Introduction and aim. The prevalence and incidence of chronic liver disease is increasing resulting, in substantial direct and indirect medical costs. Overuse of investigations, treatments and procedures contribute to rising health care costs and can expose patients to unnecessary harm and delay in receiving care. The Choosing Wisely Canada (CWC) campaign has encouraged professional societies to develop statements that are directly actionable by their members in an effort to promote higher-value health care that will lead to downstream effect on how other practitioners make decisions.
Material and methods. The Canadian Association for the Study of the Liver (CASL) established its Choosing Wisely top five list of recommendations using the framework put forward by CWC. CASL convened a task force that developed a list of draft recommendations and shared this with CASL membership electronically with eventual ranking of the top five recommendations by consensus at Canadian Digestives Disease Week (CDDW) 2017. Following revisions, the CASL Executive Committee endorsed the final list, which was disseminated online by CWC (July 2017).
Results. The top five recommendations physicians and patients should question include: 1) Don’t order serum ammonia to diagnose or manage hepatic encephalopathy (HE). 2) Don’t routinely transfuse fresh frozen plasma, vitamin K, or platelets to reverse abnormal tests of coagulation in patients with cirrhosis prior to abdominal paracentesis, endoscopic variceal band ligation, or any other minor invasive procedures. 3) Don’t order HFE genotyping based on serum ferritin values alone to diagnose hereditary hemochromatosis. 4) Don’t perform computed tomography (CT) or magnetic resonance imaging (MRI) routinely to monitor benign focal liver lesions. 5) Don’t repeat hepatitis C viral load testing in an individual who has established chronic infection, outside of anti-viral treatment.
Conclusion. The Choosing Wisely recommendations will foster patient-physician discussions, reduce unnecessary treatment and testing, avert adverse effects from testing and treatment along with reducing medical expenditure in hepatology.
The prevalence of chronic liver disease (CLD) is increasing in many countries around the world. It has been estimated that between 1990 and 2010 the overall prevalence of chronic liver disease has increased by 15% in the entire population and with that CLD has become one of the top 12 causes of hospitalizations, liver cancers and death among all gastrointestinal, liver and pancreatic diseases.1–4 CLD can affect quality of life through debilitating symptoms, reduction in ability to work and social stigma. Moreover, the burden CLD places on the individual health care system, and society is staggering. The overall cost of > $2 billion dollars is expected to continue to rise in annual direct medical costs of treating persons with CLD and indirectly with lost productivity.5,6 However, rising health care costs may partly be due to the inappropriate overuse of investigations, treatments, and procedures that can also contribute to or cause patient harm.7
The American Board of Internal Medicine (ABIM) launched the Choosing Wisely campaign in 2012, which sought to create discussions between patients and providers that promote better value care.8 One of the most visible platforms for these discussions has been the dissemination of lists created by professional societies of “Five Things that Physicians and Patients Should Question”. In the form of declarative statements, the lists identify practices that should be reduced or eliminated because they lack proven benefit or may cause harm to patients. This campaign was introduced in Canada in 2014 and by mid-2017 over 40 medical and surgical society lists have been created.9 Choosing Wisely Canada has encouraged professional societies to preferentially focus on practices that are within their purview of practice to develop statements that are directly actionable by their members. Currently, there are variations in Hepatology practice that lead some patients to receive therapies or investigations that lack benefit and may be potentially harmful.10
This position statement describes the initiative led by the Canadian Association for the Study of the Liver (CASL) to develop a Choosing Wisely Canada list of “Five Things that Physicians and Patients Should Question in Hepatology”.
