tests [191]. The evidence does not suggest any direction in favor or against any specific modality (open vs. laparoscopic LR) [192,193] but anatomical resections have been associated with lower HCC recurrence rates [194].
In BCLC-0 or A stages, LR was associated with lower 5-year recurrence rates (63.8%) compared to RFA 71.7% or PEI 76.9% [150,197,198] but with longer hospitalization and a higher rate of serious adverse events [199]. Cost-effective analysis and Markov modeling have shown that for single HCC <2cm, RFA is as comparable as LR in overall survival. For single lesion BCLC-A, LR is more cost-effective and for BCLC-A with 2–3 nodules, both techniques had similar overall survival, RFA was more cost-efective but LR had lower recurrence rate [200]. Finally, multipolar RFA presented similar survival and recurrence rates for single HCC <40mm compared to LR [201].
Additionally, LR may be performed by expert surgeons in “non-ideal” candidates [182,185,186,189]. However, the quality of the evidence is low. A MELD score less or equal than 9 points may select candidates for minor resections (less than 3 liver segments) [182,185,186,189]. Other authors have proposed LR among BCLC-B patients but with a significant risk of bias [181–183,202]. Recently, a low to moderate quality metanalysis compared LR vs. TACE in BCLC-B/C patients [204]. In the only RCT included, median survival was significantly higher for LR (41 months vs. TACE 14 months) [204]. However, unbalanced co-interventions and an indefinite number of TACEs might have led to ischeamic injury and liver decompensation [204]. Additionally, LR was not superior to sorafenib in patients with macrovascular invasion [205] (Table 2).
3.5.3Management of HCC recurrence following ablative therapies and liver resectionHCC recurrence after locoregional therapies or LR ranges from 40% to 70% at 5 years, either intra or extrahepatic [149,206–208]. Pathology variables associated with a higher risk of recurrence are macro or microvascular invasion, satellites or undifferentiated tumors [152,208,209]. The quality of the evidence supporting either one or the other therapeutic modality for HCC recurrence is low. RFA or re-resection [206,210], “salvage” LT [211,212] or preventive LT or “abb initio”[152,208] have been proposed. Salvage LT has shown similar survival but lower recurrence rates vs. re-resection [212–214]. In spite of being novel approach, preventive or “abb initio” LT needs to be externally validated [152,208]. Several strategies and therapeutic modalities of adjuvance and neo-adjuvance have failed to show any clinical benefit [215], including sorafenib (STORM trial) [216]. Other on-going RCT including pembrolizumab (MK-3475, NCT03867084), nivolumab (CheckMate-9DX, NCT03383458), dendritic cells based therapy [217] and induced natural killer cells [218] have been reported.
3.5.4Locoregional-endovascular therapies- (a)
Transarterial chemoembolization (TACE) consists in the instillation through arterial hepatic branches of a chemotherapeutic agent with lipiodol (e.g. doxorubicin or epirrubicin, among others) followed by arterial embolization either conventionally (e.g. alcohol or spongostan) or through microspheres or drug-eluting beads (DEB) [220,229]. TACE is the standard treatment for BCLC-B patients [155–157]. Neither chemoinfusion alone, “lipiodolization” nor bland embolization (without chemotherapeutic agent) have shown any survival benefit [155,157,221,222]. Some authors have questioned these results in low quality studies with significant risk of bias [223,224]. Eligible candidates for TACE are those with preserved liver function, with a tumor diameter <10cm, less than 50% of total liver involvement, without vascular or biliary tumoral or non-tumoral obstruction, without portosystemic shunts, impaired kidney function or other major comorbidities [220]. Main non-tumoral portal trunk obstruction precludes conventional TACE but it may be done with hyperselective TACE or with DEB.
In one RCT, TACE with DEB of 300–500μm was not superior to conventional TACE (PRECISION V trial) in overall survival and tumor control [220]. Systemic adverse events were lower in Child–Pugh B >7 patients with DEB [220]. Other uncontrolled studies have shown median survival of 47 months with DEB [225]. Nevertheless, there is lack of evidence comparing conventional TACE vs. smaller DEB (75–100μm).
The most common adverse event is the post-chemoembolization syndrome, ocurring in 20% of the patients (pain, fever, increasing liver enzymes). Less frequently observed are those associated with the chemotherapeutic agent such as alopecia, mucositis, bone marrow suppression or cardiac dysfunction (more frequently observed with conventional TACE) [220].
- (b)
Transarterial Radioembolization (TARE) consists of the endovascular administration of Ytrium-90 (Y-90) radiospheres selectively within the tumor [226]. In some patients with unresectable large tumors (>7cm) or with portal vein thrombosis, TACE maybe not feasible or efficient enough. In these cases, TARE may be more radical with less therapeutic sessions. Ideal candidates are unresectable BCLC-A or B, with preserved liver function, total bilirubin <2mg/dl, absence of extrahepatic metastasis and with up-to 5 HCC liver lesions, a total diameter <20cm or single lesion <10cm, with or without portal vein obstruction [226–232]. Before TARE, other procedures such as arterial liver angiography, a Tecnecium 99 (Tc-99) scan to evaluate intrahepatic-pulmonary shunts (if >15%, TARE is not recommended) or the equivalent to lung exposure of no more than 30Gy for 1 session or 50Gy for >1 sessions should be evaluated [226–232].
TARE is not associated with a post-embolization syndrome; the most serious adverse event is the Radioembolization Induced Liver Disease or “REILD”; occurring in less than 10% of the patients, within 4 to 8 weeks after TARE including jaundice, ascites and liver decompensation. Radiotoxicity may affect other abdominal organs [226,232].
- (c)
Radioembolization vs. TACE. The evidence comparing these techniques is of low to very low quality [227–232]. Two metanalyses with high statistical heterogeneity and significant risk of bias have shown that TARE may be associated with better 1-year survival rate [HR 0.74 (CI 0.61–0.90)], similar time to progression (TTP), shorter length of hospitalization and fewer adverse events [234,235]. However, a small pilot randomized, open-label, controlled trial (SIRTACE) showed no significant differences in 1-year overall survival (TARE 46.2% vs. TACE 66.7%), TTP and rate of adverse events [236]. Other small randomized trials showed longer TTP in favor of TARE but without a significant survival benefit [237,238]. There is an on-going phase III RCT (TRACE trial; NCT01381211) (Table 3).
Table 3.Quality of the evidence and level of recommendations for HCC endovascular and systemic therapies.
Topic Statement Grade of Recommendation Quality of the evidence TACE It is recommended as first line option in BCLC-B patients. Strong High DEB TACE is not superior to conventional TACE1 Strong High DEB TACE is suggested for Child Pugh B <9 Conditional High TARE Insufficient evidence to recommend or suggest TARE over TACE as first option for BCLC-B patients Conditional Low to very low In some patients with unresectable large tumors, with portal vein obstruction TARE may have a therapeutic role. Conditional Low It is uncertain to recommend or suggest TARE after TACE failure in BCLC-B. Conditional High TARE is not recommended for BCLC-B patients with tumor progression or BCLC-C patients (with vascular invasion) over sorafenib. Strong High Systemic therapies with TACE/TARE/other Sorafenib or other TKI agents in combination with TACE for BCLC-B patients are not recommended to avoid tumor progression. Strong High There is no recommendation to support the combination of TARE with sorafenib for BCLC-B patients to avoid tumor progression. Strong High Intra-hepatic arterial chemotherapy in combination with sorafenib is not recommended for BCLC-B or C patients Strong High First line systemic therapy The combination of atezolizumab plus bevacizumab is recommended as first-line systemic therapy2,3 Strong High If atezo+beva is not available or approved: sorafenib or lenvatinb are first-line systemic options4 Strong High Nivolumab is not recommended for first-line systemic therapy. Strong High Second line systemic therapy Regorafenib, cabozantinib and ramucirumab are recommended second-line systemic therapies for post-sorafenib progression. Strong High Cabozantinib or ramucirumab are recommended for intolerant-sorafenib patients. Strong High Ramucirumab is only recommended for patients with AFP values ≥400ng/ml. Strong High Pembrolizumab may be use in patients with AFP values <400ng/ml, without main portal vein invasion (tolerant or intolerant to sorafenib). Conditional High Nivolumab may be suggested as second line (tolerant or intolerant to sorafenib). Conditional Moderate There is lack of robust evidence supporting second line options after lenvatinib but these options may be use as for sorafenib. Conditional Very Low Third line systemic therapy Cabozantinib is recommended as third line (only with prior sorafenib plus other systemic therapy). Strong High There is lack of robust evidence supporting any other third line options but some first or second-line agents may be used. Conditional Very Low Notes: 1 – Only one RCT compared conventional TACE vs. DEB using large microspheres; uncertain efficacy regarding tiny microspheres. 2- Waiting for approval. 3-Without main portal trunk invasion or any prior autoimmune disease or uncontrolled arterial hypertension for atezolizumab+bevacizumab. 4- Without main portal trunk invasion or no more than 50% liver involvement or uncontrolled arterial hypertension for lenvatinib.
