Background and Aims. Acute-on-chronic liver failure has been recognized as a sudden deterioration of cirrhosis, with a high short-term mortality. Prognostic scores are used to assess liver dysfunction. However, there is not enough information on a score to predict short term mortality in those patients. We aimed to investigate the prognostic value of bilirubin concentration in predicting the 1-week outcome of patients with acute-on-chronic liver failure.
Material and methods. We performed a retrospective analysis with a cohort of 65 patients (33 women/32 men), age average of 64 years, diagnosed with acute-on-chronic liver failure with at least 1 week follow-up. Demographics, clinical and biochemical variables were analyzed. Most patients died (59 %) within 1 week of follow-up.
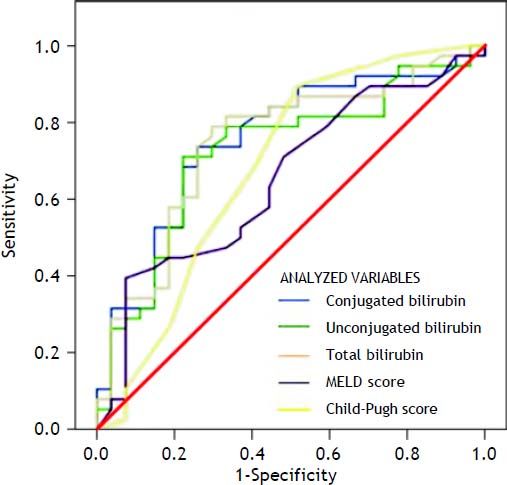
Results. In univariate logistic regression analysis, admission to the intensive care unit, use of vasoactive drugs, need for parenteral nutrition, and levels of conjugated, unconjugated, and total bilirubin at the time of hospital admission were significantly associated with 1-week mortality; in a multivariate logistic regression, conjugated (p = 0.01), unconjugated (p = 0.01), and total bilirubin (p = 0.009) were independently associated with 1-week mortality. In ROC curve analysis, conjugated (0.751, p < 0.05) and total bilirubin (0.746, p < 0.05) levels were significantly the best short-term mortality predictors.
Conclusions. High levels of bilirubin are able to predict short-term mortality in these patients. Also, we suggest that bilirubin can be used as a biochemical marker to improve triage of patients with acute-on-chronic liver failure especially with emerging interventions such as extracorporeal liver assist devices and possibly improved early phase pharmacological therapies.
Liver cirrhosis is the outcome of the long clinical course of all chronic liver diseases. Liver cirrhosis complications are the most common reason for hospital admission or the need for liver transplantation.1 Cirrhosis-related complications can decompensate homeostasis in the fragile cirrhotic liver. Liver failure associated with cirrhosis has been recognized recently as acute-on-chronic liver failure (ACLF), defined as a type of cirrhosis accompanied by jaundice and coagulopathy that may be complicated by ascites and/or encephalopathy and which precedes the development of organ failure with or without an identifiable precipitating event. ACLF has a rapid progression and high mortality rate.2
Instruments such as the Child-Pugh classification, the Model for End-Stage Liver Disease (MELD), and the King’s College criteria (for acute failure) are used to assess liver dysfunction clinically and biochemically.3 These scales use biochemical markers involved in hepatic synthetic function (bilirubin and albumin levels, and prothrombin time), renal function (creatinine and sodium levels), and clinical sequelae of cirrhosis (ascites and encephalopathy) to evaluate disease severity.
At present, the King’s College criteria are considered the most valuable tool for assessing prognosis in patients with acute liver failure.4 The MELD score has been found to be an excellent predictor of 3-month mortality in patients with chronic liver disease listed for liver transplantation.5,6 The Child-Pugh score is used to stratify cirrhotic patients into risk groups for surgical treatment, sclerotherapy, or placement of a transjugular intrahepatic portosystemic shunt. These scales have shown inconsistent reproducibility, and their poor prognostic accuracy prevents their use in predicting short-term mortality reliably in ACLF patients. Thus, there is a need for a better prognostic tool.7
The aim of this study was to investigate the prognostic value of bilirubin concentration in predicting the 1-week outcome of patients with ACLF.
