Liver injury in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant- and Omicron subvariant-infected patients is unknown at present, and the aim of this study is to summarize liver injury in these patients.
Patients and MethodsIn this study, 460 SARS-CoV-2-infected patients were enrolled. Five severe or critical patients were excluded, and 34 patients were also excluded because liver injury was not considered to be related to SARS-CoV-2 infection. Liver injury was compared between Omicron and non-Omicron variants- and between Omicron subvariant-infected patients; additionally, the clinical data related to liver injury were also analyzed.
ResultsAmong the 421 patients enrolled for analysis, liver injury was detected in 76 (18.1%) patients, including 46 Omicron and 30 non-Omicron variant-infected patients. The ratios did not differ between Omicron and non-Omicron variant-, Omicron BA.1, BA.2 and BA.5 subvariant-infected patients (P>0.05). The majority of abnormal parameters of liver function tests were mildly elevated (1-3 × ULN), the most frequently elevated parameter of liver function test was γ-glutamyl transpeptidase (GGT, 9.5%, 40/421), and patients with cholangiocyte or biliary duct injury markers were higher than with hepatocellular injury markers. Multivariate analysis showed that age (>40 years old, OR=1.898, 95% CI=1.058–3.402, P=0.032), sex (male gender, OR=2.031, 95% CI=1.211–3.408, P=0.007), serum amyloid A (SAA) level (>10 mg/ml, OR=3.595, 95% CI=1.840–7.026, P<0.001) and vaccination status (No, OR=2.131, 95% CI=1.089–4.173, P=0.027) were independent factors related to liver injury.
ConclusionsLiver injury does not differ between Omicron and non-Omicron variants or between Omicron subvariant-infected patients. The elevations of cholangiocyte or biliary duct injury biomarkers are dominant in SARS-CoV-2-infected patients.
Since the first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in Wuhan city of Hubei Province, China, it was subsequently confirmed to be capable of human-to-human transmission and was highly pathogenic, which resulted in high mortality [1, 2]. By July 14, 2022, approximately 563 million coronavirus disease 2019 (COVID-19) were reported, resulting in 6.3 million deaths. To date, COVID-19 has affected 228 countries and territories worldwide (https://www.worldometers.info/coronavirus) and has become a serious public health hazard.
SARS-CoV-2 is a positive-strand RNA virus. The virus replication depends on RNA-dependent RNA polymerase, which lacks proofreading capability. Therefore, large numbers of mutations are naturally produced during each replicative cycle or occur in a given replicative environment [3]. As the mutations accumulate, new lineages are formed, some of which have been defined as variants of concern by the World Health Organization (WHO). To date, five variants of concerns have been reported, including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529) [4]. According to data retrieved from the web-based portal, the SARS-CoV-2 sequence database (GISAID), the Omicron variant has become the dominant variant worldwide. The Omicron variant is more transmissible than the other variants [5, 6], and the prevalence of the Omicron variant has introduced more significant challenges for pandemic prevention and control.
SARS-CoV-2 primarily affects the lungs and the respiratory tract [7–9]; however, mounting evidence shows that the liver could also be involved, resulting in liver injury [10–12]. The possible pathogenesis of liver injury in SARS-CoV-2-infected patients is complex. It could be related to direct viral injury, hyper-inflammatory cytokine storm, hypoxia-ischemic, and drug-induced liver injury [13]. Liver injury has been reported in 14.8–53.0% of SARS-CoV-2-infected patients [14–17]. However, most of the patients enrolled in the studies were infected with the wild-type strain of the virus, and the status of liver injury in Omicron variant-infected patients is still unknown. In addition, the Omicron subvariants BA.5 is the dominant subvariant worldwide at present, and the difference in liver injury between Omicron BA.5 and other subvariant-infected patients is also unknown. Previous studies showed that the ratio of liver injury is significantly higher in severe/critical SARS-CoV-2-infected patients than in non-severe/critical SARS-CoV-2-infected patients [18], and it is likely that strong inflammatory reactions or hypoxic-ischemic in these patients could be associated with a higher ratio of liver injury. However, it is important to note that patients infected with the Omicron variant of SARS-CoV-2 rarely had severe/critical outcomes. Therefore, this study aims to summarize and compare liver injury in non-severe/critical patients infected with the Omicron and non-Omicron variants. Further, we compared the liver injury in non-severe/critical patients infected with Omicron BA.1, BA.2, and BA.5 subvariants. In addition, we analyzed the clinical data related to liver injury to better understand the clinical characteristics of liver injury in SARS-CoV-2-infected patients.