Abbreviations: BCLC: Barcelona Clinic Liver Cancer; DEB: drug eluting beads; TACE: transarterial chemoembolization; TARE: transarterial radioembolization.
- (d)
Radioembolization vs. systemic therapy. Two phase III trials have compared the superiority of TARE over sorafenib in BCLC-B upon tumor progression or failure following sequential TACE and BCLC-C without extrahepatic disease (SARAH and SIRveNIB) [239,240]. Both showed negative results in terms of survival with median survival of 8.0 months for TARE and 9.9 months for sorafenib [HR of 1.17 (CI 0.94;1.41)] (SARAH). Median TTP was similar and although there was a lower rate of intrahepatic tumor progression with TARE, a lower and similar disease control rate (DCR) vs. sorafenib was observed in SARAH and SIRveNIB, respectively [239,240]. A similar rate of all adverse events were observed in SARAH; although there was a higher incidence of grade ≥3 related adverse events with sorafenib (63% vs. TARE 41%) [239]. There was a higer rate of all adverse events as well as higher rate of serious adverse events with sorafenib in SIRveNIB [240]. Opposite results were observed with quality of life, better in SARAH and similar in SIRveNIB [239,240]. Some differences between studies should be underlined. A larger proportion of BCLC-C patients were included in SARAH and total bilirubin was up-to 3mg/dl vs. <2mg/dl in SIRveNIB. In SARAH there were significant cross-overs, un-standardized radiation dose and inexperienced participating centers [239]; whereas there were logistical issues reported in SIRveNIB [240]. In both trials, the median treatment duration with sorafenib was shorter than expected [241,242] (Table 3).
Different combinations of TACE with antiangiogenic drugs have been explored aiming at better TTP or disease-free survival (DFS) and in some of them, overall survival. The rationale for this combination is that higher expression of vascular endothelial growth factor (VEGF) following TACE was associated with worse survival [243].
Studies evaluating sorafenib+TACE with TTP as primary outcome include two non-randomized phase II trials (SOCRATES and START trials) [244,245] and three phase III RCT, the double-blinded SPACE trial [246], HELviCA study [247] and the open-label TACTICS trial [248]. The SOCRATES study enrolled patients with unpreserved liver function reporting higher adverse events [244]; whereas the combination was tolerable in patients with preserved liver function (START trial) [245]. Both studies used conventional TACE; DEB was used in another uncontrolled phase II trial [249]. In SPACE and HELviCA trials, the combination of TACE with DEB plus sorafenib did not show better TTP than TACE alone+placebo [247,247]. On the contrary, a longer DFS was observed in the TACTICS trial [median TTP 25.2 months vs. 13.5 months; HR 0.56 (CI 0.38;0.83)] [248]. These opposite results may be due to different elegibility criteria and different definition of tumor progression between trials. However, in both trials the combination showed a longer time to extrahepatic progression. The STAH trial evaluated overall survival as primary outcome with negative results but it included BCLC-C patients [250].
Other antiangiogenic agents in combination with TACE were negative including brivaniv (BRISK-TA phase III RCT) [251], bevacizumab [252] and axitinib [253]. More recently, intra-arterial chemotherapeutic agents in combination with sorafenib have also failed to show any survival benefit vs. sorafenib alone [254]. In another RCT including BCLC-C patients with main portal trunk invasion, the combination of intra-hepatic arterial infusion of FOLFOX plus sorafenib vs. sorafenib alone showed promising survival but with higher myelotoxicity [255]. Finally, the combination of sorafenib plus TARE did not show any survival benefit (SORAMIC trial) [242,256]. There are on-going trials exploring the TACE plus immunotherapy with nivolumab (IMMUTACE NCT03572582 and NCT03143270) (Table 3).
3.6Liver transplantation3.6.1Transplant eligibility criteria and outcomes following LTTaking into account that it has the potential to cure not only HCC but also the underlying disease, LT is the HCC treatment with the highest survival probability. As it deals with donor scarcity and social justice, it should only be offered to patients with a minimum 60% 5-year survival rate. Moreover, HCC recurrence, occuring in approximately 10–30% of the cases, is the most frequent cause of death after LT in these patients [257,258]. Thus, to select the best candidates with the lowest risk of HCC recurrence is another main transplant end-point.
Although Milan criteria (single nodule up-to 5cm or up-to 3 nodules none of them more than 3cm in diameter) have been the gold standard for transplant eligibilty, some issues should be addressed [259,260]. On the one hand, tumor burden based only on number and diameter has a 25% discordance compared to explant pathology analysis [261–263]. On the other hand, other variables are associated with post-LT outcomes [136,264–269]. Different authors have proposed extended criteria beyond Milan including pre-LT AFP values as a biomarker for candidate selection [136,138,266–268] or histological pre-LT evaluation assessing tumor differentiation (Toronto criteria) [269]. Nevertheless, pre-LT histological evaluation is not always feasible [270].
The fact is that when extending criteria from beyond Milan without including AFP as a biomarker, higher HCC recurrence and lower post-LT survival have been reported [136,138,266–268,271]. AFP values are independently associated with post-LT survival and HCC recurrence and correlate with biologically aggressive tumor features at explant pathology [136,138,266–268,271]. Particularly, AFP levels >1000ng/ml have been proposed as a contraindication for LT [135]. Consequently, composite criteria have been recently proposed. Duvoux et al. included AFP values to number and tumor diameter (French AFP model: range 0 to 9 points with a threshold of up-to 2 points) [136]. This model was externally validated in Latin America and afterwards, in other regions around the world [271–273]. Other composite models included the sum of the largest diameter plus the total number of HCC nodules with the logarithmic transformation of AFP values (the Metroticket 2.0) [138]. Finally, dynamic AFP changes, as well as tumor progression in the waitlist period, should also be considered [274–277] (Table 2).
3.6.2Locoregional therapies as adyuvant treatment and “downstaging” before LT“Bridging” or adjuvant locoregional therapies before LT, either TACE or RFA/PEI among others, aim to avoid tumor progression and remain within LT criteria [277–279]. Tumor progression beyond Milan criteria is 7–11% at 6 months and 38% at 12 months of listing [72,265,275,276]. Variables associated with a higher risk of waitlist tumor progression are tumors beyond Milan criteria, AFP >400ng/ml and unfeasible or unresponsive to “bridging” therapies [275,276]. On the contrary, patients with complete response (CR) following locoregional treatments have lower rates of tumor progression and waitlist drop-out when compared to stable disease (SD) or progressive disease (PD) (1% vs. 10% at 12 months and 1% vs. 51% at 24 months) [275,280].
“Downstaging” consists of reduction of tumor burden, either in tumor diameter or number, to values within Milan criteria [276,281–285]. However, there is no consensus on the “upper limits” beyond Milan for downstaging. Most popular LT downstaging protocol are those proposed by the University of California San Francisco (UCSF) (1 nodule >5cm but ≤8cm or 2–3 nodules all ≤5cm and sum of diameters ≤8cm or 4–5 lesions all ≤3cm and sum of diameters ≤8cm, plus AFP values below 1000ng/ml) [285,286]. There was a significant statistical heterogeneity in a metanalysis reporting the effect of “downstaging” upon survival [287]. On the one hand, effective “downstaging” was 48% (CI 39;58%), even lower in those beyond UCSF (“all-comers”) [288]. This latter group was associated with higher drop-out rate, lower probability of tumor reduction and worse outcomes after LT [288]. It seems that there should be a limit in tumor burden for “downstaging”. In this regard, AFP values below 100ng/ml were recently proposed to select better candidates within the UCSF protocol [289]. Also, a minimum observation period of 3 months to assess stable disease is required [290]. Finally, there is no robust data supporting the superiority of any therapeutic modality [178,287,291] (Table 2).
3.6.3Systemic treatment as adjuvant therapy before or after LTThere is no robust data in favor or against the use of sorafenib or other multikinase inhibitors, or other chemotherapeutic agents before or after transplantation as adjuvant therapy to reduce the risk of HCC recurrence [247,292–294]. Although immunosuppression with mTOR inhibitors was promising [295–297], there was neither survival nor recurrence benefit in a recent RCT [298].
