Hepatocellular carcinoma (HCC) is the sixth leading cause of cancer and the third-ranking contributor to cancer-related mortality worldwide, averaging about 830000 deaths per year [1]. HCC is insidious at onset and difficult to diagnose at an early stage. As a result, most diagnosis occurs at an intermediate or late period of disease. HCC is typically treated with local ablation or surgical treatment. Although this eliminates the lesion in the short term, most HCC patients experience disease recurrence and distant metastasis, which leads to extremely poor prognosis for HCC patients [2,3]. The treatment plans for hepatocellular carcinoma may vary based on tumor burden, liver function, comorbidities, and patient performance. For early diseases, surgical resection and liver transplantation are the main potential treatment options with good long-term outcomes. The emergence of immune checkpoint inhibitors (ICIS) improves the prognosis of patients with advanced HCC. ICIS monotherapy or combined targeted therapy has great potential, but it also has many shortcomings, such as more complications [4–7]. Despite significant advancements in liver cancer therapy, unfortunately, effective options are still limited [8]. Cavin-1, otherwise known as polymerase I and transcript release factor (PTRF), has garnered much attention in malignant disease research. Cavin-1 is a key constituent of the caveolae structure on the plasma membrane [9], and it serves as a positive regulator of lipolysis in adipocytes [10]. However, its function in malignant tumor is rather controversial. Multiple research reported that Cavin-1 acts as a tumor suppressor, particularly to certain types of lung and breast cancers [11,12]. Cavin-1 also behaves as a novel modulator of cellular senescence via p53/p21 and caveolar networks [13]. Cavin1 overexpression is detrimental to pancreatic cancer patient survival, and Cavin1 knockdown suppresses pancreatic cancer cell infiltration and metastasis [14]. To date, the role of Cavin1 in HCC still needs to be clarified. Hence, the possibility of targeting Cavin1 in HCC therapy requires further examination.
Several studies revealed that aberrant Wnt pathway activation accelerates cancer progression [15–17]. In the canonical Wnt/β-catenin pathway, β-catenin resides at the adherens junctions and in the cytoplasm, where it is ultimately degraded by the adenomatous polyposis coli (APC) complex. Upon activation, β-catenin escapes degradation, collects in the cytoplasm, and then transfers to the nucleus to serve a gene-modulatory role [18]. Wnt pathway could be modulated by multiple factors, like noncoding RNAs, transcription factors, and other agents [19]. Dysregulated Wnt axis could potentially influence numerous physiological processes, such as cellular proliferation, migration, differentiation, and autophagy. Emerging evidences suggested that the Wnt axis activation is closely related to the occurrence and progression of HCC. Nevertheless, the association between Cavin1 and the Wnt/β-catenin axis in HCC remains undetermined.
2Materials and methods2.1Cell culture and clinical samplesThe human HCC cell lines Huh-7, SK-HEP1, Hep 3B, HepG2, the human derived fetal hepatocyte cell line L-02 and HEK-293T were acquired from Cell Bank, Chinese Academy of Sciences (CBTCCCAS, China). The HCC LM9 cells were provided by the Medical Research Center of Zhongnan Hospital of Wuhan University. All cell lines were maintained in Dulbecco's modified Eaglemedium (Gibco) with 10% fetal bovine serum (Gibco) at 37°C in 5% CO2 Clinical specimens, namely, clinical information, as well as HCC and normal liver samples were obtained from surgical patients at the Zhongnan Hospital of Wuhan University between 2016 and 2020. Informed consent forms were acquired from all patients before tissue collection, and a pathologist verified all clinical samples before use. This work received ethical approval from the Zhongnan Hospital of Wuhan University.
2.2RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR) analysisTotal RNA isolation was performed using TRIzol (Invitrogen, Carlsbad, CA), quantification via a NanoDrop ND2000 (Thermo Fisher Scientific, CA), and qRT-PCR using a CFX96TM Real-Time System (Bio-Rad, CA). GAPDH served as the internal control for Cavin1 normalization. Relative gene expression was assessed using the (Ct) formula (2−ΔCt). All experiments were conducted in triplicates. The employed primers are summarized in Supplementary Table 1.
2.3Western blot evaluationRIPA buffer was used to lyse cells and tissues, and protein quantification was done via the BCA Protein Assay Kit (Thermo Scientific). Equal protein amounts were loaded onto 10% sodium dodecyl sulfate polyacrylamide gel for electrophoretic separation, prior to transfer to PVDF membrane, 1 h blocking in skimmed milk-TBST solution, overnight exposure to primary antibody at 4℃, then three TBST rinses, 10 minutes each, followed by a 1 h incubation in corresponding secondary antibody at room temperature (RT), and lastly, protein visualization using ECL chemiluminescence reagent (Bio-Rad). A detailed antibody list is presented in Supplementary Table 2.
2.4Immunohistochemistry (IHC) assessmentThe paraffin-embedded tissues were sectioned into 4μm slices, before IHC staining. In brief, the sections underwent deparaffinization, then hydration with xylene and ethanol, followed by immersion in citrate, and then heating to fix the antigens. Upon two rinses, the sections were blocked with serum, then incubated with primary antibodies 4 ℃, rinsed again, before a 1 h incubation with corresponding specific antibodies at RT, and rinsed again, before color development using DBA solution. The aforementioned steps strictly followed directions from the UltraSensitiveTM SP kit (Maixin, China). The employed antibodies are listed in Supplemental Table 2.
2.5Hematoxylin-eosin staining (H & E staining) assessmentUpon xylene-based deparaffinization, sections underwent hydration via a series of different alcohol concentrations. Following hematoxylin staining, hydrochloric acid ethanol was introduced to remove the excess dye. Upon ammonia neutralization blue eosin staining, the slides were blocked for observation.
2.6Plasmid and lentiviral constructions, and cellular transfectionsThe lentiviral plasmid was employed as a vector for Cavin1 gene sequence insertion. The recombinant plasmids were incorporated into 293T cells using lentiviral vectors and the Pei reagent for viral supernatant solution preparation. Lentiviruses were transfected into LM9 and SK hep1 cells using polymebrene, and following 48 hours of incorporation, puromycin was employed for cell selection and establishment of stable cell lines. The efficacy of stable cell generation was then assessed using GFP tag expression using fluorescence microscopy and Cavin1 expression using qRT-PCR. Small interfering RNAs (siRNAs) against Cavin1 were obtained from GENECREAT (Wuhan, China), and incorporated into HCC cells using GenMute (SignaGen, Maryland, USA). RNA and protein extractions were completed 36-48 hours later. The employed siRNA sequences are presented in Supplementary Table 3.