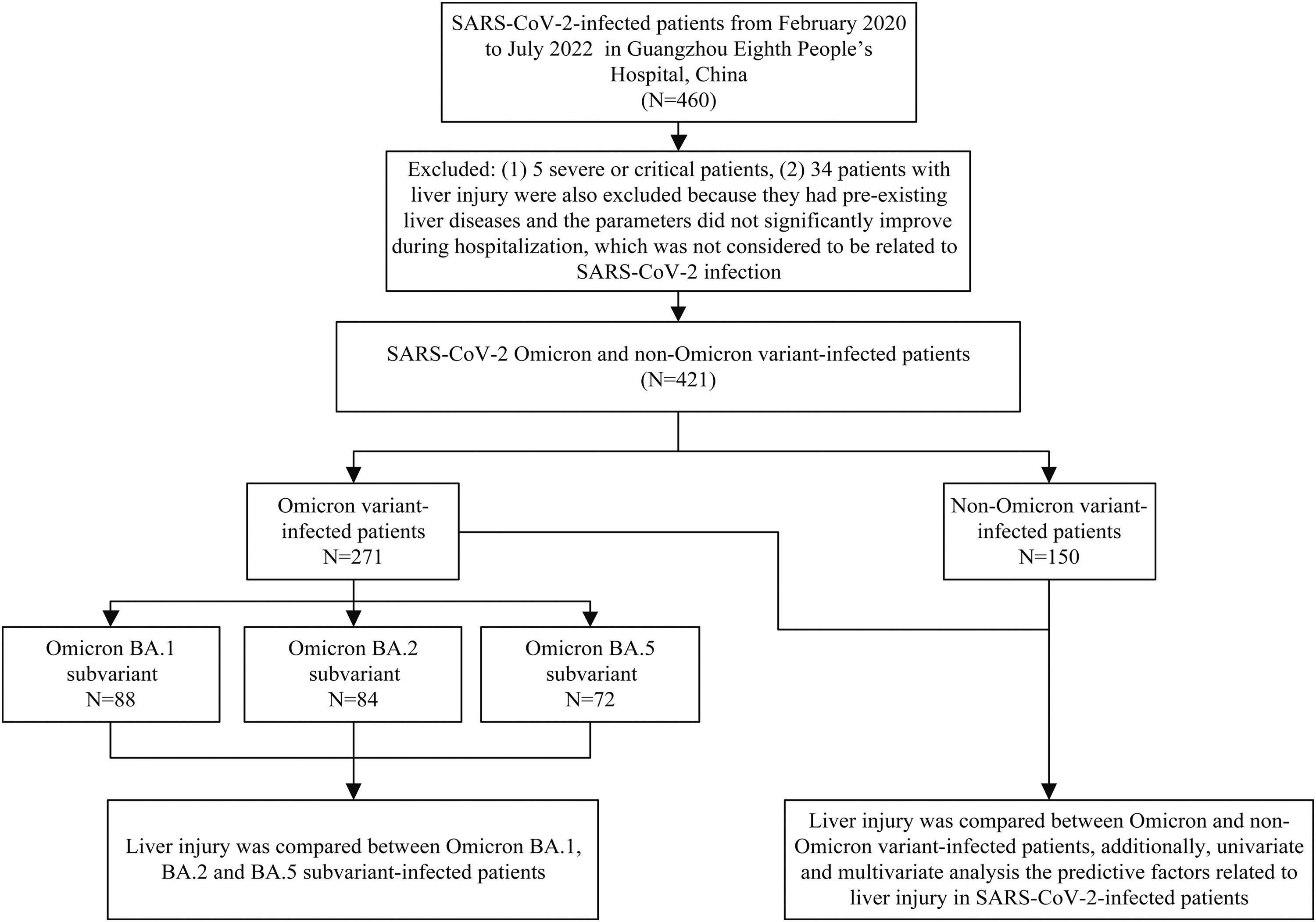
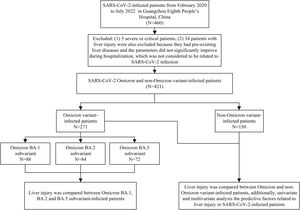
2Material and methods2.1Study subjectsThis was a cross-sectional study. A total of 460 SARS-CoV-2-infected patients hospitalized in isolation wards at Guangzhou Eighth People's Hospital from February 2020 to July 2022 were enrolled for the study. The results of liver function tests were collected and compared between Omicron and non-Omicron variants and between Omicron subvariant-infected patients. In addition, clinical data were compared between patients with and without liver injury. Prior to hospitalization, none of the SARS-CoV-2 infected patients had received any antiviral drugs for the treatment of SARS-CoV-2. Severe/critical patients were excluded based on the fact that the ratio of liver injury is significantly higher in severe/critical than non-severe/critical patients, and severe/critical patients were rare in Omicron variant-infected patients. Patients with pre-existing liver diseases and the abnormal parameters of liver function tests, which did not significantly improve during hospitalization and during follow-up after discharge, were also excluded from the study since the liver injury, in this case, was more related to pre-existing liver diseases than SARS-CoV-2 infection. The study design flow chart is shown in Fig. 1.