3.6.4Surveillance and management of HCC recurrence after LTThe risk of HCC recurrence should be reassessed at explant pathology analysis. Presence of extensive tumor burden (beyond Up-to 7 criteria) [270], microvascular invasion, undifferentiated tumors or absence of complete necrosis are associated with higher recurrence risk [137,299,300]. Surveillance for HCC recurrence is controversial since it is considered an advanced stage with a high mortality rate independently from its location and there is no robust data to support any particular therapy. Moreover, most recurrences occur during the first 18 months after LT [137,257] and its earlier detection has been associated with worse survival [257,258]. Nevertheless, international consensus recommends surveillance for HCC recurrence with CT or MRI scans plus AFP values with a minimum interval of 6 months up-to the 3rd to 5th years following LT [299]. Locoregional therapies have been reported in retrospective studies for single intra or extrahepatic lesions [258,301], re-transplantation is strongly not recommended and in patients in whom locoregional therapies are not feasible, sequential systemic therapy has prolonged overall survival in retrospective studies [302–306]. No robust data supports any change in the type of immunosuppression [302–306] (Table 2).
3.7Systemic therapies for advanced HCC3.7.1First-line systemic treatment agentsDuring the last decade, an enormous improvement in the treatment of these patients has been achieved, with unthinkable survival rates years ago. Systemic conventional chemotherapeutic agents such as FOLFOX or GEMOX [307–309] or other agents as tamoxifen [157] have failed to show any survival benefit.
Sorafenib, a tyrosine multikinase inhibitor (TKI) was the first drug to show a survival benefit over placebo in two double-blind, phase III RCT (SHARP and Asia-Pacific) [241,310]. The exact mechanism of action is unknown with anti-angiogenic, hypoxic and antiproliferative effects. Eligibility criteria included preserved liver function, ECOG 0-1 and BCLC-C or BCLC-B under tumor progression [241,310]. Sorafenib (400mg bid) had a 70% relative risk reduction of death [HR of 0.69 (CI 0.55;0.87)] [241,310] and better TTP. However, radiological CR or PR were infrequently observed with a DCR of 43–53% [241,310]. Other real-world uncontrolled studies have been published so far [311], suggesting that sorafenib may be extended up-to Child–Pugh B7; however, lower survival and higher rate of adverse events with unpreserved liver function were reported [312–315].
Since then, other non-inferiority [e.g. sunitinib [316] and brivanib [317] or superiority trials [erlotinib [318], linifanib [319], dovitinib [320]] have failed to show any survival benefit or have shown excessive toxicity vs. sorafenib [bevacizumab+sorafenib [321]. More recently, lenvatinib showed non-inferiority (REFLECT trial) [322] and atezolizumab plus bevacizumab superiority over sorafenib (IMbrave trial, NCT03434379).
The REFLECT phase III, open-label RCT, showed non-inferior survival of lenvatinib (8mg day if <60kg or 12mg day if >60kg) vs. sorafenib [322]. This TKI blocks VEGF as well as FGF and PDGF pathways. In this trial, eligibility criteria excluded patients with main portal trunk tumor invasion and those with >50% of total liver volume involvement [322,323]. Median survival was 13.6 months with lenvatinib vs. 12.3 months with sorafenib [HR 0.92 (CI 0.79;1.06)] [322]. TTP, as well as higher rates of partial response and objective response rates (ORR) were observed with lenvatinib. Higher rates of severe adverse events were observed in lenvatinib arm (57% vs. 49%), mainly hypertension, hypothyroidism and proteinuria (Table 3). In Argentina, lenvatinib was approved in late 2018.
3.7.2Immunotherapy alone or in combination for first-line systemic therapyImmunotherapy for cancer treatment has evolved into a complete novel paradigm. Cancer cells avoid lymphocyte T cell activation and proliferation, highly expressing “programmed cell death ligands” PD-1 ligand 1 and 2 (PD-L1, PD-L2) or “cytotoxic T lymphocyte protein 4” ligands (CTLA-4 and its ligands). Blockade of these unions through monoclonal antibodies (anti-PD-(L)1 or anti-CTLA-4) unblocks the anti-cancer immune host response (“immune check-point inhibitors”). The first anti-PD1 IgG4 monoclonal antibody was nivolumab. Later on, pembrolizumab (IgG4 anti-PD1), tremelimumab and ipilimumab (IgG1 anti CTLA-4) and atezolizumab, durvalumab and avelumab (IgG1 anti PD-L1).
Recently, the CheckMate-459 phase III, open-label RCT (NCT02576509) for first-line therapy in patients with advanced HCC naïve of systemic therapy, Child A, ECOG 0-1, BCLC-B or C in the absense of main portal trunk invasion did not show superior survival of nivolumab (240mg iv every 3 weeks) over sorafenib [median survival nivolumab 16.4 months vs. sorafenib 14.7 months; HR 0.85 (CI 0.72–1.02); P=0.07]. There was not a significant difference of DFS, even showing a higher rate of ORR in favor of nivolumab (15% vs. 7% with sorafenib). Nivolumab was associated with a lower rate of grade 3/4 adverse events (22% vs. 49%).
More recently, another phase III, open-label RCT, IMbrave-150 (NCT03434379) showed superiority of atezolizumab 1200mg iv (anti-PD-L1) plus bevacizumab 15mg/kg iv every 3 weeks vs. sorafenib [median survival nor reach vs. 13.2 months for sorafenib; HR 0.58 (CI 0.42–0.79); P=0.0006] [324]. Eligibility criteria included preserved liver function, systemic-naïve advanced HCC, ECOG 0-1 in the absense of main portal trunk invasion. Longer progression free survival (PFS) with a significant higher ORR rate in the combination arm 27% vs. 12% and DCR of 74% vs. 55% (P<.0001) were observed. Similar incidence of all grade adverse events and a lower incidence of grades 3/4 related adverse events were observed with the combination arm (36% vs. 46%). The most frequent adverse events were systemic hypertension, diarrhea, proteinuria, hyporexia, elevated liver enzymes and infusional reaction. However, treatment discontinuation was higher in atezolizumab+bevacizumab arm (16% and 10%, respectively) (Table 3).
Other combinations are being explored in phase III trials: pembrolizumab+lenvatinib vs. lenvatinib (LEAP-002, NCT03713593), sorafenib vs. durvalumab (anti PDL-1) vs. durvalumab+tremelimumab (CTLA-4) (HIMALAYA, NCT03298451), cabozantinib with/without atezolizumab vs. sorafenib (COSMIC-312; NCT03755791) and nivolumab+ipilimumab vs. sorafenib or lenvatinib (CheckMate-9DW; NCT04039607).
3.7.3Second-line systemic treatment agentsTumor progression under sorafenib is the natural course of the disease in most of the patients and 10–20% of the patients may not tolerate this treatment. Thus, several second-line phase III trials have been explored after sorafenib progression or intolerance. Some agents failed to show any survival benefit vs. placebo including brivanib (BRISK-PS, phase III RCT) [325], axitinib (phase II trial) [326], everolimus (EVOLVE-1, phase III RCT) [327] and tivantinib (METIV, phase III RCT) [328].
The RESORCE trial included patients with advanced HCC, preserved liver function, with tumor progression and tolerant to sorafenib (a minimum dose of 400mg daily during the last month). Dose was scheduled in 160mg day in a 4 week-cycle (3 weeks ON-1 week OFF). Median survival was 10.6 months for regorafenib and 7.8 months for placebo [HR of 0.62 (CI 0.50;0.79)]; this survival benefit was observed in all stratified analyses even in patients with worse baseline prognosis (AFP >400ng/ml or macrovascular invasion) [329]. A benefit on TTP was also observed, with a significant difference in DCR (65% vs. 36%). Adverse events were common for TKIs (fatigue, hyporexia, hypertension, diarrhea and hand-foot skin reaction-HFSR); 46% were grade III and 4% grade IV, with a discontinuation rate of 10% [329]. In Argentina, it was approved during 2018.
The CELESTIAL trial compared cabozantinib 60mg/day vs. placebo [134] including unresectable BCLC-B or C patients, Child–Pugh A, ECOG 0-1, with previous sorafenib treatment (either tolerant or intolerant), with tumor progression after up-to 2 prior systemic therapies. Median survival was 10.2 months for cabozantinib and 8 months for placebo [HR of 0.76 (CI 0.63;0.92)]; this survival benefit was also observed in all post hoc analyses, even in patients with sorafenib as the only prior systemic therapy [HR 0.74 (IC 0.59–0.92)]. However, the efficacy of cabozantinib in tolerant vs. intolerant to sorafenib is uncertain. TTP was also in favor of cabozantinib with a DCR of 64% vs. 33%. Dose reductions and discontinuations were more common in cabozantinib arm, likewise for adverse events such as HFSR, asthenia and diarrhea [134].