2.7Cell proliferation evaluationCell proliferation was assessed using cell counting kit-8 (CCK8). In short, 5000 cells/well were plated in 96-well plates, followed by the introduction of 10μl CCK8 solution (Dojindo, Kumamoto Ken, Japan) after 24 h. After a 1-hour incubation at 37 °C, absorbance was determined at 450nm via a microplate reader.
2.8Wound healing assessmentFollowing plasmid or lentiviral incorporation in six-well plates, a scratch was formed with a pipette tip on the monocellular surface. After 24 hours, images were captured and the healing rate was computed as follows: Scratch healing rate = (healing area at 0 hours – the same at 24 hours) /healing area at 0 hours.
2.9Transwell assayMigratory evaluation was conducted in 24-well transwell chambers with an aperture of 8 micrometers (BD Biosciences). Serum-free medium was introduced to the top chambers, while serum medium was placed in the bottom chambers. The chambers were removed after 48h and underwent a 15 min fixation in 4% formaldehyde, then 20 min staining in 0.1% crystal violet, before cell counting under a light microscope.
2.10In vivo experimentsMale Balb/c mice (4–5 weeks, 18–20 g) were bred and maintained at the Wuhan University Center for Animal Center Experimentation. The right armpits of mice were administered with 100μl of LM9 cell suspensions (5×10^7 cells/mL) with stable Cavin1 or GFP overexpression. All tumor sizes were measured using a vernier caliper every five days once they became visible. Four weeks post cellular implantation, the mice were euthanized. Subcutaneous tumors and lung tissues underwent fixation in paraformaldehyde, prior to paraffin-embedding for IHC staining. All animal protocols abided by the Wuhan University Center for Animal Center Experiment criteria, and received ethical approval from the same institution.
2.11Statistical analysisData analyses employed the GraphPad Prism 7.0 and SPSS 26.0 software. Data are presented as mean ± SD. Inter-group comparisons were assessed via the student's t-test. The Cavin1 content and patient clinical profile associations were assessed via the χ2 test. The patient overall survival (OS) rate was determined using Kaplan Meier Plotter (http://kmplot.com/analysis/index.php?p=service&cancer=liver_rnaseq) [20]. Lastly, two‐sided *p < .05, **p < .01, or ***p < .001 were deemed significant.
2.12Ethical statementThe use of human tumor tissues in this study complied with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University. Our research was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (Approval no. 2019036, March 4, 2019). Animals used in the experimental work of this study were treated humanely, with regard to the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
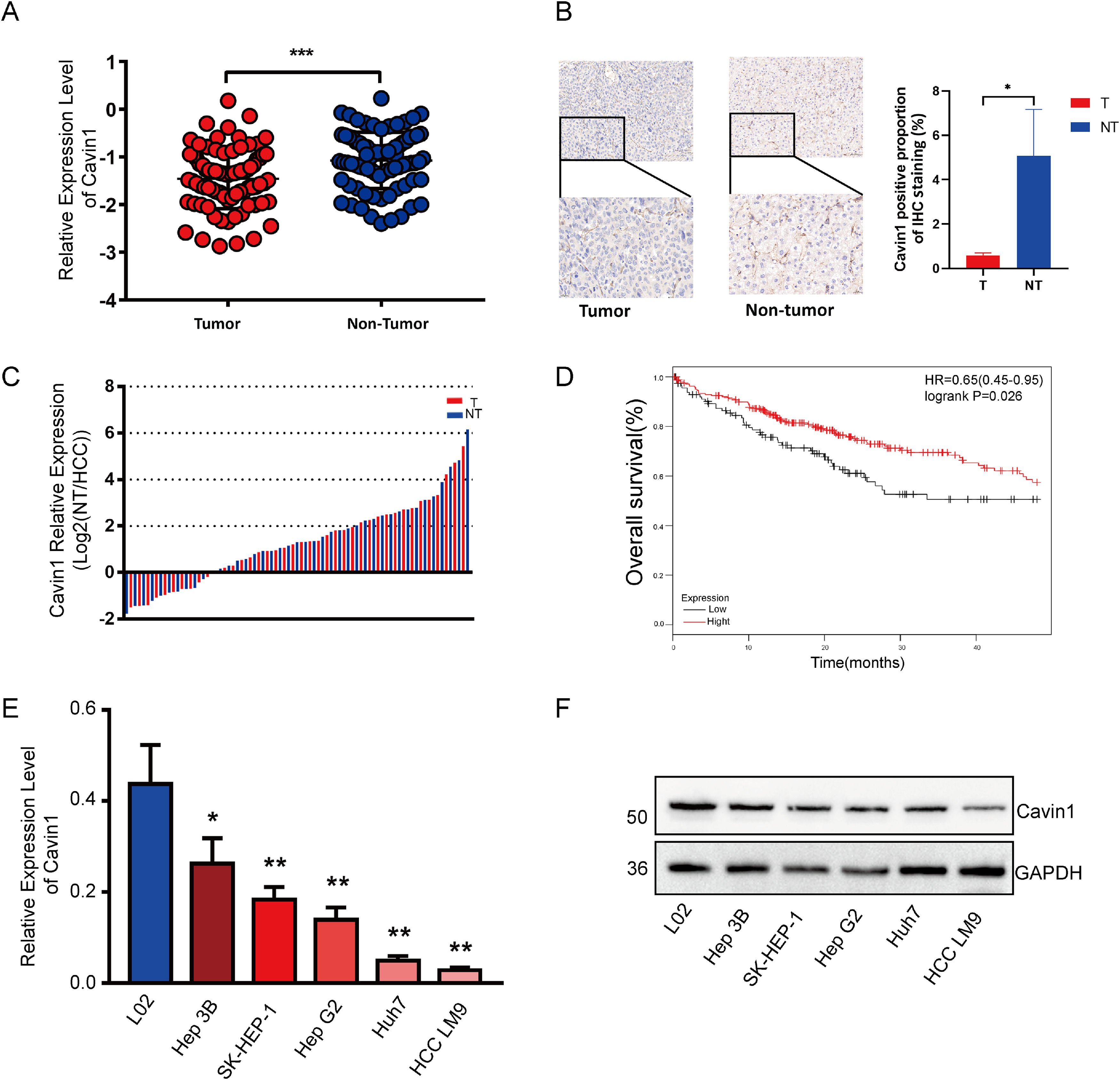
3Results3.1Reduced expression of Cavin1 in HCC tissues was detrimental to patient survivalTo elucidate the Cavin1-mediated regulation of HCC, we assessed the Cavin1 expression using qRT-PCR among tumor and adjoining healthy tissues from 81 HCC patients. Based on our observation, Cavin1 transcript and protein contents were markedly diminished in HCC versus healthy tissues (Fig. 1A and 1B). Moreover, the reduced Cavin1 expression was evident in 75% of examined HCC tissues (Fig. 1C). We next analyzed the clinical profile of the aforementioned patients, and among the 61 patients with reduced Cavin1 content, 47 patients exhibited more differentiated tumors, and 34 patients displayed advanced TNM stage (Table 1). Furthermore, most patients with reduced Cavin1 expression exhibited enhanced alpha fetoprotein (AFP) levels and diminished carcino-embryonic antigen (CEA) levels. However, our analysis revealed that Cavin1 was only strongly associated with serum alkaline phosphatase (ALP) levels in HCC patients (Table 1). Based on the survival data from TCGA LIHC cohort20, using Kaplan – Meier analysis, we next revealed that the reduced Cavin1 expression was more conducive to patient OS, compared to elevated Cavin1 expression (Fig. 1D). Based on the qRT-PCR and Western blot analyses of multiple HCC cell lines, we established that Cavin1 expression was strongly reduced in cells, such as, SK-Hep1 and LM9, compared to the L02 cell line (Fig. 1E and 1F).