The flow chart of the study design.
A total of 460 SARS-CoV-2-infected patients enrolled from February 2020 to July 2022 in Guangzhou Eighth People's Hospital. Five severe or critical patients were excluded, and 34 patients with liver injury were also excluded because they had pre-existing liver diseases and the abnormal parameters of liver function tests did not significantly improve during hospitalization and during follow-up after discharge, which was considered to be related to pre-existing liver diseases than SARS-CoV-2 infection. Liver injury was compared between patients infected with SARS-CoV-2 Omicron and non-Omicron variant (N=421), and liver injury was compared between patients infected with Omicron BA.1, BA.2, and BA.5 subvariant. Predictive factors related to liver injury in SARS-CoV-2-infected patients were also analyzed in this study.
All the patients enrolled in the study were positive for SARS-CoV-2 RNA (nasopharyngeal or pharyngeal swab specimen). The SARS-CoV-2-infected patients were clinically classified based on the "Diagnosis and treatment plan for COVID-19 (trial version 9 revision)" issued by the National Health Commission of the People's Republic of China on March 14, 2022 [19]. They were classified based on the following definition: Asymptomatic SARS-CoV-2-infected patients were defined as SARS-CoV-2 RNA positive for the upper respiratory tract samples but without any clinical symptoms and signs of pneumonia detected using computed tomography (CT) imaging. Mild COVID-19 infection was defined as patients with mild clinical symptoms but no signs of pneumonia on CT imaging. Moderate COVID-19 infection was defined as patients presenting clinical symptoms along with pneumonia on CT imaging. Severe COVID-19 infection was defined if the patients met any of the following criteria: (1) respiratory distress and respiratory rate ≥ 30 times/minute; (2) oxygen saturation ≤ 93% at the resting state; and (3) an arterial blood oxygen partial pressure (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg. Patients were categorized as critical COVID-19 infection if they met any of the following criteria: (1) respiratory failure requiring mechanical ventilation; (2) the state of shock; and (3) the requirement of intensive care unit monitoring and treatment because of complications associated with multiple organ failures. Currently, in China, all the patients who tested positive for SARS-CoV-2 RNA by PCR test (the same sample simultaneously positive in an independent testing agency and a center for disease control and prevention) are required to undergo hospitalization (quarantine) in an isolation ward.
2.3Routine clinical examinationsRoutine clinical examinations were performed for all the study subjects. Serum amyloid A (SAA) and C-reactive protein (CRP) were tested using an automated specific protein analyzer (Ottoman, Shanghai City, China). Hepatitis B virus (HBV) surface antigen and hepatitis C antibody IgG were tested using chemiluminescence immunoassays (HISCL-800, Sysmex Corporation, Kobe, Japan). Chest and upper abdominal CT scans were performed using a 128-slice dual-source CT scanner (CT680, Optima, GE, USA). Serum HBV DNA and hepatitis C virus (HCV) RNA levels were measured using the TaqMan PCR assay (DaAn Gene, Guangzhou City, China). SARS-CoV-2 RNA (nasopharyngeal or pharyngeal swab samples) was also measured using the TaqMan PCR assay (DaAn Gene, Guangzhou City, China) according to the manufacturer's instructions. The cycle threshold (Ct) value of less than 40 was defined as positive. The SARS-CoV-2 variants and subvariants were determined using the in-house next-generation sequencing or Oxford Nanopore sequencing.
2.4Definition of liver injury in SARS-CoV-2-infected patientsLiver function tests were performed using commercially available kits using an AU2700 automatic biochemical analyzer (Olympus, Tokyo, Japan). The parameters included were as follows: alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bile acid (TBA), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), total bilirubin (TBIL) and albumin (ALB) levels. The normal ranges of the liver function tests were as follows: ALT level (male: 9–50 U/L, female: 7–40 U/L); AST level (male: 15–40 U/L, female: 13–35 U/L); TBA level (0–10 μmol/L); ALP level (45–125 U/L); GGT level (male: 10–60 U/L, female: 10–45 U/L); TBIL level (10–26 μmol/L); and ALB level (40–55 g/L). Liver injury was defined if, among these liver function test parameters, the levels of ALT, AST, TBA, ALP, GGT, or TBIL exceeded the upper limit of normal value (ULN) or ALB was lower than the low limit of normal value.
