Direct-acting antiviral (DAA) agents are highly effective for treatment of chronic hepatitis C virus (HCV) yet access to treatment remains a serious challenge. The aim of this study was to identify barriers to treatment initiation with DAA-containing regimens in an urban clinic setting.
Materials and methodsA retrospective cohort of all chronic HCV patients seen in an urban academic practice in Jacksonville, FL, USA from 1/2014 to 1/2017 was analyzed. Baseline characteristics were recorded and a review of medical records was performed to identify barriers to treatment initiation and overall success rates.
ResultsTwo-hundred and forty patients with chronic HCV were analyzed. Fifty-six percent of patients were African-American and 63% were insured through Medicaid/county programs or uninsured. Sixty-nine percent had barriers to initiating antiviral therapy categorized as psychosocial (n=112), provider (n=26), medical (n=20), and insurance-related factors (n=7). The most commonly encountered psychosocial barriers included failure to keep appointments (79/240, 33%), active substance abuse (18/240, 8%), and failure to obtain laboratory testing (11/240, 5%). Overall, only 27% of patients evaluated were initiated on DAA-containing regimens with 18% reaching SVR12 within the 36-month study period.
ConclusionIn conclusion, only 27% of patients who presented to an urban academic practice with chronic HCV received DAA-containing regimens over a 36-month period. Psychosocial issues were the major barriers to antiviral therapy. These findings illustrate the need for an integrated approach that addresses psychosocial factors as well as comorbidities and adherence to care in order to increase rates of HCV treatment in at risk patients.
Chronic hepatitis C virus (HCV) affects approximately 2.7 million persons in the United States based on data obtained from the National Health and Nutrition Examination Survey (NHANES) [1]. Data including high-risk persons who were incarcerated, homeless, institutionalized, hospitalized, or in active military duty suggested a peak HCV prevalence of about 1.6% of the US population [2]. Highly effective direct acting antiviral (DAA) agents have the potential to eradicate HCV [3]. However, new cases of HCV are increasing in the United States due to injection drug use and barriers remain to treatment of many affected individuals [4]. An earlier review article cited reasons that persons with HCV remain untreated including deficiencies in population screening, poor patient/physician awareness, contraindications to treatment, and access to care [5].
Barriers to HCV therapy were studied in the interferon (IFN) era, when potential side effects limited treatment candidacy. In previous studies, only about 30% of chronic HCV patients were initiated on IFN-based treatment and psychosocial factors were a major barrier to treatment including active alcohol/substance abuse and poorly controlled psychiatric disorders due to concerns for non-adherence and exacerbation of psychiatric disease [6,7]. In another study, psychosocial factors were a more common cause of IFN treatment ineligibility than medical contraindications (44% vs. 19%) [8]. Financial considerations provide another barrier to HCV treatment. Hepatitis C positive persons were found to have a higher rate of being uninsured than those without HCV and only 36% of a cohort of treatment eligible patients were initiated on IFN therapy, largely based on payer status [9].
The introduction of DAA regimens in 2014 resulted in vastly improved sustained viral response (SVR) rates with shortened treatment duration and minimal adverse effects, even in populations historically considered difficult to treat [10–13]. Despite advances in HCV therapy, barriers to treatment initiation persist. Hepatitis C treatment frequently is delayed or deferred in eligible patients, some of whom are subsequently lost to follow-up care.
With the high cost of DAA therapy, payer restrictions have played an important role in limiting access to treatment. Many state Medicaid programs limit DAA treatment to patients with advanced fibrosis (e.g. Metavir stage F3 or F4) and some commercial payers have followed suit [14]. Given the serious challenges to treating HCV in health systems which provide care to low-income or uninsured populations, the aim of this study was to identify barriers to HCV treatment initiation with DAA-containing regimens in an urban academic practice.