Reduced expression of Cavin1 in HCC tissues is detrimental to patient survival. A Cavin1 content was assessed via qRT-PCR in tumor and adjoining healthy tissues from 81 HCC patients. B Elevated Cavin1 expression in non-HCC tumor tissues detected by IHC. C Cavin1 is underexpressed in 75% of HCC tissues. D Reduced Cavin1 expression is adverse to HCC patient survival. E and F Analysis of Cavin1 expression in HCC cell lines using qRT-PCR. and Western blotting. All trials were performed in three independent experiments and all data represent the means ± SD. *p < .05, **p < .01, ***p < .001. Scale bar = 50mm.
Associations between Cavin1 and HCC patient clinical profile
| Clinical pathologic characteristics | n | Cavin1 | P | |
|---|---|---|---|---|
| High (%) | Low (%) | |||
| Gender | 0.157 | |||
| Male | 75 | 17 (22.7) | 58 (77.3) | |
| Female | 6 | 3 (50) | 3 (50) | |
| Age (years) | 0.126 | |||
| <60 | 50 | 15 (30) | 35 (70) | |
| ≥60 | 31 | 5 (16.1) | 26 (83.9) | |
| Differentiation | 0.265 | |||
| high | 63 | 16 (25.4) | 47 (74.6) | |
| low | 15 | 2 (13.3) | 13 (86.7) | |
| Missing | 3 | |||
| TNM | 0.18 | |||
| Stage I - II | 36 | 11 (30.6) | 25 (69.4) | |
| Stage III-Ⅳ | 42 | 8 (19) | 34 (81) | |
| Missing | 3 | |||
| ALT (u/L) | 0.576 | |||
| ≤45 | 48 | 12 (25) | 36 (75) | |
| >45 | 33 | 8 (24.2) | 25 (75.8) | |
| AST (u/L) | 0.371 | |||
| ≤35 | 28 | 8 (25.6) | 20 (74.4) | |
| >35 | 53 | 12 (22.6) | 41 (77.4) | |
| TBIL (μmol/L) | 0.194 | |||
| ≤21 | 53 | 11 (20.8) | 42 (79.2) | |
| >21 | 28 | 9 (32.1) | 19 (67.9) | |
| DBIL (μmol/L) | 0.214 | |||
| ≤7 | 62 | 13 (21) | 49 (79) | |
| >7 | 18 | 6 (33.3) | 12 (66.7) | |
| Missing | 1 | |||
| UBIL (μmol/L) | 0.145 | |||
| ≤18 | 60 | 12 (20) | 48 (80) | |
| >18 | 20 | 7 (35) | 13 (65) | |
| Missing | 1 | |||
| TP (g/L) | 0.524 | |||
| <65 | 30 | 7 (23.3) | 23 (76.7) | |
| ≥65 | 51 | 13 (25.5) | 38 (74.5) | |
| ALB (g/L) | 0.374 | |||
| <40 | 41 | 9 (22) | 32 (78) | |
| ≥40 | 40 | 11 (27.5) | 29 (72.5) | |
| GLB (g/L) | 0.171 | |||
| <20 | 6 | 0 (0) | 6 (100) | |
| ≥20 | 75 | 20 (26.7) | 55 (73.3) | |
| GGT (u/L) | 0.202 | |||
| ≤57 | 40 | 12 (30) | 28 (70) | |
| >57 | 41 | 8 (19.5) | 33 (80.5) | |
| ALP (u/L) | 0.041 | |||
| ≤120 | 59 | 11 (18.6) | 48 (81.4) | |
| >120 | 22 | 9 (40.9) | 13 (59.1) | |
| TBA (μmol/L) | 0.134 | |||
| ≤15 | 69 | 15 (21.7) | 54 (78.3) | |
| >15 | 12 | 5 (41.7) | 7 (58.3) | |
| GLU (mmol/L) | 0.131 | |||
| ≤6.1 | 67 | 19 (28.4) | 48 (71.6) | |
| >6.1 | 12 | 1 (8.3) | 11 (91.7) | |
| Missing | 2 | |||
| PT (s) | 0.386 | |||
| ≤12.5 | 64 | 17 (26.6) | 47 (73.4) | |
| >12.5 | 16 | 3 (18.8) | 13 (81.2) | |
| Missing | 1 | |||
| INR | 0.449 | |||
| ≤1.15 | 65 | 17 (26.2) | 48 (73.8) | |
| >1.15 | 15 | 3 (20) | 12 (80) | |
| Missing | 1 | |||
| APTT (s) | 0.663 | |||
| ≤36.5 | 75 | 19 (25.3) | 56 (74.6) | |
| >36.5 | 5 | 1 (20) | 4 (80) | |
| Missing | 1 | |||
| TT (s) | 0.098 | |||
| ≤16.6 | 69 | 15 (21.7) | 54 (78.3) | |
| >16.6 | 11 | 5 (45.5) | 6 (54.5) | |
| Missing | 1 | |||
| AFP (ng/L) | 0.59 | |||
| ≤8.78 | 15 | 4 (26.7) | 11 (73.3) | |
| >8.78 | 62 | 16 (25.8) | 46 (74.2) | |
| Missing | 4 | |||
| CEA (ng/L) | 0.244 | |||
| ≤5 | 58 | 13 (22.4) | 45 (77.6) | |
| >5 | 4 | 2 (50) | 2 (50) | |
| Missing | 19 | |||
| Smoking | 0.53 | |||
| Yes | 40 | 8 (20) | 32 (80) | |
| No | 27 | 6 (22.2) | 21 (77.8) | |
| Missing | 14 | |||
| Alcohol abuse | 0.483 | |||
| Yes | 29 | 7 (24.1) | 22 (75.9) | |
| No | 36 | 10 (27.8) | 26 (72.2) | |
| Missing | 16 | |||
| Hepatocirrhosis | 0.402 | |||
| Yes | 39 | 11 (28.2) | 28 (71.8) | |
| No | 7 | 1 (14.3) | 6 (85.7) | |
| Missing | 35 | |||
Abbreviation: ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; UBIL, urine bilirubin; TP, total protein; ALB, albumin; GLB, globulin; GGT, glutamyl transpeptidase; ALP, alkaline phosphatase; TBA, total bile acid; GLU, glucose; PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time; TT, thrombin time; AFP, alpha fetoprotein; CEA, carcino-embryonic antigen.