The REACH I failed to show any survival benefit of ramucirumab (8mg/kg iv every 2 weeks), a monoclonal antibody that blocks VEGF receptors, vs. placebo. However, in a post hoc analysis there was a specific survival benefit among patients with baseline AFP values ≥400ng/ml, a group with an expected lower survival rate [133]. This motivated to performed the REACH II RCT including patients with histologically or cytologically confirmed HCC with AFP ≥400ng/ml, with prior sorafenib therapy – tolerant or intolerant – and tumor progression [330]. Median survival was 8.5 months for ramucirumab and 7.3 months for placebo [HR of 0.71 (CI 0.53;0.95)]. Stratified analyses confirmed the efficacy of ramucirumab in all groups but uncertain in prior-intolerant to sorafenib. TTP was longer and there were higher ORR and DCR rates over placebo (60% vs. 39%). Serious adverse events were similar between arms; ramucirumab presented more frequently hypertension (13%) and hyponatremia (6%) with a discontinuation rate due to adverse events of 11% [330]. Neither cabozantinib nor ramucirumab has been approved for HCC in Argentina (Table 3).
3.7.4Immunotherapy for second-line systemic therapyTremelimumab has been explored in an uncontrolled phase II trial in patients with HCV+; it was well tolerated with no HCV biochemical flares, even with decreasing HCV viral loads [331]. In another phase I/II, uncontrolled trial of nivolumab (CheckMate-040) in Child–Pugh A-B <9 patients under progression with sorafenib (tolerant or intolerant) [332]. There were escalating and expansion cohorts (3mg/kg iv every 3 weeks). The survival rate at 9 months was 74% (CI 67%;79%) with ORR of 20% and a DCR of 64%. PD-L 1 expression on tumor biopsies (≥1% vs. <1%) was not associated with a better survival but higher ORR was observed. Nivolumab showed a very good safety profile; common adverse events were rash, pruritus, diarrhea or colitis and liver or pancreatic enzymes elevation. This granted FDA temporary approval and motivated the CheckMate-459 at frontline (NCT02576509).
More robust data comes from a phase III, double-blind, RCT of pembrolizumab 200mg iv every 3 weeks vs. placebo (KEYNOTE-240) in patients under progression with sorafenib or intolerant to sorafenib, Child A, ECOG 0-1, BCLC B or C without main portal trunk invasion and AFP levels <400ng/ml [333]. The trial was negative in terms of survival benefit according to its null hypothesis alfa level; however, median survival was significantly longer for pembrolizumab vs. placebo [13.9 vs. 10.6 months, HR of 0.78 (CI 0.71–0.99); P=0.024]. DFS did not reach its primary efficacy endpoint too, although there was a higher ORR in favor of pembrolizumab (18.3% vs. 4.4%; P=0.00007), with a longer median duration of response. The incidence of immune-related adverse events (irAEs) was 18.3% with pembrolizumab, 7.2% grade 3/4 and in 3.6% motivated treatment discontinuation. The most frequent adverse events were liver enzymes elevation, fatigue, pruritus, hyporexia and diarrhea. In a stratified analysis, patients with AFP <200ng/ml or with post-progression (vs. intolerant to sorafenib) or with HBV had the highest benefit of pembrolizumab [333]. In Argentina, nivolumab and pembrolizumab were approved in late 2019 (Table 3).
3.8Clinical and radiological assessment after treatments for HCCThe main outcome of the treatment of HCC patients is overall survival. Secondary outcomes should be cancer erradication for curative therapies or delaying tumor progression with palliative therapies [334]. Liver decompensation events are competing events (jaundice, ascites, hepatic encephalopathy) leading to higher mortality and lower survival. These events are considered to be “untreatable progression”[335,336]. On the other hand, TTP or DFS are not surrogate markers of overall survival in patients with HCC; both are lower robust end-points with different risk of bias [291,317].
“Target lesions” are those potentially treatable with at least APHE [337,338]; which should not be confounded with inflammatory signals. In cases of CR for curative therapies, imaging controls should be done every 3 months thereafter. Tumor response evaluation should be assessed with triphasic CT or MRI scans 4–6 weeks following TACE; particularly, with MRI after conventional TACE avoiding confounding effect with lipiodol. TARE response should be assessed not earlier than 8 weeks; with the best response usually occurring even after 6 months. Tumor re-assessment for systemic therapies is done every 8–12 weeks if second or third lines are available. Different imaging criteria to address tumor response have been proposed through the Response Evaluation Criteria for Solid Tumors (RECIST), including different versions (RECIST 1.0, RECIST 1.1), modified RECIST (mRECIST) [337,338]. Other criteria include the EASL or WHO [337,338]. mRECIST and EASL consider residual hyperenhacement diameters [337,338]. For locoregional therapies, mRECIST or EASL are preferable and for systemic therapies, RECIST 1.1 or mRECIST have been validated in RCT [339]. LIRADS tumor response assessment has not been validated in RCT [108,118]. Finally, evaluation after immunotherapy should consider that inflammatory infiltrates may increase tumor diameters at the very beginning (iRECIST).
3.8.1Clinical management after locoregional endovascular therapiesAlthough it has been shown that TACE number of sessions is directly proportional to tumor diameter [340] and CR has been associated with better survival [340], it is important to underline the risk of liver decompensation with unnecessary sequential TACEs when no response or tumor progression is observed, due to ischemic injury on the non-tumoral liver. Thus, it may be prudential to perform no more than 2 or 3 TACE sessions to achieve local tumor control and if not achieved, start systemic therapy [337,341]. Although the risk of liver ischemic injury with TARE is much lower, potential REILD should be evaluated.
Some scoring models have been published to identify candidates for sequential re-TACE, including the ART, STATE-ART and HAP scores [342–344]. However, significant risk of selection and information bias precluded these scores to be robust enough. Patients with “untreatable progression” are neither candidates for sequential TACE nor systemic therapy [335,336]. The rate of progression from Child–Pugh A to B or C following TACE was 13.8% and 15.9%, respectively (RELPEC cohort study) [345]. In Argentina, patients with “untreatable progression” following TACE had lower survival compared to those with sequential TACE-systemic therapy [311]. Data from first and second line RCT considered two intrahepatic tumor progression following TACE in BCLC-B as eligible for systemic therapy [241,317,322]. TARE as a rescue therapy following TACE failure was reported in the SARAH and SIRveNIB trials [240,241].
3.8.2Clinical management and radiological assessment after systemic therapiesClinical evaluation and management of patients receiving systemic therapies should include “untreatable progression”, PD and tolerability. Any type of PD may not defined prognosis [134,329,330,332]. Whereas vascular invasion, new or growing extrahepatic lesions are prognostic markers, at least two consecutive new intrahepatic or growing intrahepatic lesions may be needed to start a second line [335].
Adverse events following TKIs should be addressed at every clinical visit; the need for dose reduction or treatment discontinuation. Intolerance to sorafenib was differently defined in the RESORCE and KEYNOTE-224 trials [329,333]. Treatment discontinuation rates were 9% with lenvatinib [322], 10% with regorafenib [329], 11% with sorafenib [241,310] and 16% with cabozantinib [134]. This comparison should consider different elegibility criteria and assessment of adverse events [346]. Most common adverse events for TKIs are fatigue, hyporexia, diarrhea, dermatologic events including rash and HFSR, hypertension, hypothyroidism, elevated liver enzymes, elevated total bilirubin and proteinuria [134,241,310,322,329]. Ramucirumab presented hypertension and hyponatremia [330]. Dermatological adverse events during the first 45 days of sorafenib therapy have been associated with better overall survival [314,347]. In general, grades I-II adverse event should prompt specific treatment and may continue with TKI dose until resolution of symptoms; grades III demand TKI reduction or transient interruption until resolution and grades IV definite treatment discontinuation [346]. Schemes of dose reduction according to RCT are shown in Table 4.