2Material and methodsThe study protocol was approved by the Institutional Review Board at the University of Florida. Study patients were seen at University of Florida Health gastroenterology and hepatology clinics located in the Urban Core neighborhood of Jacksonville, Florida, USA starting in January 2014. Medical records were reviewed from all patients with a diagnosis of hepatitis C seen at the clinics until January 2017. In each case, hepatitis C viremia was confirmed and treatment decisions were made by a gastroenterologist or hepatologist based on the IDSA/AALSD guideline recommendations at the time of evaluation (http://www.hcvguidelines.org/). In July 2015, a multidisciplinary HCV treatment team was developed which included a hepatologist, clinical pharmacists, a pharmacy technician, a social worker, and a research coordinator with goals of identifying barriers to treatment initiation, obtaining DAA therapy, managing patients to end-of-treatment (EOT) and maintaining follow-up to determine response to therapy.
All patient data were reviewed retrospectively. Demographic data included age, sex, ethnicity, race, marital status, and distance from the medical center. Prior history of HCV treatment was reviewed. Estimation of hepatic fibrosis was obtained by FibroSURE (Labcorp, Burlington, NC, USA) or FibroTest (Biopredictive: Paris, France), liver biopsy, or by transient elastography (FibroScan; Echosens, Paris, France). The diagnosis of cirrhosis was based on the non-invasive studies, cross sectional imaging showing characteristic features of cirrhosis, or clinical evidence of cirrhosis including history of decompensated liver disease (i.e. history of variceal bleeding, ascites, or hepatic encephalopathy).
Barriers to initiating or continuing antiviral therapy were identified on the basis of a careful review of medical records and were categorized as: psychosocial, medical, provider, or insurance-related. Treatment status including reasons for failure to initiate therapy, and in patients who were treated, DAA agents used, and treatment outcomes were documented.
2.1Statistical analysisDescriptive summaries consisted of frequencies and percentages for categorical variables and means and standard deviations for numeric variables. Categorical variables were compared by Pearson's Chi-square test (or Fisher's exact test when appropriate). The magnitude of effects was described using odds ratios (OR), along with their 95% confidence intervals (CI). Non-normally distributed continuous outcomes were compared using the Wilcoxon rank sum test. All analyses were performed with SAS® for Windows Version 9.4.
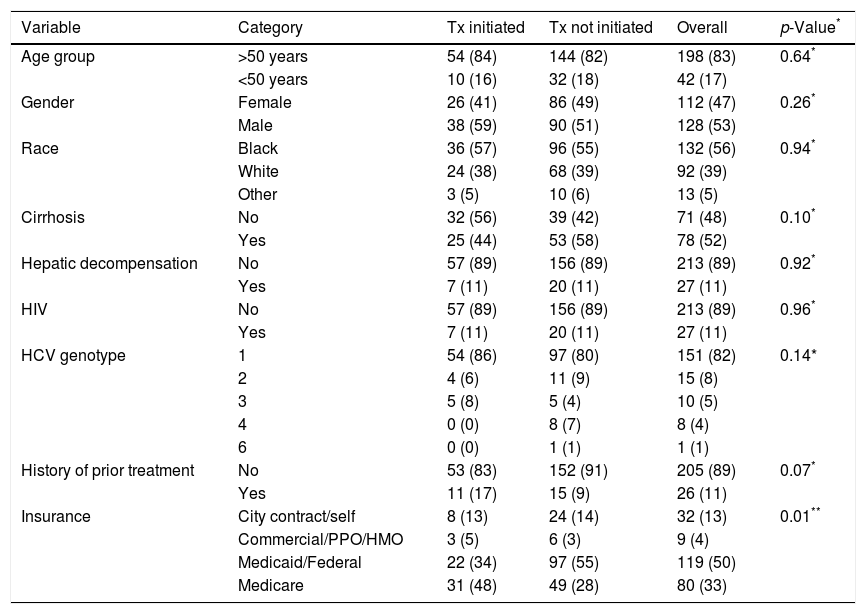
3ResultsThree hundred and twenty-two patients were referred to the gastroenterology or hepatology clinics in the Urban Core neighborhood of Jacksonville, Florida for management of HCV during the study period (Fig. 1). Eighty-one patients were excluded on the basis of negative HCV antibody testing, negative HCV-RNA, treatment with an interferon-containing DAA regimen, or failure to present for their initial appointment. One patient presented with an acute HCV infection and subsequently developed viral clearance. The remaining 240 patients were included in the analysis. Demographic data are detailed in Table 1. The mean age was 56±10 years and 53% of patients were male. The racial distribution included 56% African-American, 39% white, 2% Asian, 1% Native-American, and 4% other races. Sixty-three percent of patients were either insured through Medicaid/county programs or uninsured. Eighty-two percent of patients had HCV genotype 1 and 43% were classified as having F3 or F4 fibrosis. Eighty-nine percent were treatment naïve.