Material and MethodsWe performed a retrospective analysis of medical records and selected a cohort of 65 patients diagnosed with ACLF admitted to the emergency unit at a university hospital (Medica Sur Clinic & Foundation, Mexico City, Mexico) from March 2005 to August 2012. The diagnosis of chronic liver disease before decompensation was classified as follows: hepatitis C virus chronic infection (n = 19), cryptogenic cirrhosis (n = 27), alcoholic liver disease (n = 16), and hepatocellular carcinoma (n = 3). The cirrhosis diagnosis was confirmed by clinical, biochemical, or imaging data, or histological confirmation. Patients who met one of the following criteria were excluded: coexisting human immunodeficiency virus infection, cholestatic liver disease, autoimmune liver disease, past or current extrahepatic malignant tumor, liver transplantation, or severe systemic or mental disease. All patients were followed up for at least 1 week.
The patient records were reviewed, and the findings for the clinical parameters at the time of admission were documented. Results of standard laboratory tests were also recorded on the day of admission (day 1) and between days 6 and 9 after admission. The outcome of the admission episode and short-term (1-week) mortality were also documented. All patients underwent combined medical treatment during their hospital stay.
We analyzed the following variables: demographics (age and sex); biochemical parameters (leukocyte and platelet counts; plasma concentrations of ammonia; serum concentrations of hemoglobin, sodium, albumin, alanine aminotransferase, aspartate aminotransferase, gamma glutamyl transpeptidase, alkaline phosphatase, creatinine, conjugated, unconjugated, and total bilirubin; and international normalized ratio); presence of acute complications (ascites, encephalopathy, and infections); and clinical complications (admission to the intensive care unit (ICU), intubation, use of parenteral nutrition, and use of pressor amines). The scores on measures of the severity of liver disease (MELD, MELD-Na, and Child-Pugh) were calculated for each patient at admission time.
The study protocol was conducted in accordance with the standards of the Declaration of Helsinki and current ethical guidelines, and was approved by ethical committee of Medica Sur Clinic & Foundation.
Statistical analysisContinuous variables are expressed as mean and standard deviation (SD), and categorical variables are expressed as frequency and percentages. To compare the data at hospital admission with those 1 week after admission, the χ2 test or Fisher’ s exact test was used for qualitative variables, and Student’s t test or Mann-Whitney U test was used for quantitative variables. Parameters that reached significance in univariate analyses were included in a multivariate logistic regression analysis to identify the independent factors associated with 1-week mortality. Estimations of risks were made using 95% confidence intervals and their associated p-values. To categorize the variables, we used the median value as a cutoff point.
Receiver-operating characteristic (ROC) curves were used to assess the performance of the model in predicting the 1-week mortality of these ACLF patients. The accuracy to predict short-term mortality of bilirubin was compared with the MELD score, the MELD-Na score and the Child Pugh score through the analysis of the area under the ROC curve (AUC). The predictive ability of the model was evaluated using the AUC. For a prognostic factor, the AUC ranges from 0 to 1. An AUC between 0.8 and 0.9 indicates excellent diagnostic accuracy, and > 0.7 is generally considered useful, whereas an AUC of 0.9 or greater is seldom seen. Data analysis was performed using the SPSS Statistical Package (version 15.0; SPSS, Chicago, IL). A p value < 0.05 was considered significant.
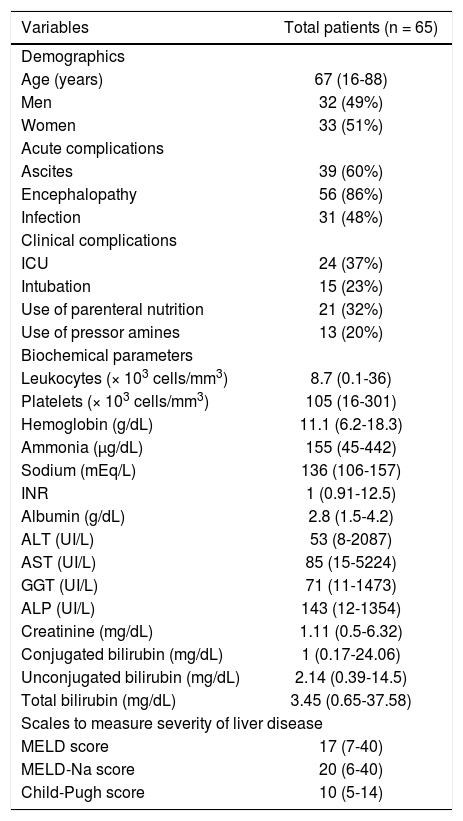
ResultsCharacteristics of the patientsThe total cohort was of 65 patients diagnosed with ACLF. There were 33 women and 32 men, with an average age of 64 ± 14 years. The clinical, demographic, and biochemical characteristics of the whole group of patients are shown in table 1. Most patients died (38, 59%) within 1 week of follow-up. Of all patients with ACLF, the most common acute complications were ascites, upper gastrointestinal bleeding, infection, hepatic encephalopathy, hepatorenal syndrome, and multiorgan failure.