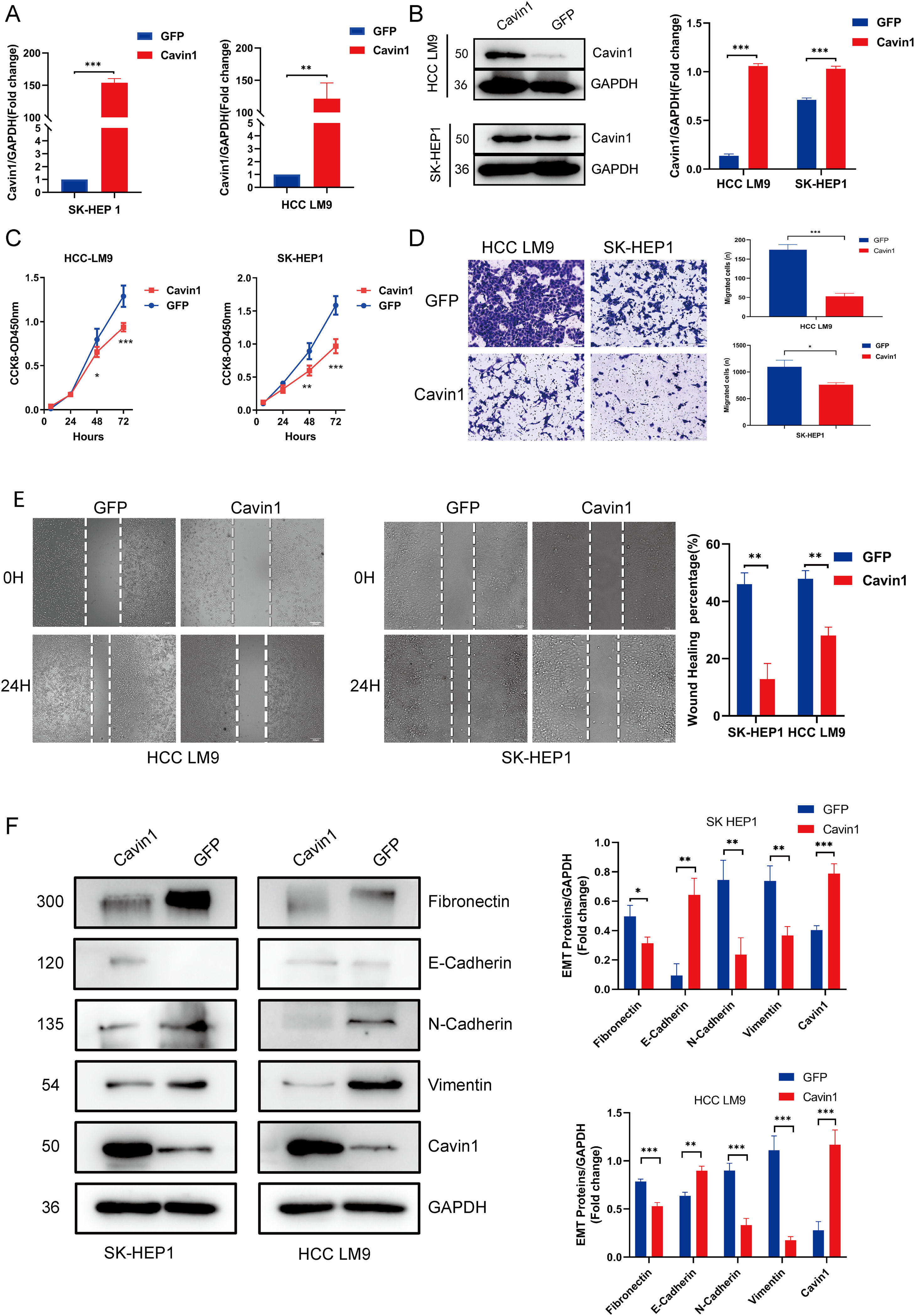
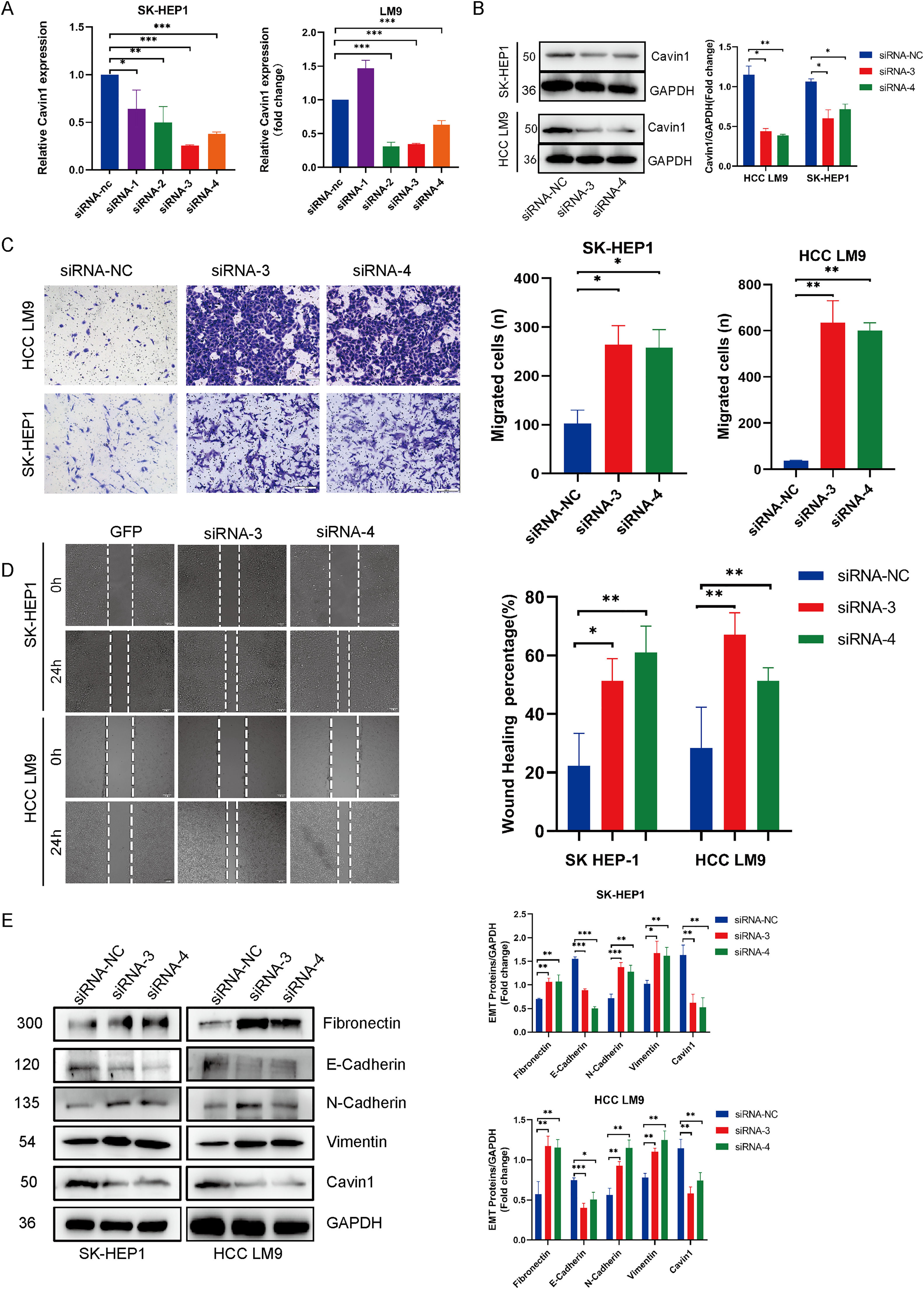
To investigate the anti-tumor properties of Cavin1, we next established SK-Hep1 and LM9 cells with stable overexpression of Cavin1, as verified by qRT-PCR and Western blot analyses (Fig2 A, Fig2 B). Meanwhile, we designed four small interfering RNA sequences, and following validation, siRNA-3 and siRNA-4 were selected for further analyses. Using CCK8, we revealed that Cavin1 overexpression strongly suppressed HCC LM9 and SK-Hep1 cell proliferation (Fig. 2C). Next, we employed transwell and wound healing assays to demonstrate that Cavin1 overexpression markedly diminished the migratory ability of HCC cells, relative to controls (Fig. 2D and 2E). In contrast, Cavin1 deficiency produced opposite results (Fig. 3C and 3D). Since the invasive migratory property of tumors is typically associated with epithelial-mesenchymal transition (EMT), we next explored alterations in EMT-associated proteins in Cavin1-overexpressed HCC cells. Based on our western blot results, Cavin1 overexpression considerably upregulated E-cadherin levels, while downregulating