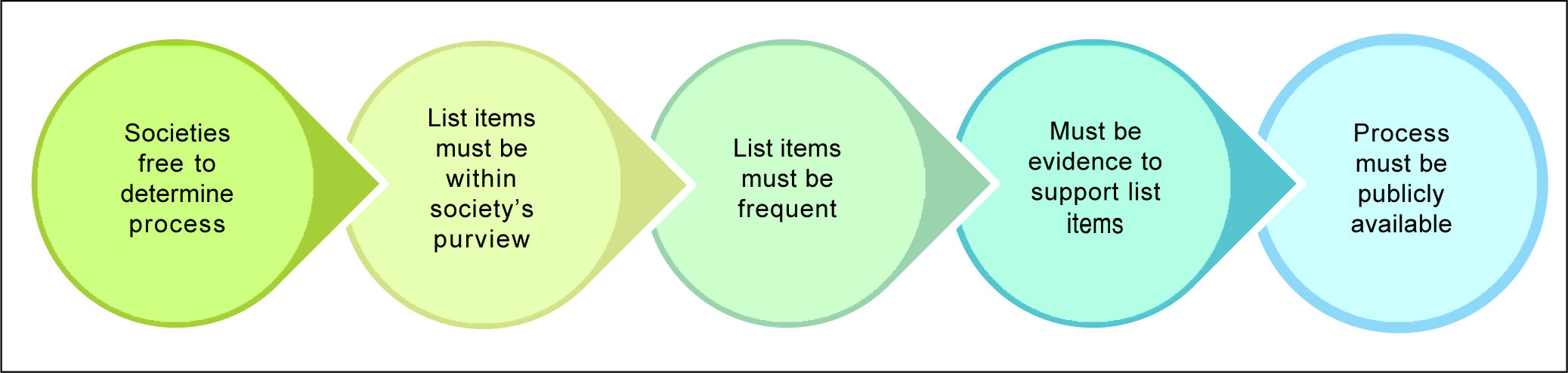
Material and MethodsThe CASL education committee struck a working group to develop the CWC top five list of recommendations using the framework proposed by Choosing Wisely Canada (Figure 1).
Initial survey of meSmbershipThe Choosing Wisely Canada (CWC) framework was first introduced to CASL members through an e-mail communication that provided an overview of the campaign as well as a request to members to submit recommendations on tests, treatments or procedures that should be reduced or eliminated based on members opinions that represent low-value care (June 2015). From the initial survey 8 recommendations were obtained that were compatible with CWC’s mission statement and aims.
CASL Choosing Wisely Task ForceIn July 2016, a task force of seven members was convened representing a diverse group of adult Hepatologists with a broad range of clinical experience from several geographical regions, practice settings and institution types. Over a six-month period this task force conducted four teleconferences to develop a draft list of recommendations. The task force began by reviewing the initial suggestions and also added additional statements to this list. In total, there were eight statements incorporated from the membership survey and five new statements added by the task force members, for a total of thirteen suggestions that were developed into formal statements. The thirteen statements related to practices that met the following four criteria:
- •
Within the purview of Hepatology practice.
- •
Frequently seen in practice.
- •
Significant potential for uptake by other physicians and societies.
- •
Likely to have significant overall impact on the value of care provided by the members of our profession.
Discussions were held until consensus was reached regarding the top eight statements. All members contributed edits to both the eight statements and the accompanying rationales.
Vetting and endorsement of list by the CASL membershipThe draft recommendations were shared electronically with CASL membership via online survey sent in February and March, 2017 to provide an opportunity for feedback and to vote on a top five list. The task force based on member suggestions made changes and the revised recommendations were presented at a clinical symposium held at the Canadian Digestive Disease Week (CDDW) annual conference in Banff, Alberta, on March 5, 2017. During this forum members were provided the opportunity to engage in open discussion to review and rank the eight statements in order to generate a top five list. Real-time polling software (surveymonkey.com) was used to enable members to vote anonymously. Among those in attendance, none believed that any alternative statements should replace any of the eight statements presented. On final voting, all agreed that the top five declarative statements should be endorsed and disseminated by both CASL and Choosing Wisely Canada.
Dissemination by CASL and Choosing Wisely CanadaFollowing the annual meeting, minor refinements to the declarative statements were made by the task force based on feedback from the session. The CASL Executive Committee provided full endorsement of the final list that was disseminated on July 19, 2017.
ResultsThe final top five declarative statements of Hepatology practices that physicians and patients should question are listed in table 1. In this section, we provide the reasoning behind the selection of each recommendation and how the statement may ultimately be incorporated into Hepatology practice to improve the value of care provided.