Baseline characteristics.
| Variable | Category | Tx initiated | Tx not initiated | Overall | p-Value* |
|---|---|---|---|---|---|
| Age group | >50 years | 54 (84) | 144 (82) | 198 (83) | 0.64* |
| <50 years | 10 (16) | 32 (18) | 42 (17) | ||
| Gender | Female | 26 (41) | 86 (49) | 112 (47) | 0.26* |
| Male | 38 (59) | 90 (51) | 128 (53) | ||
| Race | Black | 36 (57) | 96 (55) | 132 (56) | 0.94* |
| White | 24 (38) | 68 (39) | 92 (39) | ||
| Other | 3 (5) | 10 (6) | 13 (5) | ||
| Cirrhosis | No | 32 (56) | 39 (42) | 71 (48) | 0.10* |
| Yes | 25 (44) | 53 (58) | 78 (52) | ||
| Hepatic decompensation | No | 57 (89) | 156 (89) | 213 (89) | 0.92* |
| Yes | 7 (11) | 20 (11) | 27 (11) | ||
| HIV | No | 57 (89) | 156 (89) | 213 (89) | 0.96* |
| Yes | 7 (11) | 20 (11) | 27 (11) | ||
| HCV genotype | 1 | 54 (86) | 97 (80) | 151 (82) | 0.14* |
| 2 | 4 (6) | 11 (9) | 15 (8) | ||
| 3 | 5 (8) | 5 (4) | 10 (5) | ||
| 4 | 0 (0) | 8 (7) | 8 (4) | ||
| 6 | 0 (0) | 1 (1) | 1 (1) | ||
| History of prior treatment | No | 53 (83) | 152 (91) | 205 (89) | 0.07* |
| Yes | 11 (17) | 15 (9) | 26 (11) | ||
| Insurance | City contract/self | 8 (13) | 24 (14) | 32 (13) | 0.01** |
| Commercial/PPO/HMO | 3 (5) | 6 (3) | 9 (4) | ||
| Medicaid/Federal | 22 (34) | 97 (55) | 119 (50) | ||
| Medicare | 31 (48) | 49 (28) | 80 (33) |
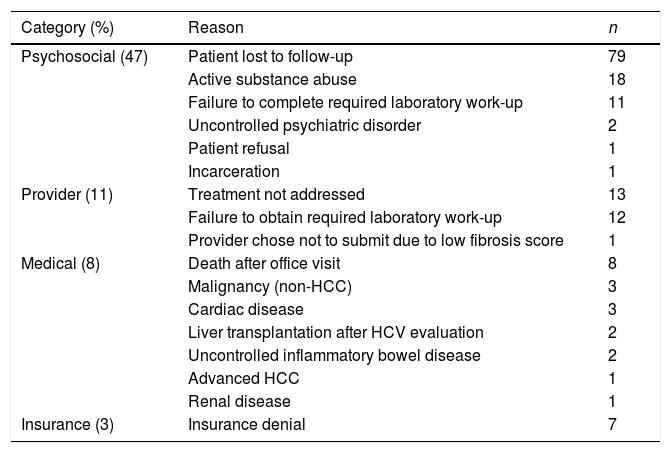
A majority of HCV patients (72%, n=176) were not initiated on DAA therapy during the study period. Seven patients were awaiting insurance authorization and four patients were pending initiation of treatment at the time of analysis. Barriers to treatment initiation in the remaining 165 patients were categorized as psychosocial, medical, provider, or insurance-related factors. (Table 2)
Barriers to treatment initiation (n=165).