Radiological tumor response assessment, management of adverse events and dose scheme for HCC systemic therapies.
| Topic | Statement | Grade of Recommendation | Quality of the evidence |
|---|---|---|---|
| Radiological response assessment | mRECIST is recommended for post-locoregional therapies tumor reassessment. | Strong | Moderate to high |
| RECIST 1.1 or mRECIST are recommended to assess tumor response under systemic treatments. | Strong | High | |
| There is no robust evidence to support tumor reassessment by LIRADS. | Strong | Low to very low | |
| Stopping rules | Grade 4 adverse events for any therapy. | Strong | High |
| Unresolved grade 3 adverse events for any therapy. | Strong | High | |
| Grades 2 or higher for irAEs1 | Strong | High | |
| “Untreatableprogression”2 for locoregional and systemic therapies. | Strong | High | |
| Dosing schedules for systemic therapies | Dose is fixed for atezolizumab 1200mg iv+bevacizumab 15mg/kg iv; cycles every 21 days may be postpone or interrupted for adverse events. | Strong | High |
| Dose scheme reduction for sorafenib is 400mg bid to 400mg/day and 400mg every other day. | Strong | High | |
| Dose scheme reduction for lenvatinib depends or initial weight but it is 12mg/day to 8mg/day, 4mg/day to 4mg every other day. | Strong | High | |
| Dose scheme reduction for regorafenib is 160mg/day to 120mg/day and 80mg/day (ON-OFF cycles). | Strong | High | |
| Dose scheme reduction for cabozantinib is 60mg/day to 40mg/day and 20mg/day. | Strong | High | |
| Dose is fixed for ramucirumab 8mg/kg iv; cycles every 14 days may be postpone or interrupted for adverse events. | Strong | High | |
| Dose is fixed for pembrolizumab 200mg iv; cycles every 21 days may be postpone or interrupted for adverse events. | Strong | High | |
| Dose is fixed for nivolumab 3mg/kg or up-to 240mg; cycles every 14 days may be postpone or interrupted for adverse events. | Strong | High | |
Notes: 1-Specific management for each irAE should be addressed with other practice guidelines. 2- Defined as jaundice, ascites, hepatic encephalopathy and other related complications (hepatorenal syndrome, among others).
Abbreviations: iv: intravenous infusion. irAE: immune-related adverse event.
Management of irAEs should be addressed following other international guidelines [348]. The irAEs have been reported in 83% of treated patients with less than 10% being grades 3 or 4 [332,333,349,350]. Although well-tolerated, irAEs could be life-threatening and demand a low threshold of suspicion [348]. Every organ can be involved, with a median occurrence between 3 to 6 months of initiation, earlier for anti-CTLA-4 compared to anti PD-(L)1 [348]. Most frequently involved organs are the skin (rash, pruritus), gastrointestinal (diarrhea or colitis) and the liver (hepatitis) [333]. Most of irAEs respond to steroid treatment and resolve between 6 to 8 weeks; steroid treatment has not been associated with lower efficacy in other tumors. However, steroids should be cautiously used in patients with cirrhosis due to a higher risk of infections or liver decompensation [351]. Recurrence of irAEs has been reported 55% of the cases, associated with time of occurrence with the first event [348]. Viral load for HBV before immunotherapy should be controlled with antivirals below 100 or 500UI/ml because of the risk of HBV flares [332].
3.9Special populationsHepatocelluar carcinoma in non-cirrhotic or non-advanced fibrotic patients, mixed hepato-cholangio carcinoma and fibrolamellar HCC are special populations with low quality evidence to support for or go against any specific recommendations and these patients have been excluded from RCT. Although the BCLC could be applied, these patients should be discussed in multidisciplinary boards and LR should be the first-line option.
3.10Final considerationsWith the coming new diagnostic and novel therapies for HCC, these patients will demand multidisciplinary team-working specifically trained on liver-related complications and HCC including hepatologist, gastroenterologists, liver surgeons, oncologists, radiologists, palliativies and pathologists [7]. Hepatologists have had a key role in research in this field [73,91,136,138,155,157,228,241,285,322,328]. Consequently, international associations and guidelines should not only underline the main role of hepatologists but also the need for multidisciplinary team-working [352–355]. Similarities and differences between this and other guidelines are described on Table 5. Differences between these practice guidelines with the former may be related to feasibility and availability of diagnostic and therapeutic modalities but also to cost-effective, and healthcare system barriers. Cost-effective analysis in countries with limited health resources should be done, as well as local patient's preferences. Approval of some interventions in these countries should be carefully addressed. For this reason, it is important to have local or regional data, analyzing access to health care [358,359]. In this clinical practice guideline, in 7.5% of the total included references, Argentinian colleagues participated as first authors, or as participating co-authors from international observational and RCT. However, the first authorship rate was 4.4%, this is a common problem in Latin America, a region where there is a low rate in the clinical setting [356–358].
Comparison between A.A.E.E.H and other international guidelines.
| Topic/Guideline | AAEEH | AASLD [1] | EASL [2] | ALEH [3] | APASL [4] |
|---|---|---|---|---|---|
| General organization | Consensus meeting 2015Key clinical questions.GRADE approach | Ten key clinical questions.GRADE approachPublished in 2018 | GRADE approachPublished in 2018 | GRADE approachPublished in 2014 | Consensus meetingGRADE approachPublished in 2017 |
| Epidemiology | Definition of the population at high risk for HCC. | Definition of the population at high risk for HCC. | Definition of the population at high risk for HCC. | Definition of the population at high risk for HCC. | Definition of the population at high risk for HCC.Special focus on HBV HCC. |
| Primary, secondary and tertiary prevention detailed. | Primary, secondary and tertiary prevention detailed. | Primary and secondary prevention detailed. | Primary, secondary and tertiary prevention detailed. | ||
| Strongly recommends treatment of liver disease to avoid progression of liver disease.HCV therapy after curative therapies for HCC: benefit vs. recurrence risk to be individualized.Uncertainty for recommending coffee consumption. | Strongly recommends treatment of liver disease to avoid progression of liver disease.HCV therapy after curative therapies for HCC: benefit vs. recurrence risk to be individualized.Strongly recommends coffee consumption. | Universal HBV vaccination in infants, specially HBV-endemic areas.Long-term antiviral HBV therapy as secondary prophylaxis.Recommends achievement of viral eradication for HCV but in high risk patients warrants surveillance.Further studies to assess risk and benefits of DAA after curative therapies for HCC. | |||
| Surveillance | Recommends surveillance with US.Suggests surveillance with US with AFP in cases of unknown or inexpert US operators based on 3 AFP cut-off values. | Suggests surveillance with US with or without AFPSuggest not performing surveillance in ChildPugh C if not listed for LT. | Recommends surveillance with US.Does not recommend biomarkersRecommended in patients with preserved liver function or listed for LT. | Recommends US. | Recommends US with AFP (suggested cut-off 200ng/ml) or even a lower cut-off after HBV or HCV viral eradication.Suggests simultaneous use of biomarkers: AFP, AFP-L3 and DCP. |
| Every 6 months. | Time-frame for surveillance every 4–8 months. | Every 6 months. | Every 6 to 12 months. | Every 6 months. | |
| Imaging Diagnosis | Imaging diagnosis strongly recommended either with triphasicCT or MRI.Suggests using LI-RADS in a specific algoritm. | Imaging diagnosis strongly recommended either with triphasicCT or MRI. | Imaging diagnosis strongly recommended either with triphasic CT or MRI or contrast-US.PET-CT not recommended.MRI with hepatobiliary contrast agents included in algorithm but recalling lower specificity. | Imaging diagnosis strongly recommended either with triphasic CT or MRI | Imaging diagnosis strongly recommended either with triphasic CT or MRI or gadoxetic acid MRI for non-hypervascular nodules and contrast US. |
| Tumor biopsy | Tumor biopsy for non-cirrhotic and indeterminate nodules.Maybe assessed after gadoxetic acid contrast MRI.Tumor biopsy only in non-cirrhotic patients. | Tumor biopsy conditional.Against routine biopsy of every indeterminate nodule. | Tumor biopsy only in non-cirrhotic patients. | Tumor biopsy in non-cirrhotic patients. | Tumor biopsy for non-cirrhotic and indeterminate nodules after gadoxetic acid contrast MRI. |
| Staging | BCLC staging. | BCLC not included. | BCLC staging. | BCLC staging. | Asia-Pacific treatment algorithm. |
| Includes specific key question for LT candidates in BCLC-B and D stages. | Staging based on TNM. | “Not optimal surgical candidate” with portal hypertension may be done including extension of hepatectomy and MELD <10. | |||
| Endovascular therapies | Strongly recommends TACE for BCLC-B patients.TACE with DEB not recommended over conventional TACE.Suggests evaluating TARE in patients with unresectable large tumors, with/without portal vein thrombosis (non-tumoral invasion). | Strongly recommends with TACE, TARE or external radiation over BSC for T2 or T3 HCC not candidates for surgery or LT.Does not recommend one form of therapy over another. | Strongly recommends TACE, either conventional or with DEB, over BSC.Does not recommend TARE or other therapy for intermediate HCC. | Strongly recommends TACE over BSC.TACE with DEB although “showing benefits over conventional TACE, not recommended due to higher costs”. | TACE, either conventional or with DEB, over BSC in multinodular, un-resectable and without extrahepatic or vascular disease HCCTARE or SBRT conditional options.Concept or “TACE failure/refractoriness” (2 or more consecutive ineffective responses or 2 or more intrahepatic progressions). |
| Locoregional therapies | Either RFA or LR for very early HCC. PEI if RFA not feasible.Does not recommend cryoablation.Uncertain recommendation for SBRT or IRE.CSPH and preserved liver function recommended to select candidates for LR. | Suggests resection over RFA: for T1 or T2. | Either RFA or LR for very early HCCSuggests “resection” in non-ideal candidates: MELD <10. | Either RFA or PEI for very early HCC not suitable for surgery. | RFA first line for single <2cm, Child A or B.RFA as alternative for LR. PEI for cases in which RFA is not feasible in patients with up-to 3 nodules <3cm each. |
| Liver Transplantation | Suggests using composite criteria for candidate selection.Suggests bridging therapies while on the waitlist period depending on tumor burden, AFP >400ng/ml and expected time on the waitlist.Suggests downstaging for patients beyond Milan criteria but with AFP <100ng/ml. | Suggest for T1 HCC listed for LT to undergo observation period.Suggests bridging therapies for T2 while on the waitlist period; without any specific treatment recommendation.Suggests downstaging for patients beyond Milan criteria within T3 stage. | Recommends Milan criteria for appropriate candidate selection but recalls for composite models including biomarkers.Suggests bridging therapies while on the waitlist period; without any specific treatmentWeak recommendation for downstaging. | Recommends Milan criteria for appropriate candidate selection.Conditional recommendation for downstaging.Recall for AFP cut-offs for LT selection. | Resectability vs. LT based on Child Pugh and “surgeons evaluation”. |
| Adjuvance | Against adjuvant therapy after LR or ablation. | Against adjuvant therapy after LR or ablation. | Against adjuvant therapy after LR or ablation. | Not up-dated. | |
| Systemic treatment | Specific recommendations for available options in first-line (sorafenib, lenvatinb) and second line (regorafenib, pembrolizumab and nivolumab).Not yet approved: atezolizumab+bevacizumab in first-line and cabozantinib in second-line.Against TARE over sorafenib. | Strongly recommends systemic therapy over BSC for Child A or B in advanced HCC patients.No specific recommendation for first or second lines. Not up-dated. | Specific recommendations for first and second lines (sorafenib, regorafenib, cabozantinib).Against TARE over sorafenibNot up-dated. | Only sorafenib.Not up-dated. | Sorafenib for first line therapy.Regorafenib as second line.Not up-dated. |
| Other remarks | Specific recommendations for adverse events, stopping rules and radiological response assessment.Recalls resection as main therapy in non-cirrhotic patients.Multidisciplinary team working. | Multidisciplinary team workingRecalls resection as main therapy in non-cirrhotic patients. | Recalls resection as main therapy in non-cirrhotic patients.LT may be offer in these patients.HCC in pediatric population. | Multidisciplinary team working. | |
Notes:
[1] Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–80. doi:10.1002/hep.29086/suppinfo.