Canadian Association for the Study of Liver Diseases-Choosing Wisely Canada Physician Recommendations for Hepatology.
| Recommendation | Rationale |
|---|---|
| Don’t order serum ammonia to diagnose or manage hepatic encephalopathy (HE). | High blood-ammonia levels alone do not add any diagnostic, staging, or prognostic value in HE patients known to have chronic liver disease. |
| Don’t routinely transfuse fresh frozen plasma, platelets or give Vitamin K to reverse abnormal tests of coagulation in patients with cirrhosis prior to abdominal paracentesis, endoscopic variceal band ligation, or any other minor invasive procedures. | Routine tests of coagulation do not reflect bleeding risk in patients with cirrhosis and bleeding complications of these procedures are rare. |
| Don’t order HFE genotyping based on serum ferritin values alone to diagnose hereditary hemochromatosis. | Serum ferritin values reflect an increase in hepatic iron content and have a significant false positive rate because of elevations due to inflammation. Thus, in patients with evidence of liver disease, hemochromatosis genotyping should only be performed among individuals with an elevated ferritin and fasting transferrin saturation >45% (TSat) or a known family history of hemochromatosis-associated hereditary hemochromatosis. |
| Don’t perform computed tomography (CT) or magnetic resonance imaging (MRI) routinely to monitor benign focal liver lesions (ex. focal nodal hyperplasia, hemangioma). | Patients with benign focal liver lesions who do not have underlying liver disease and have demonstrated clinical (asymptomatic) and radiologic stability do not need repeated imaging as the likelihood of evolving into neoplastic lesions is very low. In contrast, patients with radiologic evidence of hepatocellular adenoma may have an increased risk of complications and/or neoplasia thus warranting closer observation. |
| Don’t repeat hepatitis C viral load testing in an individual who has established chronic infection, outside of antiviral treatment. | Highly sensitive quantitative assays of hepatitis C RNA are appropriate at the time of diagnosis (to confirm infection) and as part of antiviral therapy, which is typically at the beginning and after therapy is completed to confirm sustained virological response at week 12 (SVR 12). Outside of these circumstances the results of virologic testing do not usually change clinical management or outcomes. |
This statement was the most highly ranked. Members of the task force and those surveyed stated that ordering serum ammonia is highly prevalent within the practice of Hepatology and other specialties and that there was potential for significant uptake of this recommendation by other physicians and societies. The liver mostly clears ammonia with some extra-hepatic metabolism in muscle tissue. HE is caused by accumulation of unmetabolized ammonia resulting in neuropsychiatric toxicity and encephalopathy. However, elevated ammonia levels also occur in urea cycle disorders, Porto systemic shunting, gastrointestinal bleeding, shock, renal disease, etc.11 Moreover, the accuracy of ammonia measurement is influenced by many factors including whether it is a venous or arterial sample, fist clenching, tourniquet use, and whether the sample is placed on ice and transfer time, as well as the analytical assay technique (direct or indirect) used. This may possibly prevent accurate measurements of ammonia in most clinical settings and rendering the results inaccurate/inconclusive. Thus, elevated ammonia levels do not add any diagnostic, staging, or prognostic value. The diagnosis of HE should be based on clinical judgment, and a trial of medications such as lactulose and rifaximin.12–16 If there is an inadequate response to medical therapy, the diagnosis of HE should be questioned and other etiologies considered.