Demographic, clinical, and biochemical parameters in ACLF patients at hospital admission.
| Variables | Total patients (n = 65) |
|---|---|
| Demographics | |
| Age (years) | 67 (16-88) |
| Men | 32 (49%) |
| Women | 33 (51%) |
| Acute complications | |
| Ascites | 39 (60%) |
| Encephalopathy | 56 (86%) |
| Infection | 31 (48%) |
| Clinical complications | |
| ICU | 24 (37%) |
| Intubation | 15 (23%) |
| Use of parenteral nutrition | 21 (32%) |
| Use of pressor amines | 13 (20%) |
| Biochemical parameters | |
| Leukocytes (× 103 cells/mm3) | 8.7 (0.1-36) |
| Platelets (× 103 cells/mm3) | 105 (16-301) |
| Hemoglobin (g/dL) | 11.1 (6.2-18.3) |
| Ammonia (µg/dL) | 155 (45-442) |
| Sodium (mEq/L) | 136 (106-157) |
| INR | 1 (0.91-12.5) |
| Albumin (g/dL) | 2.8 (1.5-4.2) |
| ALT (UI/L) | 53 (8-2087) |
| AST (UI/L) | 85 (15-5224) |
| GGT (UI/L) | 71 (11-1473) |
| ALP (UI/L) | 143 (12-1354) |
| Creatinine (mg/dL) | 1.11 (0.5-6.32) |
| Conjugated bilirubin (mg/dL) | 1 (0.17-24.06) |
| Unconjugated bilirubin (mg/dL) | 2.14 (0.39-14.5) |
| Total bilirubin (mg/dL) | 3.45 (0.65-37.58) |
| Scales to measure severity of liver disease | |
| MELD score | 17 (7-40) |
| MELD-Na score | 20 (6-40) |
| Child-Pugh score | 10 (5-14) |
All values are expressed as medians (range) or number (%). ACLF: acute-on-chronic liver failure. ALP: alkaline phosphatase. ALT: alanine aminotransferase. AST: aspartate aminotransferase. GGT: gamma-glutamyl transpeptidase. ICU: intensive care unit. INR: international normalized ratio. MELD: Model for End-Stage Liver Disease. Na: sodium.
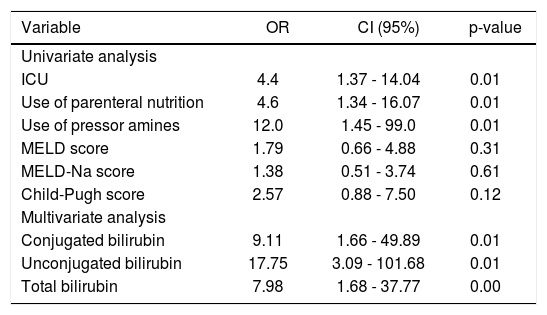
The univariate logistic regression analysis identified that admission to the ICU, use of vasoactive drugs, need for parenteral nutrition, and levels of conjugated, unconjugated, and total bilirubin at the time of hospital admission were significantly associated with 1-week mortality in these patients with ACLF (Table 2).
Results of univariate and multivariate logistic regression analysis.
| Variable | OR | CI (95%) | p-value |
|---|---|---|---|
| Univariate analysis | |||
| ICU | 4.4 | 1.37 - 14.04 | 0.01 |
| Use of parenteral nutrition | 4.6 | 1.34 - 16.07 | 0.01 |
| Use of pressor amines | 12.0 | 1.45 - 99.0 | 0.01 |
| MELD score | 1.79 | 0.66 - 4.88 | 0.31 |
| MELD-Na score | 1.38 | 0.51 - 3.74 | 0.61 |
| Child-Pugh score | 2.57 | 0.88 - 7.50 | 0.12 |
| Multivariate analysis | |||
| Conjugated bilirubin | 9.11 | 1.66 - 49.89 | 0.01 |
| Unconjugated bilirubin | 17.75 | 3.09 - 101.68 | 0.01 |
| Total bilirubin | 7.98 | 1.68 - 37.77 | 0.00 |
CI: confidence interval. ICU: intensive care unit. MELD: Model for End-Stage Liver Disease. Na: sodium. OR: odds ratio.
Factors independently associated with 1-week mortality identified by multivariate logistic regression were the levels of conjugated, unconjugated, and total bilirubin (p = 0.01, p = 0.01, and p = 0.009, respectively) (Table 2).