N-cadherin, Fibronectin, and Vimentin levels (Fig2 F). The opposite results were obtained with Cavin1 deficiency (Fig. 3E). Together, these results suggested that Cavin1 overexpression strongly suppresses EMT alterations within HCC cells.
HCC cell proliferation and migration were inhibited following Cavin1 overexpression. A and B The successful establishment of stably transfected Cavin1- overexpressing SK-HEP1 and HCC LM9 cell lines. C HCC LM9 and sh-hep1 cell proliferation is suppressed following Cavin1 overexpression, as evidenced by CCK8 assay. D and E Cavin1 overexpression suppressive HCC cell migration, as evidenced by transwell and wound healing assays. F Cavin1 overexpression downregulates fibronectin and N-cadherin expressions while upregulating E-cadherin and vimentin expressions, as evidenced by western blot analysis. Data is presented as means ± SD of 3 separate experimentations. *p < .05, **p < .01, ***p < .001. Scale bar = 200μm.
Cavin1 silencing markedly enhanced HCC invasion and migratory abilities. A and B Four small interfering RNAs (siRNAs) were constructed and validated in two HCC cell lines, sk-hep1 and LM9, using qRT-PCR and western blot analysis. Sirna-3 and 4 depicts the highest knockdown efficiency. C and D enhances HCC invasion/migratory capacity, as evidenced by the transwell and wound healing assays. E Cavin1 silencing upregulating EMT-related fibronectin, vimentin, and N-cadherin expressions, while downregulates E-cadherin levels. Data is presented as means ± SD of 3 separate experimentations. *p < .05, **p < .01, ***p < .001. Scale bar = 200μm.
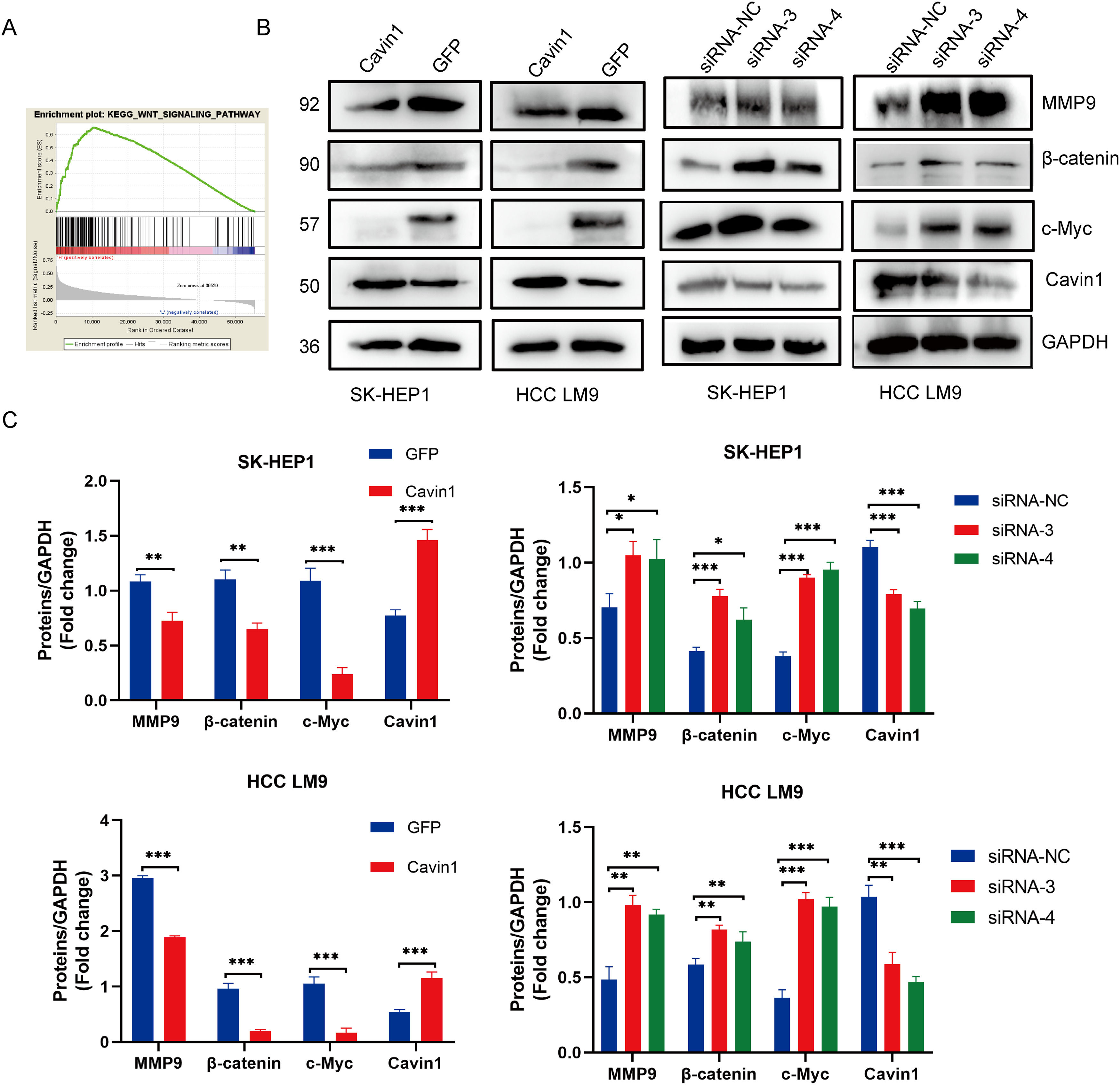
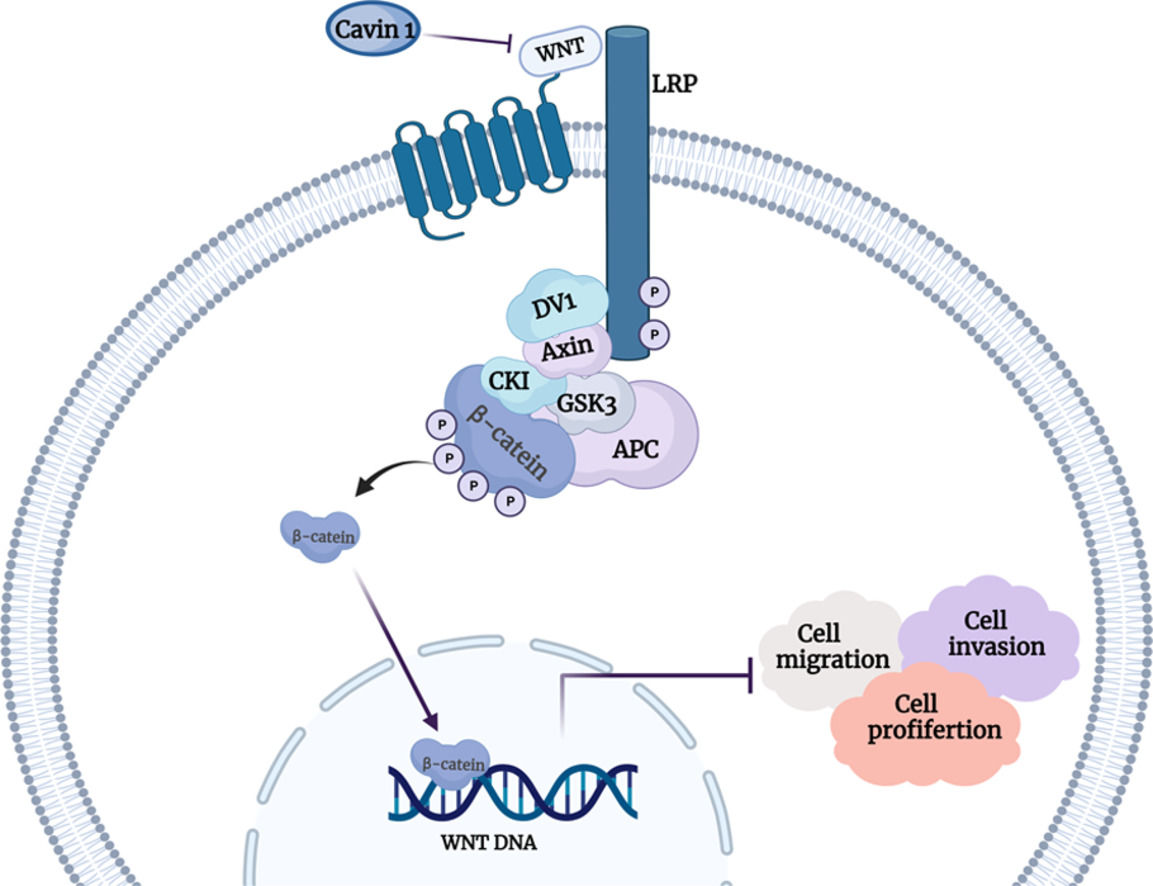
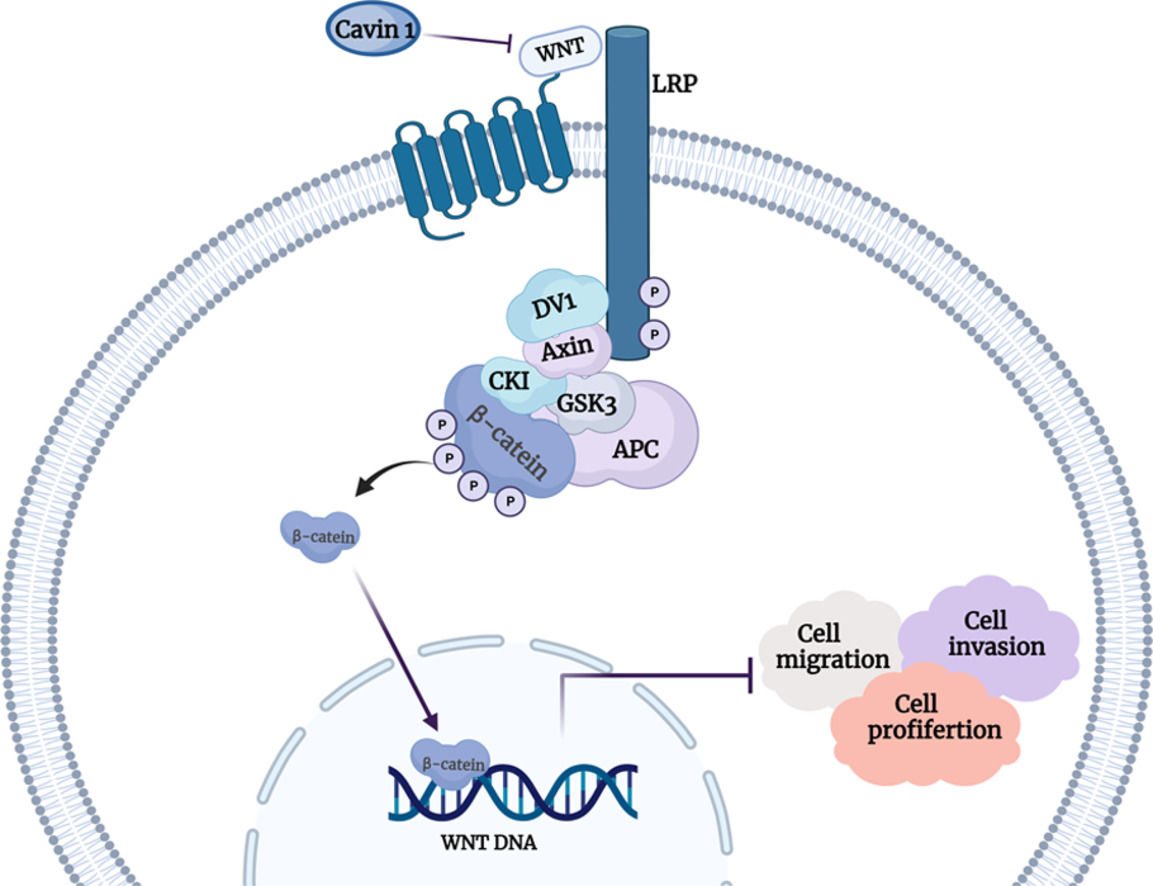
To explore the underlying mechanism behind Cavin1 action, we performed gene set enrichment analysis (GSEA) analysis of gene sets from the TCGA database. We demonstrated that Cavin1 was strongly associated with the Wnt axis in HCC (FIG. 4A). Subsequently, using western blot analysis, we revealed that Cavin1 overexpression strongly diminished β - catenin, c-Myc, and MMP9 expressions within the Wnt axis. Alternately, Cavin1 deficiency markedly enhanced the same proteins (Fig. 4B and 4C). These findings suggested that Cavin1 enhances HCC progression via activation of the Wnt axis.