[2] Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. Journal of Hepatology 2018;69:182–236. doi:10.1016/j.jhep.2018.03.019.
[3] Méndez-Sánchez N, Ridruejo E. Latin American Association for the Study of the Liver (LAASL) Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. Annals of … 2014.
[4] Omata M, Cheng A-L, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update 2017:1–54. doi:10.1007/s12072-017-9799-9.
On the other hand, genetic regional variations should also be considered [357]. During the coming years, advancement of medical technology, based on novel genetic [357,359], epigenetic researches [360], as well as radiomics and artificial intelligence [361], may help to enhance surveillance, diagnosis and treatment response assessment in the clinical setting of HCC patients. In this regard, clinical practice guidelines should be appropriately updated [357].
Conflict-of-interestF Piñero has received Advisory Board and speaker honoraria and he is consultant of Bayer Healthcare; has received speaker honoraria from Raffo and research grants from the Argentinean National Institute of Cancer (INC ID-190), Argentinean National Ministry of Science and Technology Development (PICT 2017, FONCYT) and from the Latin American Liver Research Educational and Awareness Network (LALREAN). G Aballay Soteras has received speaker honoraria and Advisory Board fees and research grants from Bayer Healthcare, MSD; he is consultant for Bayer and has received Advisory Board fees from Roche. S Marciano has received speaker honoraria from Gador, Gilead and Abbvie; has received educational grants from Gilead, Bristol-Myers Squibb, Bayer, Biotoscana and Gador and research grants from Gilead and Bristol-Myers Squibb. M Silva has received has received speaker honoraria and is a consultant for Abbvie, Gador, Bristol-Myers Squibb, Merck, Bayer and research grants from the Argentinean National Institute of Cancer (INC ID-190), Argentinean National Ministry of Science and Technology Development (PICT 2017, FONCYT). A Ruf has received Advisory Board and speaker honoraria and he is consultant of Bayer Healthcare, Novartis, Gador, Astellas and has received research grants from the Executive Secretariat of the Group of 77 at the United Nations. M Dirchwolf has received Advisory Board and speaker honoraria and he is consultant of Bayer Healthcare and has received a research grant from Novartis. M Tanno, M Tisi Baña, E Fassio, S Mengarelli, S Borzi, N Fernandez, E Ridruejo, V Descalzi, M Anders, G Mazzolini, V Reggiardo, JC Spina, L McCormack, M Maraschio, C Lagues, F Villamil, F Cairo and B Ameigeiras do not have any conflict of interest to disclose.
On behalf of those who have participated in the development of this clinical practice guideline: (1) General Coordination: F Piñero, M Tanno, G Aballay Soteras. (2) GRADE supportive board: F Piñero, M Tisi Baña. (3) Epidemiology: E Fassio, V Reggiardo, A Gadano, S Marciano. (4) Surveillance: S Mengarelli, M Dirchwolf, S Borzi. (5) Diagnosis: M Volpachio, J P Perotti, F Diaz Telli, J C Spina. (6) Staging: N Fernández, A Ruf. (7) Pathology: C Lagues, M Amante, MT García de Dávila. (8) Treatment: L McCormack, M Maraschio, E de Santibañes, G Podestá, E Ridruejo, V Descalzi, M Anders, F Villamil, G Eiselle, R García Mónaco, G Mazzolini, F Perazzo (9) Special populations: S Marciano, F Cairo, M Silva.
To the A.A.E.E.H 2018–2020 boards: Beatriz Ameigeiras, Diana Krasniansky, Fernando Cairo, Paola Casciato, Omar Galdame and Manuel Mendizabal.