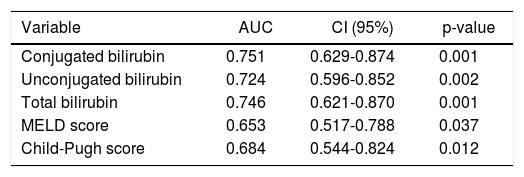
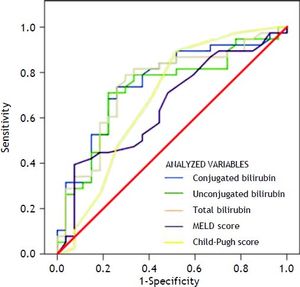
Variables that were independently associated in the multivariate analysis (conjugated, unconjugated, and total bilirubin levels) and the measures of the severity of liver disease (MELD and Child-Pugh scores) were analyzed to build a ROC curve as predictors of death at 1 week (Table 3) (Figure 1). In this analysis, conjugated, unconjugated, and total bilirubin levels (p = 0.001, p = 0.002, and p = 0.001, respectively) were significantly more accurate on comparison wih the MELD and Child-Pugh scores in predicting 1-week mortality. The AUC was significantly higher for conjugated bilirubin level than that for total and unconjugated bilirubin levels (0.751, 0.746, and 0.724, respectively) (all p < 0.05). The best predictive cutoff value was a high conjugated bilirubin level.
Results from area under the ROC curve analysis for the variables analyzed for predicting 1-week mortality in ACLF patients.
| Variable | AUC | CI (95%) | p-value |
|---|---|---|---|
| Conjugated bilirubin | 0.751 | 0.629-0.874 | 0.001 |
| Unconjugated bilirubin | 0.724 | 0.596-0.852 | 0.002 |
| Total bilirubin | 0.746 | 0.621-0.870 | 0.001 |
| MELD score | 0.653 | 0.517-0.788 | 0.037 |
| Child-Pugh score | 0.684 | 0.544-0.824 | 0.012 |
ACLF: acute-on-chronic liver failure. AUC: area under the ROC curve. CI: confidence interval. MELD: Model for End-Stage Liver Disease. ROC: receiver+operating characteristic.
This is the first study to demonstrate that a high bilirubin level (≥ 3.45 mg/dL) at hospital admission predicts short-term mortality in patients with ACLF. This is not surprising because of the wide use of bilirubin determination as a component in the prognostic scales to assess liver disease severity such as the MELD, Child-Pugh, and King’s College criteria scores,7 in which hyperbilirubinemia plays an important role in predicting a poor outcome.
At present, it is crucial to predict short-term mortality in ACLF patients because of the risk of sudden deterioration of liver function and subsequently of other end organs over a period of weeks. It is important to decide quickly whether to perform liver transplantation or to optimize the therapeutic options to avoid life-threatening complications that could lead to death.
Bilirubin is the lipophilic end product of heme breakdown8 and, at concentrations ranging from ~0.01 to 10 µM, is a potent antioxidant that may protect cells against oxidative free radicals.9,10 It also exerts immunomodulatory and anti-inflammatory effects by downregulating the expression of inflammatory cytokines and adhesion molecules, and by reducing immune cell infiltration.10,11 However, its beneficial properties are lost in patients with liver dysfunction.
One important question is how the bilirubin level can be used to predict short- term mortality outcome. It is well known that impairment of liver function, as occurs in ACLF patients, prevents the conjugation of bilirubin. This means that the less-efficient renal excretion becomes the main route of elimination for the less water-soluble unconjugated bilirubin; as a result, unconjugated bilirubin accumulates in the blood.8 In addition, biliary excretion of conjugated bilirubin is impaired markedly (cholestasis) in ACLF patients, leading to hepatic and systemic accumulation of potentially toxic biliary compounds such as bile acids and conjugated bilirubin, resulting in liver damage and jaundice.12
Because of the complications of liver dysfunction, ACLF is a critical condition that worsens over time, and most patients require special care in the ICU including implementation of parenteral nutrition and the use of vasoactive drugs.13 In this clinical context, we found significant associations between short-term mortality and admission to the ICU, use of parenteral nutrition, and use of pressor amines.