| Category (%) | Reason | n |
|---|---|---|
| Psychosocial (47) | Patient lost to follow-up | 79 |
| Active substance abuse | 18 | |
| Failure to complete required laboratory work-up | 11 | |
| Uncontrolled psychiatric disorder | 2 | |
| Patient refusal | 1 | |
| Incarceration | 1 | |
| Provider (11) | Treatment not addressed | 13 |
| Failure to obtain required laboratory work-up | 12 | |
| Provider chose not to submit due to low fibrosis score | 1 | |
| Medical (8) | Death after office visit | 8 |
| Malignancy (non-HCC) | 3 | |
| Cardiac disease | 3 | |
| Liver transplantation after HCV evaluation | 2 | |
| Uncontrolled inflammatory bowel disease | 2 | |
| Advanced HCC | 1 | |
| Renal disease | 1 | |
| Insurance (3) | Insurance denial | 7 |
Psychosocial factors were the most common barrier to treatment, affecting 47% (112/240) of cases. The three predominant psychosocial barriers were failure of patients to follow-up after the initial office visit (n=79), active substance abuse (n=18), and failure to obtain required laboratory testing (n=11). Substance use included alcohol (50%), cocaine (33%), and marijuana (17%). In addition, two patients were ineligible due to uncontrolled psychiatric disease. One patient declined therapy due to concerns over side effects and one patient was incarcerated after initial evaluation.
Provider-related barriers were identified as the reason for lack of HCV treatment in 11% (26/240) of cases. In half of these cases (n=13), providers failed to address HCV positive status during office visits for primary gastrointestinal issues (i.e. colorectal cancer screening). Other barriers included incomplete laboratory orders leading to insufficient information to request therapy (n=12) and one case in which the provider did not submit for therapy based on evidence of mild hepatic fibrosis.
Therapy was deferred in 8% (20/240) of – cases due to medical conditions. Six patients died from complications of end-stage-liver disease (n=4) and hepatocellular carcinoma (n=2). Two deaths occurred from complications of esophageal and colon cancer. Further reasons for not initiating HCV treatment included need for liver transplantation (n=2), uncontrolled Crohn's disease (n=2), advanced hepatocellular carcinoma (n=1), renal cell cancer (n=1), liposarcoma (n=1), prostate cancer (n=1), renal disease (n=1), coronary artery disease (n=1), atrial fibrillation (n=1), and uncontrolled hypertension (n=1). Insurance-related barriers only accounted for 3% (7/240) of cases as a result of denials from Florida Medicaid.
3.1.2Treatment and response ratesForty-four percent of treated patients had evidence of cirrhosis and 11% had evidence of decompensated liver disease. Eighty-six percent of patients had HCV genotype 1 and 17% were treatment experienced. Treatment regimens included ledipasvir–sofosbuvir (n=45), daclatasvir–sosfobuvir (n=4), dasabuvir/ombitasvir/paritaprevir/ritonavir plus ribavirin (n=4), velpatasvir–sofosbuvir (n=2), elbasvir–grazoprevir (n=2), sofosbuvir plus ribavirin (n=2), and simeprevir–sofosbuvir (n=2), daclatasvir–sofosbuvir plus ribavirin (n=1), simeprevir–sofosbuvir plus ribavirin (n=1). One patient was enrolled in an investigational drug study with an unknown oral combination agent. The mean duration of treatment was 14±5 weeks.
Seventeen cases were excluded in the calculation of treatment initiation rates including 7 who were awaiting insurance approval, 4 who were pending initiation of treatment and 6 who were on therapy at the time of analysis. Considering these exclusions, only 27% (64/240) of patients who had an initial outpatient consultation for HCV in a gastroenterology or hepatology clinic were initiated on DAA agents, with 91% (58/64) of these patients completing treatment. An additional 11 patients who had an EOT and were in post-treatment follow-up at the time of analysis and 1 patient who completed therapy but was lost to follow-up after a suicide attempt were excluded from calculation of the SVR12 rate. An SVR12 was achieved in 97% (44/46) of patients who completed therapy and had 12 weeks of follow-up. The 2 patients who did not reach SVR12 developed viral relapse. The overall SVR12 rate was 18% (44/240) and could reach as high as 30% (72/240) if the remaining (28) patients awaiting treatment, on treatment, or in post-treatment follow-up achieve SVR.