2.5Statistical analysisKolmogorov-Smirnov test and Shapiro-Wilk test were used to assess the normal distribution of data. Continuous variables are represented as the mean ± standard deviation for normally distributed data or the median (interquartile range, IQR) for data that did not follow a normal distribution (non-normal). The categorical variables were presented as numbers (percentages). The student's t-test was used for normally distributed continuous variables, and the Mann–Whitney U test was used for non-normally distributed variables between two groups. The χ2 test and Fisher's exact test were used for categorical variables between two or multiple groups. Logistic regression analysis was used for multivariate analysis. SPSS Statistic 20.0 (SPSS Inc, Illinois, USA) was used for all analyses. P<0.05 was considered statistically significant.
2.6Ethical statementWritten informed consent was obtained from each patient included in the study. All the protocols performed in this study were in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. The study was approved by the Ethics Committee of Guangzhou Eighth People's Hospital, Guangzhou Medical University (approval number: 202001134).
3Results3.1Study population characteristicsAll the 460 SARS-CoV-2-infected patients enrolled in the study were Chinese, and five severe or critical patients were excluded from the study. Additionally, 34 patients with liver injury (31 patients with nonalcoholic fatty liver disease or three patients with HBV infection) were also excluded from the study as the liver injury was considered unrelated to SARS-CoV-2 infection. Therefore, a total of 421 SARS-CoV-2-infected patients were enrolled for further analysis, including 271 patients with Omicron infection (88 patients with Omicron BA.1, 84 patients with Omicron BA.2, 8 patients with Omicron BA.2.12.1, 19 patients with Omicron BA.3 and 72 patients with Omicron BA.5 subvariant-infection) and 150 non-Omicron variant-infected patients (43 patients with wild-type infection, 33 patients infected with Alpha, and 74 patients infected with Delta variant). These basic clinical data of the patients are included in Table 1. No difference was observed in the clinical data like age, sex, body mass index (BMI), smoking history, alcohol abuse, and pre-existing liver diseases between the Omicron and non-Omicron variant-infected patients. However, significant differences (P<0.05) were observed in the vaccination status, clinical types, inflammatory markers, and viral loads of SARS-CoV-2 between patients infected with Omicron and non-Omicron variants, as shown in Table 1.
Basic clinical data of SARS-CoV-2 Omicron and non-Omicron variant-infected patients.
| Clinical data | Omicron (N=271) | Non-Omicron (N=150) | t/Z/χ2 value | P value |
|---|---|---|---|---|
| Age, years (median, IQR) | 34 (26–45) | 30 (35–44) | −1.780 | 0.075 |
| Sex (male/female) | 173/98 | 105/45 | 1.635 | 0.201 |
| BMI, kg/m2, (mean ± SD) | 23.6 ± 4.4 | 23.2 ± 3.3 | −1.010 | 0.313 |
| Smoking history, n (%) | 34 (12.5) | 22 (14.7) | 0.376 | 0.539 |
| Alcohol abuse, n (%) | 12 (4.4) | 8 (5.3) | ||
| Pre-existing liver diseases, n (%) | 6.274 | 0.057 | ||
| NAFLD | 8 (3.0) | 13 (8.7) | >0.05 | |
| HBV infection | 14 (5.2) | 7 (4.7) | >0.05 | |
| HCV infection | 1 (0.4) | 0 (0.0) | >0.05 | |
| Hepatolithiasis | 3 (1.1) | 0 (0.0) | >0.05 | |
| Vaccinated patients, n (%)a | 260 (95.9) | 75 (50.0) | 125.372 | <0.001 |
| Clinical types, n (%) | 100.510 | <0.001 | ||
| Asymptomatic | 42 (15.5) | 33 (22.0) | >0.05 | |
| Mild status | 182 (67.2) | 28 (18.7) | <0.05 | |
| Moderate status | 47 (17.3) | 89 (59.3) | <0.05 | |
| Inflammatory markersb | ||||
| SAA (mg/mL) | 22.4 (10.2–9.0) | 9.3 (5.1–32.1) | −5.723 | <0.001 |
| CRP <10/≥10(mg/mL) | 181/90 | 116/34 | 5.166 | 0.023 |
| Viral load (N gene) b | 18 (16–21) | 24 (17–32) | −6.881 | <0.001 |
| Viral load (ORF 1ab gene) b | 21 (19–23) | 26 (20–34) | −5.776 | <0.001 |
Vaccinated patients: patients who have received the COVID-19 vaccination (at least two doses of inactive or mRNA vaccine) for at least four weeks prior to infection with SARS-CoV-2.
Results on the same day of liver function test; Viral load (N and ORF 1ab gene): The viral load was determined using the Ct values obtained by PCR for the nasopharyngeal or pharyngeal swab samples. BMI, Body mass index; NAFLD, Nonalcoholic fatty liver disease; HBV, hepatitis B virus; SAA, Serum amyloid A; CRP, C-reactive protein.