Nuclear receptors and bilirubin itself could play important roles in the mechanisms involved in deterioration of liver function in these patients. Jaundice is mediated and exacerbated by altered regulation of the farnesoid X receptor (FXR) and the xenobiotic receptors, constitutive androstane receptor and pregnane X receptor,14 which participate critically in the regulation of genes involved in the detoxification and transport of bile acids and bilirubin.15,16 In this context, alterations in patients with decompensated liver disease result from the progressive and/or acute damage to hepatocytes caused by ACLF.17 Our multivariate analysis demonstrated that the levels of conjugated, unconjugated, and total bilirubin were independent factors associated with 1-week mortality in this type of patient. Unconjugated blirubin level had the highest odds ratio for predicting mortality in these patients (Table 3). These results suggest that, in patients with ACLF, bilirubin has a pathophysiological involvement in liver decompensation, including the presence of hepatic encephalopathy, ascites, variceal bleeding, infection, or even jaundice itself. However, several other pathological mechanisms that are orchestrated by bilirubin and that alter nuclear receptor signaling in hepatocytes could also be involved in ACLF complications.
Infection is a common feature of ACLF,18 which is also associated with elevated levels of multiple proinflammatory cytokines including tumor necrosis factor-alpha (TNF-α), TNF-α receptor 1, TNF-α receptor 2, interleukin (IL)-2, IL-2 receptor, IL-6 and IL-8.2,19 Alterations in liver nuclear receptor signaling (Peroxisome proliferator-activated receptors, liver X receptor and FXR)15,20,21 resulting from cholestasis and inflammation suggest increased inflammation. Liver inflammation is an important determinant of portal pressure. An increase in the production of reactive oxidative species, in particular superoxide, is also observed in ACLF and may contribute to an increase in intrahepatic resistance by reducing nitric oxide bioavailability.22,23 Hepatic nitric oxide synthase endothelial activity can be modulated by FXR.24,25 Another common manifestation of ACLF2,17,26 is hepatic encephalopathy, which ammonia is thought to play a key role in the development of; however, no direct relationship between the severity of hyperammonemia and hepatic encephalopathy has been observed.27In vitro studies have demonstrated that a high unconjugated bilirubin level is neurotoxic and can activate apoptotic and necrotic pathways in astrocytes and neurons.28 ACLF is accompanied by hypoalbuminemia, which prevents binding of free bilirubin to albumin in plasma29,30 and thus contributes to the increased permeability of the blood-brain barrier because of a high ammonia level.31 We suggest that, in ACLF, bilirubin probably passes freely into the brain, where it causes neurotoxic effects, which may increase hepatic encephalopathy and precipitate death.
It is well known there are several scoring systems available to assess severity and prognosis of alcoholic hepatitis which use bilirubin determination, such as the modified Maddrey Discriminant Function, the Glasgow Alcoholic Hepatitis Score, the Lille score and the the ABIC (Age, serum Bilirubin, INR, and serum Creatinine) score.32 The purpose of these scoring systems has been to estimate the likelihood of short-term survival, and to determine whether the patient should be treated or not with corticosteroids.
Currently, several nonbiologic artificial liver support devices have been used to alleviate acute liver failure and hyperbilirubinemia, seeking to remove albumin-bound and water soluble substances.33 The extracorporeal liver assist device (ELAD), the only bioartificial liver system that uses the human hepatocyte cell line C3A, considers the bilirubin and ammonia follow up for determine a beneficial outcome in liver failure patients; in this context bilirubin may be used as a short-term mortality marker allowing the clinician to decide for liver transplantation or another therapeutic option.34
In consequence, monitoring bilirubin in ACLF patients in which serum bilirubin levels are markedly elevated, would beneficial for physicians use this biomarker, particularly when determining the clinical severity to initiate therapy or take decisions about liver transplantation and/or use of liver support devices in a short time.
We have proposed that the bilirubin level is an accurate prognostic marker for predicting shortterm mortality in patients with end-stage cirrhosis. The wide availability, low cost, and high reproducibility of tests to measure bilirubin levels, and due to is an impartial testing that does not require interpretation may mean that the bilirubin level is an accurate measure to help identify patients for whom liver transplantation is the only therapeutic choice.
ConclusionThis is the first study to propose that high levels of bilirubin are able to predict short-term mortality in ACLF patients. Also we suggest that bilirubin can be used as a biochemical marker in the clinical practice to improve the outcome of patients with chronic liver disease.
Abbreviations- •
ACLF: acute-on- chronic liver failure.
- •
AUC: area under the ROC curve.
- •
FXR: farnesoid X receptor.
- •
ICU: intensive care unit.
- •
IL: interleukin.
- •
MELD: model for end-stage liver disease.
- •
MELD-Na: model for end-stage liver disease sodium.
- •
ROC: receiver-operating characteristic.
- •
SD: standard deviation.
- •
TNF-α: tumor necrosis factor-alpha.
Medica Sur Clinic and Foundation.
Potential Competing InterestsNone potential conflicts.