The implementation of a multidisciplinary HCV management team was likely associated with an increase in the rate of treatment initiation. From January 2016 to January 2017, treatment rates rose from 9% to 27% (p<0.0001) while the SVR rates increased from 8% to 18% (p<0.0001) of the study population.
3.1.3Factors influencing treatment initiationThe 11 patients who did not obtain required laboratory testing were not started on therapy. Distance from clinic did not factor into treatment initiation (8.9 miles for treated vs 8.6 miles for untreated patients, p=0.1). Patients with Medicare coverage were more likely to receive treatment compared to those with Medicaid (p<0.001) [Table 1].
4DiscussionIn this retrospective cohort of HCV patients seen at an urban academic practice, only 27% of patients evaluated over a three-year period were initiated on DAA therapy with 18% reaching SVR12. The low overall success rate is sobering given the high efficacy of DAA therapy and highlights the importance of understanding and addressing barriers to HCV treatment. Psychosocial factors were the most common impediment to DAA therapy and were identified in 47% of patients including non-adherence with laboratory testing and office follow-up as well as active substance abuse. The data are reminiscent of a VA study from the IFN era in which psychosocial contraindications prevented treatment initiation in 70% of evaluated patients [8]. In contrast to the findings of the current study, substance abuse accounted for 25% of untreated cases in the VA study while issues with adherence were less common, occurring in only 3.6% of the cases [8]. In the present study, provider-related barriers were noted in 11% of untreated cases followed by medical comorbidities precluding treatment (8%) and insurance-related factors (3%).
Despite the increased prevalence of mental illness in patients with chronic HCV, uncontrolled psychiatric disease was identified as a barrier to treatment initiation in less than 1% of all patients evaluated [5]. The low rate of psychiatric contraindications with DAA therapy is a welcome change from the IFN era and reflects the lack of psychiatric adverse events with DAAs. Other commonly associated psychosocial factors including substance abuse and incarceration led to failure of treatment initiation in a minority of cases. Surprisingly, the category of provider-related factors was the second most common barrier to treatment initiation. The two predominant causes were failure of the gastroenterology provider to address HCV status and errors in ordering laboratory testing required for authorization of treatment by the insurance provider. Both of these issues could be improved with education aimed at improving provider awareness of hepatitis C status and HCV therapy submission criteria, particularly with state Medicaid programs [15].
The mean age of the study population falls within the baby boomer generation that is known to have a high prevalence of HCV and both medical and HCV-related comorbidities [1,16]. Many patients with chronic HCV suffer from coexisting comorbid conditions with an increased prevalence of illnesses associated with metabolic syndrome and advanced liver disease [17–19]. Interestingly, 25% of patients with serious medical comorbidities suffered from non-hepatic malignancies not associated with HCV. The high prevalence of tobacco use in patients with HCV might have been a contributing factor as 75% of the patients with non-hepatic malignancy and cardiac disease in previous studies reported a history of tobacco use [20,21].
Modeling data predicts a steep reduction in the burden of HCV with new treatments and a goal of near HCV eradication by 2030 [3]. However, the estimated global number of new infections in 2015 (N=1.75 million) exceeded the estimated number of deaths from end-stage HCV infection (N=399000) and cures (N=843000), meaning the global epidemic still expanded in magnitude in 2015. To achieve elimination by 2030 (65% reduction in mortality and 90% reduction in incidence), 90% of HCV infected persons need to be identified and, of those identified, 80% need to be treated [22]. Worldwide, the overall HCV treatment rate is approximately 25% in the pre-DAA era with loss of follow up and lack of specialty care serving as predominant barriers to treatment [23]. Developing countries with a high prevalence of hepatitis C and socialized healthcare systems face infrastructure related barriers including the development of nationwide screening programs and financial investment for acquisition of DAA therapies [24].