Among the 421 SARS-CoV-2-infected patients, liver injury was reported in 76 patients (18.1%). The abnormal liver function biomarkers ranging from the highest to the lowest were as follows: The level of GGT was reported to be elevated in 40 (9.5%) patients, 21 (5.0%) patients reported an increase in levels of ALT, and 18 (4.3%) patients showed an increase in TBA levels. Further, elevation in AST levels was observed in 15 (3.6%) patients, ALP levels were elevated in 10 (2.4%) patients, and the levels of TBIL increased in 3 (0.7%) patients. The results revealed mild elevation (1-3 × ULN) in most liver function tests; except for one patient with wild-type infection and two patients infected with Omicron variant reported a moderate increase in GGT level (4-6 × ULN, shown in Supplementary Tables 1-2). The frequency of patients with at least one abnormal cholangiocyte or biliary duct injury marker (TBA, ALP, or GGT) was higher than with hepatocellular injury markers (ALT or AST; 14.7%, 62/421 vs. 6.4%, 27/421).
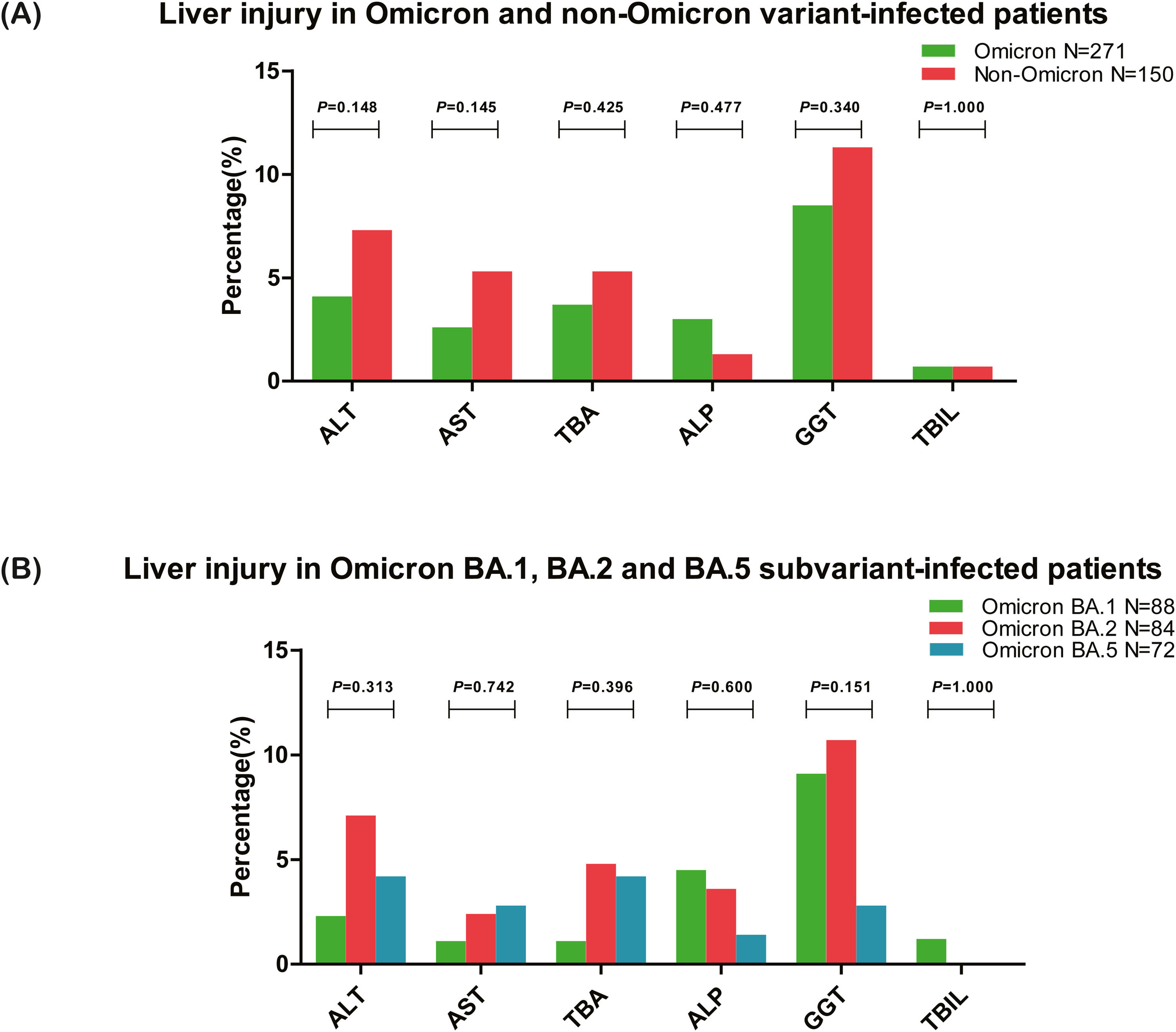
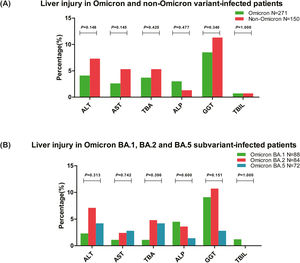
3.3Liver injury in Omicron variant- and Omicron subvariant-infected patients3.3.1Liver injury in patients infected with Omicron and non-Omicron variantsLiver injury in patients infected with Omicron and non-Omicron variant-of SARS-CoV-2 were analyzed and compared. The results revealed that liver injury was reported in 46 (17.0%) patients infected with the Omicron variant and 30 (20.0%) patients infected with non-Omicron variants (χ2=0.598, P=0.440). No significant difference was observed in the liver function test between patients infected with SARS-CoV-2 Omicron and non-Omicron variants (Table 2 and Fig. 2A). The most frequently elevated parameter of the liver function test was GGT (8.5%, 23/271 in Omicron and 11.3%, 17/150 in non-Omicron variant-infected patients), and the frequency of patients with at least one abnormal cholangiocyte or biliary duct injury markers (TBA, ALP, or GGT) was higher than abnormal hepatocellular injury markers (ALT or AST) in patients infected with Omicron variant (14.0%, 38/271 vs. 5.2%, 14/271).
Liver injury in Omicron and non-Omicron variant-infected patients (n, %).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBA, total bile acid; ALP, alkaline phosphatase; GGT, γ-glutamyl transpeptidase; TBIL, total bilirubin; ALB, albumin. ALT, AST, TBA, ALP, GGT, or TBIL level exceeding the upper limit of normal value or ALB lower than the low limit of normal value was defined as liver injury.