Statement 2: Don’t routinely transfuse fresh frozen plasma, vitamin K, or platelets to reverse abnormal tests of coagulation in patients with cirrhosis prior to abdominal paracentesis, endoscopic variceal band ligation, or any other minor invasive proceduresThis statement was ranked highly because it addresses a common problem in patients with end stage liver disease (ESLD), is highly relevant to Hepatologists, and has significant uptake potential by other specialties. Abdominal paracentesis and endoscopic variceal band ligation are common procedures in ESLD and most patients have abnormal coagulation parameters, hence the administration of prophylactic blood products (i.e., fresh frozen plasma, platelets, vitamin K) is commonplace. The transfusion of blood products pre-procedure may be unnecessary, pose increased risks of an adverse reaction, delay diagnosis, and increase the access and costs of care. Although ESLD is characterized by clinical bleeding and coagulopathy due to an imbalance between naturally occurring pro- and anti-coagulants, seminal studies have shown this is not the case and that blood coagulation is rebalanced with thrombin levels (the final enzyme of coagulation) being the same levels in both cirrhotic and normal healthy patients.17–19 Moreover, prothrombin (PT) time is expressed as an INR and this was devised initially, and validated, to standardize across laboratories the PT in patients receiving anticoagulation therapy with vitamin k antagonists such as warfarin.20 Thus, the test should be used in similar clinical scenarios and not standardized across all patient populations. Similarly, platelets provide a hemostatic plug through an interaction with von Willebrand factors (vWf) and thrombin generation, however, it has been shown vWf is up-regulated in cirrhotic patients restoring platelet adhesion while levels of ADAMTS 13 (a metalloprotease limiting vWf) are down-regulated.21,22 While the Task force acknowledged that there are clinical circumstances that warrant the use of these products (i.e., platelets < 20, INR > 2.5), their routine use should be discouraged. Moreover, since physicians are often basing treatment decisions on individualized clinical scenarios, personal experience, and inherent judgment of risk vs. benefit, the word ‘routinely’ was selected to respect these circumstances.
Statement 3: Don’t order HFE genotyping based on serum ferritin values alone to diagnose hereditary hemochromatosisHemochromatosis refers to excessive iron accumulation. The initial approach to diag-nosing hemochromatosis involves indirect markers of iron metabolism such as transferrin saturation (TS, a measure of the saturation of iron transport capacity in plasma) and serum ferritin (a measure of intracellular iron stores). Although studies have differed in use of cutoff values, a value of TS greater than 45% is often chosen for its high sensitivity for detecting C282Y homozygotes and will identify persons with minor secondary iron overload as well as some C282Y/ wild-type heterozygotes.23 Serum ferritin has a significant false positive rate because of elevations related to inflammation and can be elevated in the absence of increased iron stores in patients with other liver diseases such as excess alcohol consumption, hepatitis B and C, and nonalcoholic fatty liver disease (NAFLD). It can also be elevated in non-liver diseases such as systemic inflammatory conditions. Thus, in a patient with a suggestive history, physical findings, or family history, a combination of TS and ferritin should be obtained before ordering an HFE mutation analysis.24
Statement 4: Don’t perform computed tomography (CT) or magnetic resonance imaging (MRI) routinely to monitor benign focal liver lesions (ex. focal nodal hyperplasia, hemangioma)The widespread use of imaging modalities has steadily increased the detection rate of benign focal lesions.25 More importantly, the evaluation of liver lesions has taken on greater importance due to the increasing incidence of hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA).26 Therefore, a thorough and systematic approach to the management of focal liver lesions is important with critical components including a detailed history, physical exam, blood tests, and selective radiological tests. For example, a liver lesion in the setting of chronic liver disease/ ESLD and portal hypertension should lead to a high index of suspicion for HCC. A radiological test is the most important aspect in the evaluation of a liver lesion and should begin with a high-quality ultrasound, followed by cross-sectional imaging with either a triple-phase CT or MRI. The combined imaging will give the clinician information about the characteristics of the liver lesion, its location and relationship to anatomical structures (such as the gallbladder and hepatic vasculature), and, in the case of malignancy, allow staging. In the case of benign lesions such as Hemangiomas and FNH, routine imaging follow-up is not needed unless patients experience pain or the lesion size is larger than 10 cm (which may require intervention).27 Additionally, a hepatic adenoma (a benign lesion) would need closer follow-up to ensure size stability and no malignant transformation.