Despite the advances afforded by DAA therapy, patient-related factors and linkage to subspecialty care will continue to serve as barriers to care [25–27]. When further evaluating barriers to care in a predominantly Caucasian population, medical comorbidities surpass psychosocial issues as the predominant barrier to initiating DAA therapy [28]. In an urban clinical setting with predominantly African-American patients, the prevalence of psychosocial barriers almost doubles. These findings are representative of real-world challenges encountered when providing healthcare in urban settings. Socioeconomic constraints including financial hardship, transportation issues, poor social support, and incarceration are amongst the leading contributing factors [29]. Furthermore, concern over HCV-related stigma also can affect both patient access and adherence [30]. While a large emphasis has been placed on screening and access to care, our data demonstrates the hurdles that exist when guiding patients through treatment after access to care has been accomplished.
We convened a multidisciplinary HCV treatment team in 2015 after observing low rates of HCV treatment at our center in 2014. This change in practice coincided with a doubling of treatment rates, though the percentage of patients initiated on treatment remained quite low at 27%. In retrospect the multidisciplinary team most impacted patients who met payer criteria for treatment submission to obtain therapy but did little to address the major barriers to care outlined in this study. Furthermore, one cannot ignore other factors which may have led to improved treatment rates including new therapies approved during this time period, lower drug prices, decreased payer restrictions, and improved patient and provider knowledge.
Our finding that psychosocial factors were the most common barrier to HCV provides a basis to further develop a treatment team strategy. The high rates of failure to follow-up for office visits and laboratory testing suggest a role for a patient navigator. Prior studies have demonstrated the utility of patient navigator systems for colorectal cancer screening in urban and high risk populations [31,32]. Moreover, a study of high need patients with HCV showed that the use of patient navigators increased treatment rates, suggesting that the addition of a patient navigator could address gaps that exist in patients who fail to meet criteria for HCV submission and miss office appointments [33]. While the use of a multidisciplinary team including a patient navigator to increase HCV treatment rates requires further study, such interventions are resource intensive and taxing on resource limited health systems. State of federal programs might be needed to support comprehensive HCV treatment team initiatives.
Acquisition of DAA therapy remains time and resource intensive partly due to the need for medication prior authorization [34]. Disparities in DAA access have been reported, particularly in Medicaid populations [35,36]. Despite a low rate of insurance denials in this study, all denials occurred in patients with Medicaid coverage. Furthermore, many patients with Medicaid coverage did not reach treatment submission while those initiated on therapy were twice as likely to have Medicare coverage in comparison to those who remained untreated. In order to be effective, HCV treatment teams will need to include expertise in obtaining insurance approval.
In conclusion, psychosocial barriers were identified to be a major determinant of failure to initiate HCV therapy in an urban academic clinic despite establishment of a multidisciplinary HCV treatment team. This study demonstrates the characteristics and outcomes of a real-world urban cohort of patients treated for HCV. Weaknesses of the study include the retrospective analysis, which lacks fine detail. However, the barriers to HCV treatment identified in the current study suggest that the development of HCV treatment teams which incorporate a patient navigator and expertise in the insurance approval process have the potential to improve treatment rates in at-risk populations and deserve further prospective evaluation. This data also brings to light important healthcare barriers that exist when providing care to urban populations.
AbbreviationsDAA direct-acting antiviral chronic hepatitis C virus National Health and Nutrition Examination Survey interferon sustained viral response end-of-treatment odds ratios confidence intervals
Miguel Malespin: Receives research/grant support from AbbVie, Gilead, and Intercept
Novo Nordisk research/grant support
Ciel Harris: no conflict of interest
Ozdemir Kanar: no conflict of interest
Kelly Jackman: no conflict of interest
Carmen Smotherman: no conflict of interest
Abbey Johnston: no conflict of interest
Julie Ferm: no conflict of interest
Silvio W. de Melo Jr: no conflict of interest
James S. Scolapio: no conflict of interest
David R. Nelson: receives research/grant support from AbbVie, BMS, Gilead, and Merck
Scott J. Cotler: no conflict of interest
There was no financial support provided for this study.