Comparison of liver injury in SARS-CoV-2-infected patients. (A) Liver injury in patients infected with SARS-CoV-2 Omicron and non-Omicron variant, and all the ratios of abnormal liver function tests did not differ between the two groups (all P>0.05). (B) Liver injury in patients infected with SARS-CoV-2 Omicron BA.1, BA.2, and BA.5 subvariant, and all the ratios of abnormal liver function tests also did not differ between the three groups (all P>0.05). ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBA, total bile acid; ALP, alkaline phosphatase; GGT, γ-glutamyl transpeptidase; TBIL, total bilirubin.
Among the 271 patients infected with the Omicron variant, the liver injury was analyzed in three major subvariants BA.1, BA.2, and BA.5 (90.0%, 244/271). The results showed that liver injury was reported in 13 (14.8%) patients infected with Omicron BA.1, 17 (20.2%) patients infected with Omicron BA.2, and 8 (11.1%) patients infected with Omicron BA.5 subvariant (χ2=2.523, P=0.283). As shown in Table 3 and Fig. 2B, no difference (all P>0.05) was observed in all the parameters of liver function tests between patients infected with Omicron BA.1, BA.2, and BA.5 subvariant. The most frequently elevated parameter of the liver function test was GGT in Omicron BA.1 (9.1%, 8/88) and Omicron BA.2 (10.7%, 9/84) subvariant-infected patients, and TBA or ALT in BA.5 (4.2%, 3/72) subvariant infected patients.
Liver injury in Omicron BA.1, BA.2, and BA.5 subvariant-infected patients (n, %).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBA, total bile acid; ALP, alkaline phosphatase; GGT, γ-glutamyl transpeptidase; TBIL, total bilirubin; ALB, albumin. ALT, AST, TBA, ALP, GGT, or TBIL level exceeding the upper limit of normal value or ALB lower than the low limit of normal value was defined as liver injury.
The clinical data related to liver injury in SARS-CoV-2-infected patients were compared. Univariate and multivariate analysis was performed on 76 SARS-CoV-2-infected patients with liver injury and 345 without liver injury. The univariate analysis showed that the patient's age and inflammatory markers (SAA and CRP level) were significantly higher in patients with liver injury (P<0.05), as shown in Table 4. Binary logistic regression analysis included age (≤40/>40 years old), sex (male/female), BMI (≤25/>25 kg/m2), alcohol abuse (Yes/No), vaccination status (Yes/No), clinical types (Asymptomatic status/Mild status/Moderate status), pre-existing liver diseases (Yes/No), SAA level (≤10/>10 mg/mL), CRP level (≤10/>10 mg/mL) and viral load in the nasopharynx or pharynx (Ct value of N gene, ≤20, >20 to ≤30, >30) as the dependent variables were also performed. The results showed that age (>40 years old, OR=1.898, 95% CI=1.058-3.402, P=0.032), sex (male gender, OR=2.031, 95% CI=1.211-3.408, P=0.007), SAA level (>10 mg/mL, OR=3.595, 95% CI=1.840-7.026, P<0.001) and vaccination status (No, OR=2.131, 95% CI=1.089-4.173, P=0.027) were independent factors related to liver injury.