Statement 5: Don’t repeat hepatitis C viral load testing in an individual who has established chronic infection, outside of antiviral treatmentHighly sensitive quantitative assays of hepatitis C RNA (viral load) are widely available. The test is appropriately used to confirm infection after a positive hepatitis C antibody test, to determine the duration of treatment for certain regimens, and to confirm sustained virological response at week 12 (SVR 12) after therapy is completed. It has been suggested that assessment of viral load at week 4 of therapy may help determine initial response to therapy and adherence. However, there is no data on how to use viral load levels during treatment to determine when to stop treatment for futility, and thus far the test utility is solely based on expert opinion. The utility of using HCV RNA testing periodically in individuals with chronic infection who are not being treated is not established, as it tends to remain stable and has no link to prognosis.28
DiscussionThrough a structured consensus process, we have made recommendations to avoid 5 diagnostic and therapeutic interventions that are unnecessary, over utilized, costly, and potentially harmful.
Hepatologists play an active role in our health care system through resource stewardship. The Choosing Wisely Canada campaign has stimulated discussion within our specialty and challenged CASL members to identify further opportunities for resource stewardship that are within our purview of practice. The CASL-Choosing Wisely Canada Top Five List in Hepatology is not a guideline document, but is meant to facilitate conversations between physicians and patients, and between physicians and other health care providers related to low-value practices.
There are several important strengths of the Choosing Wisely Canada statements developed by CASL. First, this list was developed through broad consultation and engagement of CASL members with ample opportunity for contribution from members at large. Consensus was reached on the final five recommendations by the task force as well as by members of CASL who were present at our annual meeting. Second, the process created by the task force involved the application of objective criteria to rank each declarative statement. Third, all statements relate to practices within the purview of the field of Hepatology, which is important as it allows for immediate action by CASL members in effecting change in these areas, and teaching and modelling of these practices for other health care providers who provide care to patients with CLD. Additionally, although the Canada Health Act provide the framework for Canada’s universal health care system health care is administered by provincial and territorial government, highlighting the importance of developing consensus recommendations by CWC for application within a diverse health care system.
The creation of the final top five recommendations also carries limitations. First, it does not represent a comprehensive list of low-value practices within Hepatology. Additional practices could have been included, however, we limited the list to five statements to adhere to the Choosing Wisely Canada (CWC) format. Second, some of the recommendations may be of lower cost impact than others that could have been included. However, the purpose of CWC is to improve quality (as well as decrease costs) and adherence to the recommendations selected will improve the quality of Hepatology care. Lastly, we should note the task force comprised adult Hepatologists and the literature search focused on data from adult populations. Consequently, these recommendations target patients with liver disease who are 18 years and older and their respective providers.
ConclusionThe CWC statements in Hepatology endorsed by CASL represent a starting point to engage Hepatologists in a broader discussion related to healthcare utilization in Hepatology and to implement recommendations to improve care and reduce spiralling health care costs. The next step will include evaluation of the impact of these statements both at the local/provincial and national level and represents a valuable opportunity for research and collaboration among CASL members. Implementation of the CWC statements will improve healthcare utilization and delivery of care to all Canadians living with liver disease.
Abbreviations- •
ABIM: American Board of Internal Medicine.
- •
CASL: Canadian Association for the Study of the Liver.
- •
CDDW: Canadian Digestive Disease Week.
- •
CWC: Choosing Wisely Canada.
- •
CLD: chronic liver disease.
- •
CT: computed tomography.
- •
ESLD: end stage liver disease.
- •
HE: hepatic encephalopathy.
- •
HCC: hepatocellular carcinoma.
- •
MRI: magnetic resonance imaging.
- •
NAFLD: nonalcoholic fatty liver disease.
- •
PT: prothrombin.
- •
SVR 12: sustained virological response at week 12.
- •
TS: transferrin saturation.
- •
vWf: von Willebrand factors.
The authors declares that there is no conflict of interest regarding the publication of this article.
DisclosuresThe authors have no financial disclosures to declare.
AcknowledgmentsThe authors acknowledge Michelle Menard and Rebecca Swanson for providing administrative support to CASL. We would also like to thank Dr. Wendy Levinson and Karen MacDonald of Choosing Wisely Canada for their review and edits of the final Choosing Wisely statements and manuscript. Lastly, we would like to acknowledge Dr. Rick Schreiber and Dr. Marc Bilodeau from the CASL executive committee for taking an interest in promoting quality and patient safety in Canadian Hepatology practice.