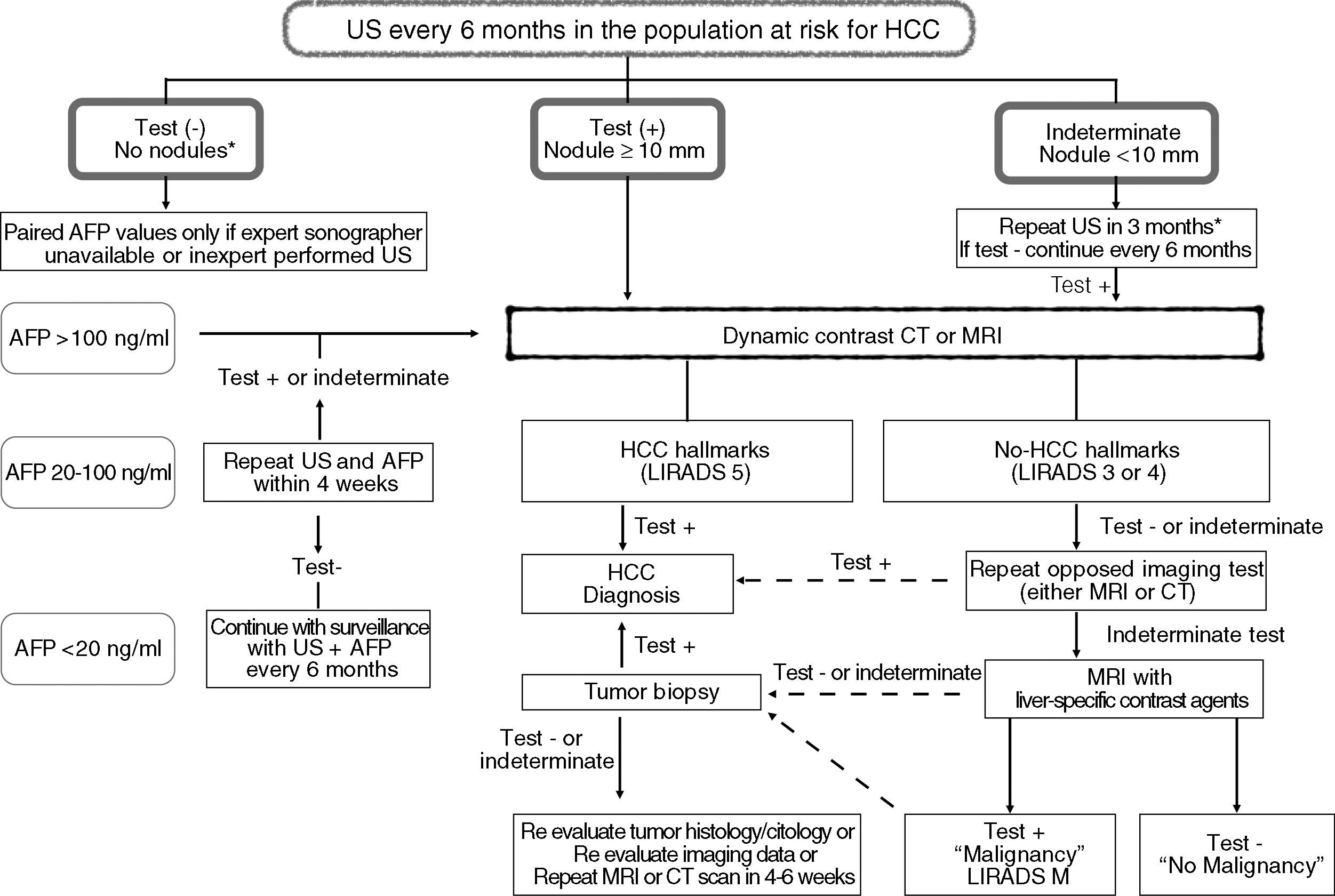

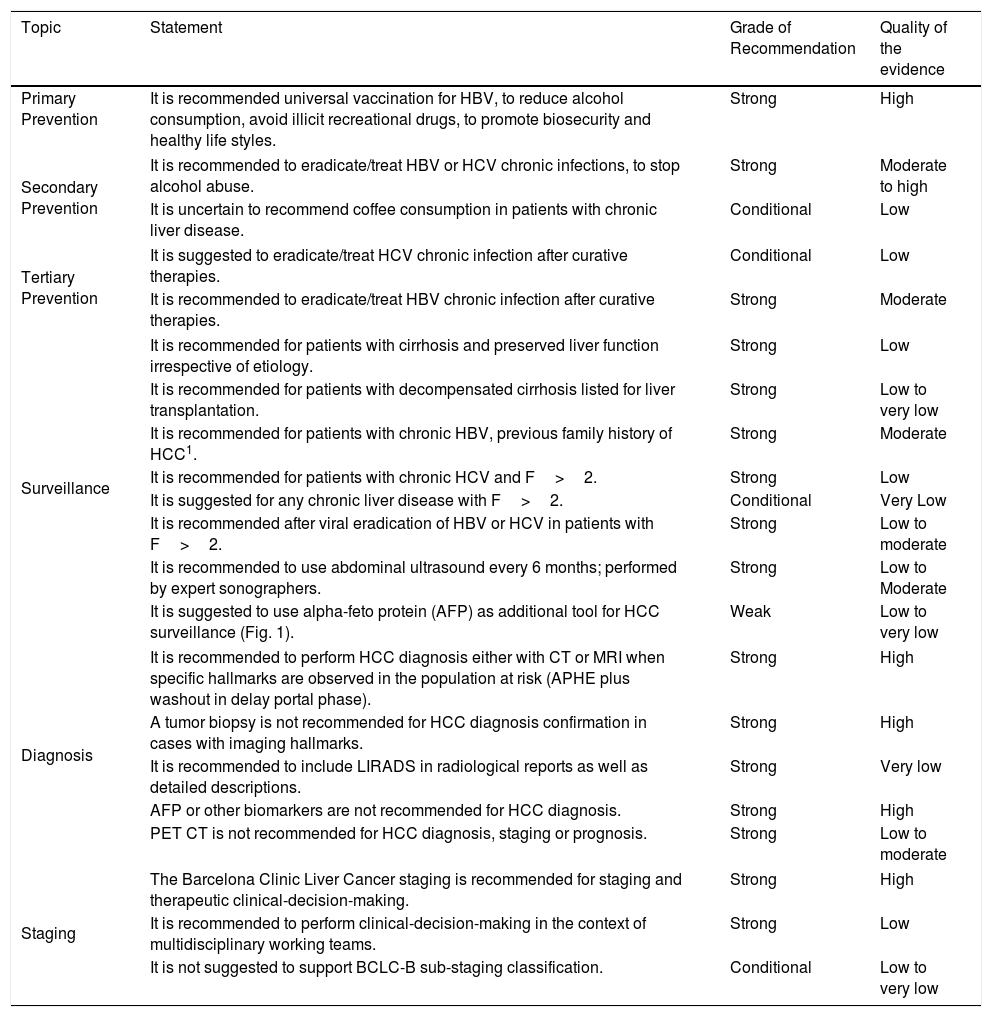
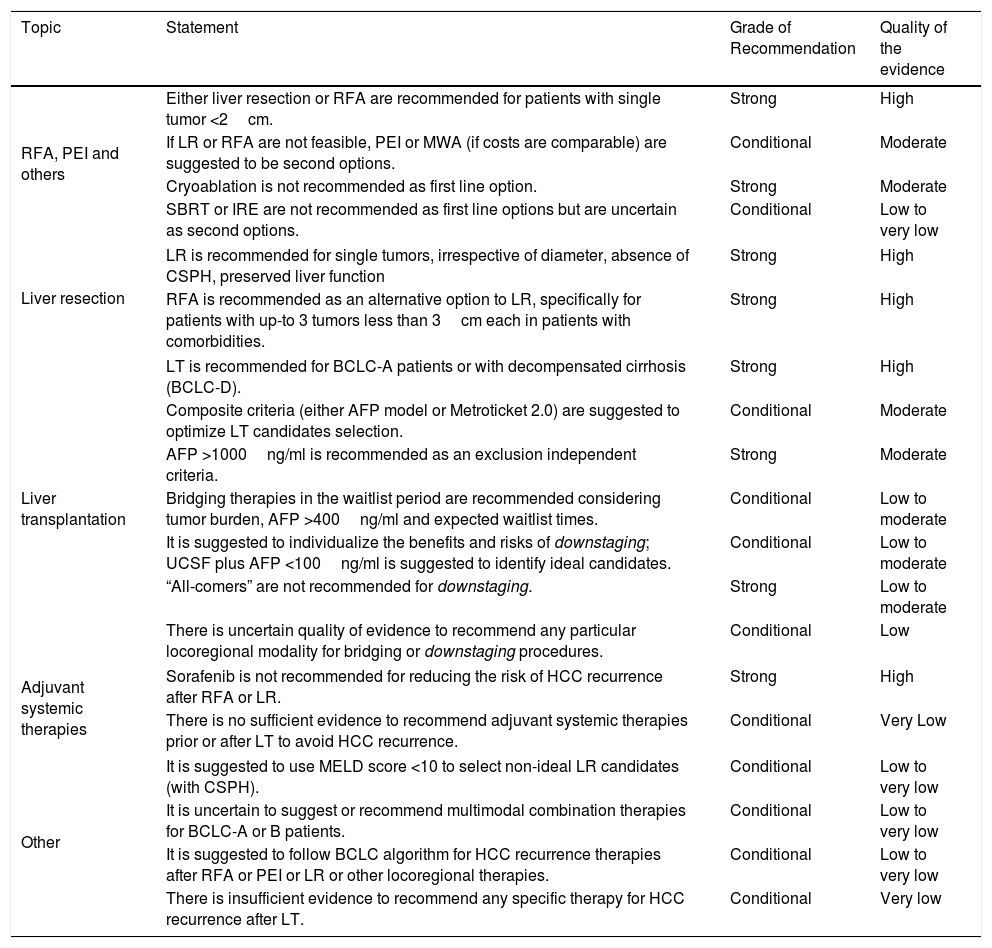
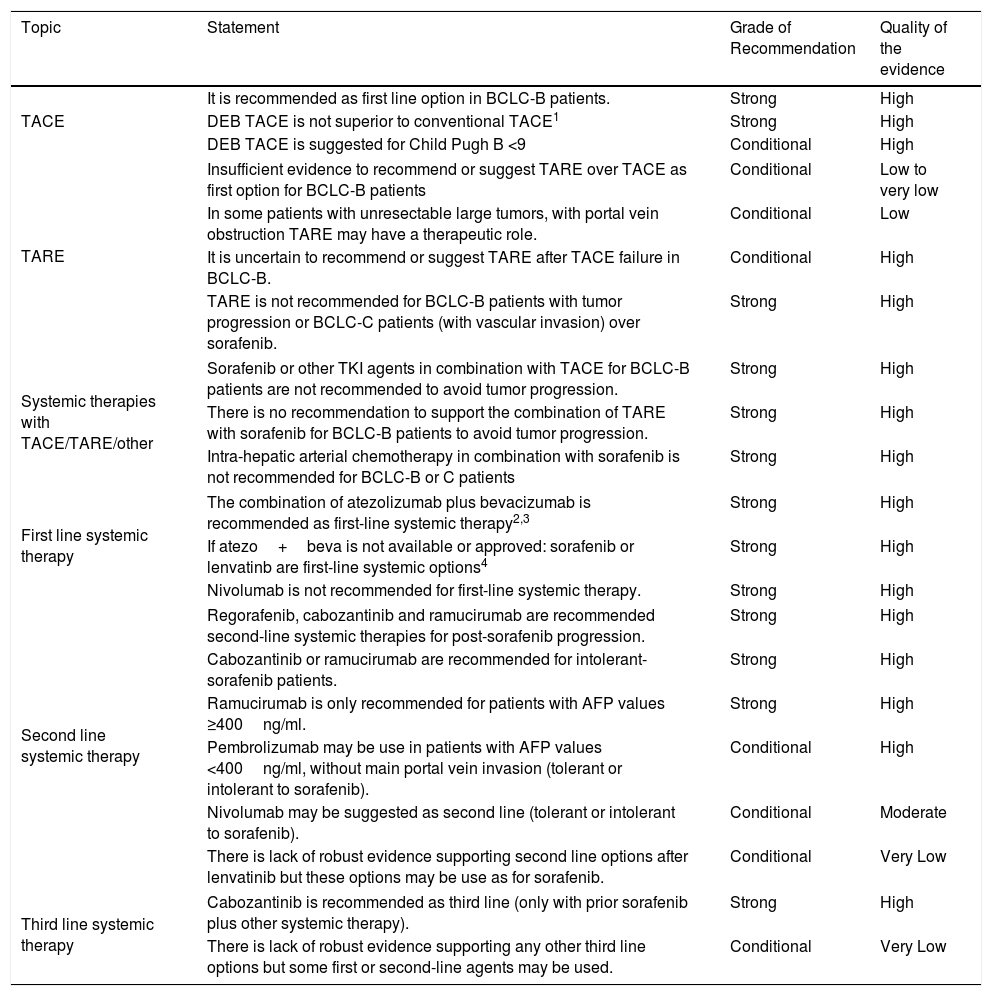
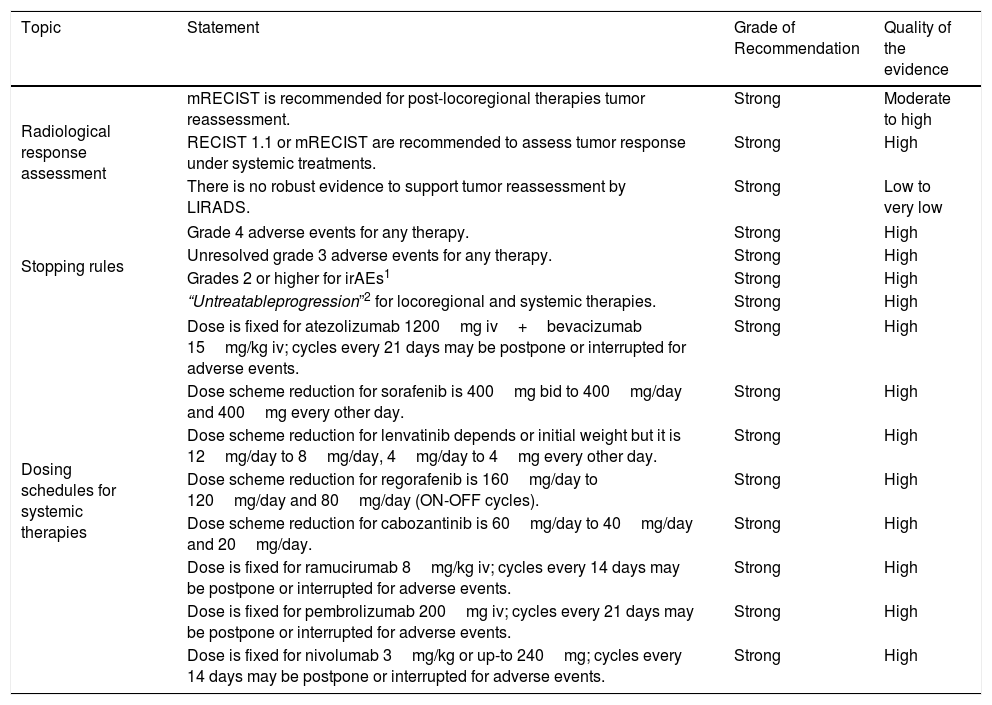
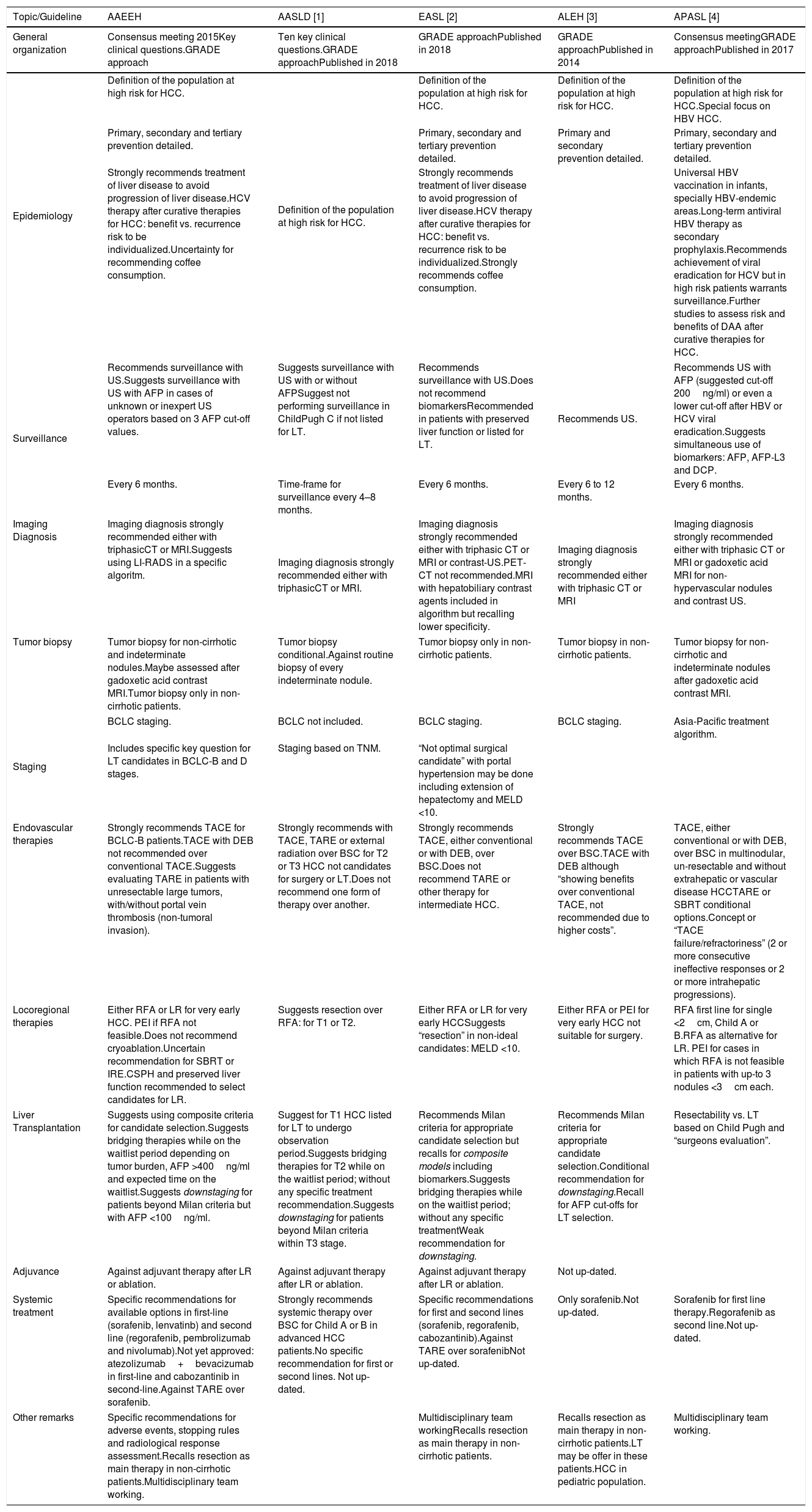

 AFP <100ng/ml). Preserved liver is equivalent to Child Pugh A or absence of any of the following decompensation events: jaundice, ascites, hepatic encephalopathy, hepatorenal syndrome, variceal bleeding.
AFP <100ng/ml). Preserved liver is equivalent to Child Pugh A or absence of any of the following decompensation events: jaundice, ascites, hepatic encephalopathy, hepatorenal syndrome, variceal bleeding. 