Clinical data compared SARS-CoV-2 infected patients with and without liver injury.
| Clinical data | Liver injury N=76 | Without liver injury N=345 | t/χ2/Z value | P value |
|---|---|---|---|---|
| Age, years (median, IQR) | 40 (31–49) | 34 (26–45) | −2.283 | 0.022 |
| Sex, Male/Female | 57/19 | 221/124 | 3.325 | 0.068 |
| BMI, kg/m2, (mean ± SD) | 24.0 ± 4.1 | 23.3 ± 4.1 | 1.430 | 0.154 |
| Vaccinated patients, n (%) a | 58 (76.3) | 277 (80.3) | 0.136 | 0.712 |
| Alcohol abuse, n (%) | 5 (6.6) | 15 (4.3) | 0.281 | 0.596 |
| Clinical types, n (%) | 0.538 | 0.787 | ||
| Asymptomatic status | 12 (15.8) | 63 (18.3) | >0.05 | |
| Mild status | 37 (48.7) | 173 (50.1) | >0.05 | |
| Moderate status | 27 (35.5) | 109 (31.6) | >0.05 | |
| Pre-existing liver diseases, n (%) | 9 (11.8) | 37 (10.7) | 0.080 | 0.777 |
| Inflammatory markersb | ||||
| SAA (mg/mL) | 23.8 (13.5-88.0) | 15.9 (7.0–49.8) | −3.020 | 0.003 |
| CRP ≤10/>10(mg/mL) | 46/30 | 251/94 | 4.481 | 0.034 |
| Viral load (N) b | 18 (16–21) | 19 (16–25) | −1.342 | 0.179 |
| Viral load (ORF 1ab gene)b | 21 (19–25) | 22 (19–27) | −1.441 | 0.150 |
| Hospital daysc | 12 (10–17) | 14 (12–19) | −1.791 | 0.073 |
Vaccinated patients: patients who have received the COVID-19 vaccination (at least two doses of inactive vaccine or mRNA vaccine) for at least four weeks prior to infection with SARS-CoV-2.
Results on the same day of liver function test; cDischarge criteria: (1) Ct values of N and ORF 1ab genes were more than 35 (SARS-CoV-2 PCR test) for nasopharyngeal or pharyngeal swab samples for at least two times (each time more than 24 h), (2) improvement in clinical symptoms and CT imaging of the lungs. Viral load (N and ORF 1ab gene): The viral load was determined by the Ct values of PCR tests in nasopharyngeal or pharyngeal swab samples. Abbreviations: BMI, Body mass index; SAA, Serum amyloid A; CRP, C-reactive protein.
Since the SARS-CoV-2 Omicron variant was first reported in South Africa in November 2021, this SARS-CoV-2 variant has spread rapidly and has become the dominant variant in different countries [20]. To date, the SARS-CoV-2 Omicron variant has become the most common variant in imported and local SARS-CoV-2 outbreaks in China, which is more transmissible than the other variants [5, 6], introducing greater challenge for pandemic prevention and control. Liver injury in SARS-CoV-2-infected patients has been reported; however, most of the data reported regarding liver injury were studied in the wild-type SARS-CoV-2-infected patients [14–17], and the clinical characteristics of liver injury in the Omicron variant-infected patients are still unknown. In addition, the Omicron BA.5 variant has become the most prevalent Omicron subvariant worldwide [21, 22], and the characteristics of liver injury in Omicron subvariant BA.5 are also unknown.
In this study, we enrolled a total of 421 non-severe/critical patients infected with SARS-CoV-2 Omicron and non-Omicron variants. The results showed that liver injury was reported in 76 patients (18.1%). There was no difference in the ratios of patients with liver injury who were infected with SARS-CoV-2 Omicron and non-Omicron variants. Further, the ratio of patients with liver injury also did not differ between Omicron BA.1, BA.2, and BA.5 subvariant infections. The most frequently elevated parameter of the liver function test was GGT, and the ratios of patients with cholangiocyte or biliary duct injury markers (TBA, ALP, or GGT) were higher than patients with hepatocellular injury markers (ALT or AST) infected with both Omicron and non-Omicron variants. The results of previous studies showed that the dominant pattern of hepatocellular, cholangiocyte, or biliary duct injury after SARS-CoV-2 infection is still disputed. For example, a report suggests that hepatocellular injury biomarkers like AST and ALT were most frequently elevated; however, another study showed an increase in cholangiocyte or biliary duct injury biomarkers like GGT was more predominant [23, 24]. The results of our study were consistent with the findings of the latter study. Further, our results are in accordance with our previous findings, which analyzed 157 Delta or Omicron variant-infected patients [25]. Patients who received antiviral drugs or hepatoxic drugs prior to liver function tests were excluded from this study. Further, non-severe/critical patients were excluded from the study to minimize the interference of the drug-induced liver injury and strong inflammatory reactions associated with the liver injury. Therefore, our study may reflect the actual situation of liver injury SARS-CoV-2 infection, such as direct viral injury. The results of this study are in accordance with the fact that the receptors (angiotensin-converting enzyme 2, ACE2) of SARS-CoV-2 are more highly expressed in cholangiocytes (59.7%) than in hepatocytes (2.6%) [26]. For our study, we enrolled patients infected with Omicron and its subvariants for analysis. The results indicate that the ratio of liver injury induced by SARS-CoV-2 infection was not significantly affected by different variants or subvariants.