Cavin1 regulates HCC invasion and migration by activating the Wnt/β-catenin axis. A GSEA identifies Cavin1 as the Wnt axis mediator in HCC. B and C Cavin1 overexpression diminishes β-Catenin, c-Myc, and MMP9 expressions, whereas, Cavin1 silencing enhances β-catenin c-Myc and MMP9 expressions. Data is presented as means ± SD of 3 separate experimentations. *p < .05, **p < .01, ***p < .001.
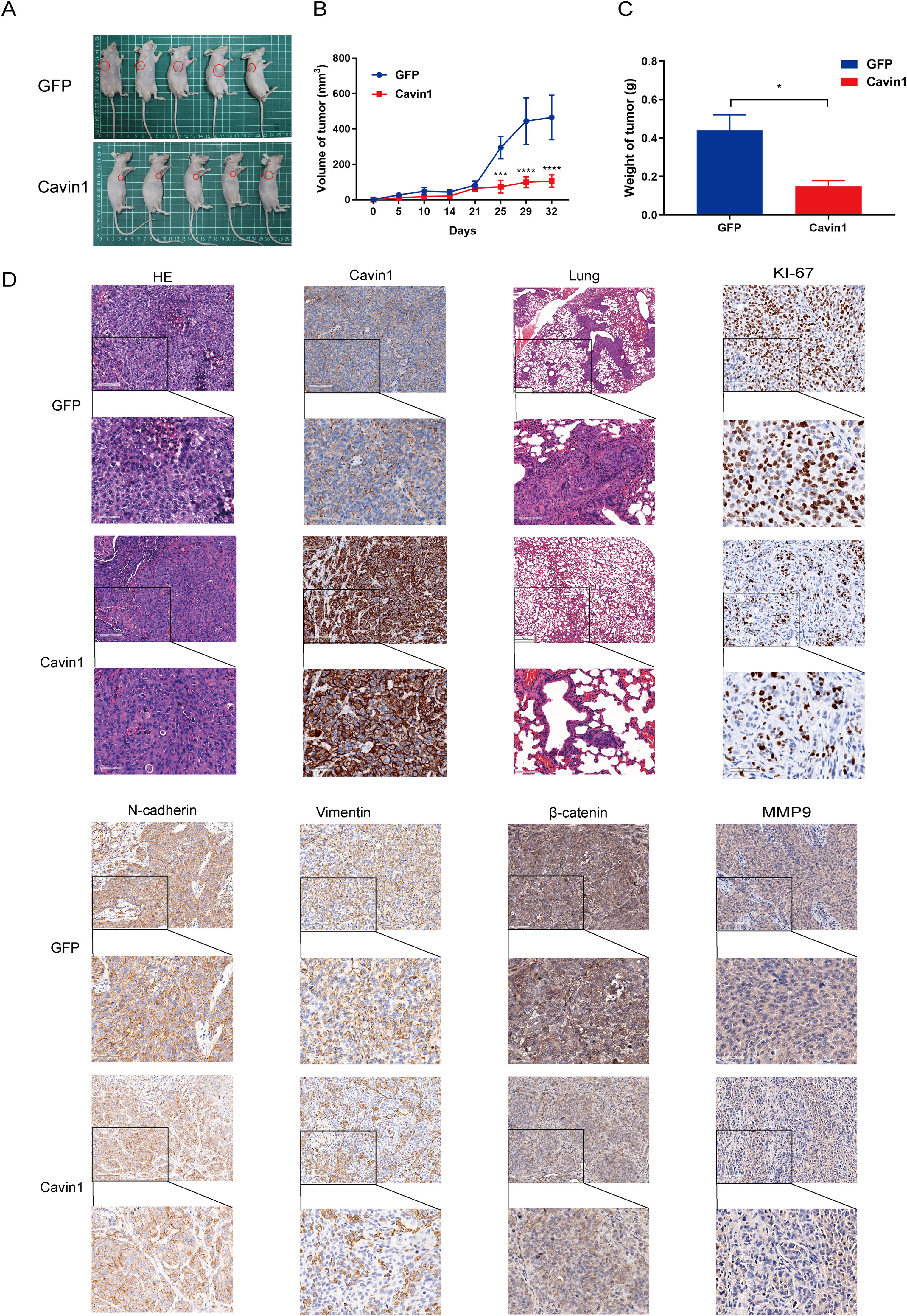
Furthermore, we randomly divided 10 Balb / c nude mice into experimental and control groups and established subcutaneous and metastatic tumor models to verify the in vivo anti-tumor effects of Cavin1. Our results revealed that Cavin1-overexpressed mice exhibited smaller subcutaneous tumor volumes and weights, compared to controls (Fig. 5A–5C). Using H&E staining of mouse lung tissues, we further revealed that the amount of lung metastases was drastically reduced in Cavin1-overexpressed mice. In addition, based on our IHC analysis, the Ki67, Vimentin, and N-cadherin contents were substantially diminished in the Cavin1-overexpressed subcutaneous tumors. Moreover, the β catenin and MMP9 levels from the Wnt axis were also decreased, relative to controls (Fig. 5D). Collectively, these data indicated that Cavin1 also suppresses tumor proliferation and metastasis in vivo.