It is worth noting that the multivariate analysis showed that age, sex, vaccination status, and SAA level as independent factors related to liver injury in SARS-CoV-2-infected patients. In our previous study, the liver injury in Delta and Omicron variant infected patients was compared (most of these patients were vaccinated), and the results revealed a relation between male patients, viral load analyzed in the nasopharynx samples and liver injury in Delta and Omicron variants [25]. The difference between the two studies could be due to: (1) The sample size of our previous study was relatively small (only 29 patients reported liver injury), which may reduce statistical efficiency. (2) Liver injury reported in SARS-CoV-2-infected patients could be affected by multiple factors, and different patients infected with different genotypes of SARS-CoV-2 infection, vaccination, and immune status, which may add to the heterogeneity. Despite excluding severe/critical patients from the study, our results still indicated that the inflammatory response is an independent factor related to liver injury; this suggests that the inflammatory response is still an important factor related to liver injury in patients infected with SARS-CoV-2 as per the previous study [27]. Additionally, the results of our study showed that the difference in vaccination status of the patients related to liver injury might be due to the inflammatory responses that were usually more severe in patients who did not receive the vaccination. However, as new lineages or variants of SARS-CoV-2 emerge, the factors associated with a liver injury needs to be further investigated.
Some limitations in this study should be noted: (1) This study was a single-center cross-sectional study and thus could have some bias. Hence a multicenter and prospective study is required to further explore the features of liver injury in SARS-CoV-2-infected patients. (2) Although we excluded most of the pathogens causing liver injury in SARS-CoV-2-infected patients, some examinations were not performed for all the enrolled patients, such as autoimmune hepatitis antibody tests. Hence, a more comprehensive review of liver injury would be needed to exclude other pathogens for prospective multiple-center studies. (3) The follow-up data of liver function tests were not analyzed due to missing information, and the results of our previous study showed that in most cases, the peak levels of liver injury occurred in non-severe/critical patients after admission and decreased during hospitalization in patients who did not receive any antiviral drugs [25]. (4) Further, the lack of information about the patient's liver function test prior to admission is also one of the limitations of the study.
This study investigated the clinical characteristics of liver injury in patients infected with the SARS-CoV-2 Omicron variant and three Omicron subvariants. Further studies are required to investigate liver injury in patients infected with SARS-CoV-2 because the characteristics of liver injury may change with the emergence of new SARS-CoV-2 lineages and variants.
5ConclusionsIn conclusion, the liver injury does not differ between patients infected with Omicron and non-Omicron variants and between Omicron BA.1, BA.2, and BA.5 subvariants. Further, the elevations of cholangiocyte or biliary duct injury markers (TBA, ALP, or GGT) are dominant in SARS-CoV-2-infected patients. In addition, this study also analyzed the factors related to liver injury in SARS-CoV-2-infected patients. The results show that age, gender, inflammatory markers level (SAA), and vaccination status are independent factors related to liver injury.
FundingThis work was supported by the Medical Scientific Research Foundation of Guangdong Province, China (grant number B2021302), and the Foundation of Key Medical Disciplines in Guangzhou City (2021–2023) - Viral Infectious Diseases (grant number 202208XK0002).
Author contributionsGJ conceived and designed the study; MY and LH made substantial contributions to the conception, design, acquisition, statistical analysis and interpretation of the clinical data; DH drafted the manuscript, acquisition, statistical analysis and interpretation of the clinical data; GJ revised the manuscript. All authors critically reviewed the manuscript and gave final approval of the version to be published.
Data availability statementThe data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of interestNone
We would like to thank Dr. Shuifeng Li in Guangzhou Eighth People's Hospital for her constant help with statistical analysis, thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript, additionally thank all the staff in Guangzhou Eighth People's Hospital for insisting on the treatment of SARS-CoV-2-infected patients for exceeded two years.