Cavin1 inhibits tumor proliferation and metastasis in vivo. A Representative images of subcutaneous tumor models in nude mice. B Subcutaneous tumor growth curves in nude mice. C Subcutaneous tumor weight in nude mice. D Hematoxylin & Eosin (H & E) staining of subcutaneous tumors and lung tissues of nude mice, IHC was performed to assess Cavin1, Ki-67, E-cadherin, vimentin, β- catenin, and MMP9 expressions in HCC. Data is presented as means ± SD of 3 separate experimentations. *p < .05, **p < .01, ***p < .001. Scale bar = 100μm.
In this study, We revealed a strong relation between Cavin1 and HCC patient prognosis as well as the malignancy of HCC cells. Moreover, we demonstrated that the Cavin1-mediated regulation of HCC cell proliferation, invasion, and metastasis was mediated through the activation of the Wnt/β-catenin axis.
Several studies reported heterogeneity in the influence of Cavin1 on different tumors. Yi et al. [21] demonstrated that Cavin1 enhances glioblastoma proliferation while suppressing tumor immunologic responses. Bai M et al. [22] revealed that mir-217 augments cutaneous squamous cell carcinoma progression by targeting Cavin1. Gould ML et al. [23] reported that Cavin1 expression was lost in prostate cancer cells but not in prostate stromal cells, and that elevated Cavin1 levels minimized prostate cancer progression [24]. Herein, we demonstrated that Cavin1 was differentially expressed in HCC tumors versus non-tumor tissues, and low Cavin1 expression was intricately linked to poor HCC patient prognosis. Based on our evidence, Cavin1 might suppress HCC progression to a certain extent.
A study by Huertas-Martínez J. et al. [25] reported that Cavin1 accelerates Ewing sarcoma cells apoptosis by activating p53. Additionally, the study of Peng J. et al. [26] revealed that Cavin1 overexpression suppresses non-small cell lung cancer (NSCLC) invasion and metastasis. Likewise, Yanjun Cai et al. [27] illustrated that Cavin1 overexpression inhibits alterations in the NSCLC EMT. In this investigation, HCC cell proliferation and migration were markedly downregulated upon Cavin1 overexpression. Alternately, the results were opposite in Cavin1-deficient HCC cells. Additionally, using in vivo experiments in nude mice, we validated that Cavin1 overexpression strongly suppressed the tumor size, weight, and number of lung metastases in subcutaneous tumors, compared to controls. These lines of evidence suggested that Cavin1 abrogated HCC progression both in vitro and in vivo.
EMT was first described by E D Hay [28] as alterations that occur during the epithelial to mesenchymal phenotype transition in the primitive streak of chicken embryos, and it was later reported in multiple tumors such as HCC [29], gastric cancer [30], breast cancer [31], colorectal cancer [32], and so on. Loss of E-cadherin-induced cell-cell adhesion is a hallmark of EMT, and upregulation of contemporaneous mesenchymal markers (N-cadherin, vimentin, and fibronectin) with reduced E-cadherin expression enhances tumor cell metastasis [33]. In this study, Cavin1 overexpression in HCC cells markedly enhanced E-cadherin levels, while diminishing N-cadherin, Fibronectin, and Vimentin levels. Together, these results suggested that Cavin1 inhibited EMT progression.
To further explore the underlying mechanism behind Cavin1 action, we conducted GSEA analysis and revealed that Cavin1 activated the Wnt axis. Prior investigations revealed that aberrant Wnt/β-catenin axis activation is a robust indicator of HCC [34]. Herein, Cavin1 deficiency markedly enhanced β-catenin content within HCC cell lines, whereas, the opposite result was observed following Cavin1 overexpression. C-Myc and MMPs are common downstream genes of the Wnt/β-catenin axis. Rilin Deng et al. [35] demonstrated upregulated β-catenin and c-Myc protein levels after Wnt/β-catenin axis inhibition. Moreover, BO QU et al. [36] detected a concomitant decrease in the MMP2 and MMP9 proteins in HCC cells after catenin knockdown. Herein, we revealed diminished c-Myc and MMP9 expressions following Cavin1 overexpression in HCC cells. Similarly, we showed marked decreases in β-catenin and MMP9 contents in the subcutaneous tumors of nude mice overexpressing Cavin1. These lines of evidence confirmed that Cavin1 suppressed the Wnt Wnt/β-catenin pathway to minimize HCC invasion and migration in vitro and in vivo. In this investigation, we demonstrated a potential relationship between Cavin1 and the Wnt/β-catenin axis. However, the underlying mechanism requires further experimental exploration.
Based on the role and mechanism of Cavin1 in tumors, we believe that Cavin1 is expected to become a new therapeutic target or biomarker for HCC in the future. However, research on Cavin1 is still in its early stages and has not fully explained its other roles in HCC. Further research is needed before its practical application in clinical work. Some studies have shown that some molecules can upregulate Wnt3 and p-GSK3 β Activating the Wnt/β-catenin pathway [37]. However, limited by the current experimental conditions, this study failed to explain the specific mechanism of cavin1 activating Wnt/β-catenin pathway.
5ConclusionsIn conclusion, this study was the first to reveal the inhibitory function of Cavin1 on HCC cells in vitro and in vivo. Cavin1 expression was decreased in HCC tissue samples relative to adjacent healthy tissues. Moreover, the reduced Cavin1 levels were unfavorable for the patient OS. Given this evidence, Cavin1 can influence HCC cell proliferation and migration by activating the Wnt/β-catenin pathway in vivo and in vitro. Hence, Cavin1 holds great promise as a novel target for HCC therapy.
Author contributionsXingyuan Hao, Jinghua Li, Xi Chen, Weijie Ma and Yufeng Yuan conceived and designed the experiments. Fushegn Liu and Yonghua Guo collected the clinical samples and parameters. Wei Jing and Bin Liu performed PCR testing and data analysis on clinical samples. Xingyuan Hao, Jinghua Li, Yonghua Guo, Xiaomian Li and Bin Liu performed the experiments. Xingyuan Hao and Jinghua Li wrote the manuscript. Weijie Ma, Xi Chen, Jinghua Li and Yufeng Yuan participated in the revision of the draft. All the authors have read and approved the final version of the manuscript for publication.
Availability of data and materialsThe data that support the findings of this study are available from the corresponding author upon reasonable request.
Data sharing statementThe data used to support the findings of this study are available from the corresponding author yuanyf1971@whu.edu.cn upon request.