Liver resection is the only curative therapeutic option for large hepatocellular carcinoma (> 5 cm), but survival is worse than in smaller tumours, mostly due to the high recurrence rate. There is currently no proper tool for stratifying relapse risk. Herein, we investigated prognostic factors before hepatectomy in patients with a single large hepatocellular carcinoma (HCC).
Material and methodsWe retrospectively identified 119 patients who underwent liver resection for a single large HCC in 2 tertiary academic French centres and collected pre- and post-operative clinical, biological and radiological features. The primary outcome was overall survival at five years. Secondary outcomes were recurrence-free survival at five years and prognostic factors for recurrence.
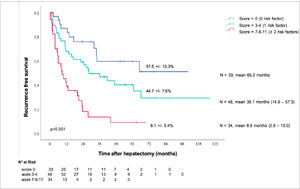
ResultsA total of 84% of the patients were male, and the median age was 66 years old (IQR 58–74). Thirty-nine (33%) had Child–Pugh A cirrhosis, and the mean Model for End-Stage Liver Disease (MELD) score was 6 (6–6). The aetiology of liver disease was predominantly alcohol-related (48%), followed by nonalcoholic steatohepatitis (22%), hepatitis B (18%) and hepatitis C (10%). The mean tumour size was 70 mm (55–110). The median overall survival was 72.5 months (IC 95%: 56.2-88.7), and the five-year overall survival was 55.1 ± 5.5%. The median recurrence-free survival was 26.6 months (95% CI: 16.0-37.1), and the five-year recurrence-free survival rate was 37.8 ± 5%. In multivariate analysis, preoperative prognostic factors for recurrence were baseline alpha-fetoprotein (AFP) > 7 ng/mL (p<0.001), portal veinous invasion (p=0.003) and cirrhosis (p=0.020). Using these factors, we created a simple recurrence-risk scoring system that classified three groups with distinct disease-free survival medians (p<0.001): no risk factors (65 months), 1 risk factor (36 months), and ≥2 risk factors (8.9 months).
ConclusionLiver resection is the only curative option for large HCC, and we confirmed that survival could be acceptable in experienced centres. Recurrence is the primary issue of surgery, and we proposed a simple preoperative score to help identify patients with the most worrisome prognosis and possible candidates for combined therapy.
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second most frequent cause of cancer-related death worldwide [1]. While it has a geographic imbalance, with almost 75% of the 841 000 new cases occurring in Asia in 2018 (610 000), its incidence is increasing globally, and the number of cases in the West (Europe and North America) is estimated to reach 146 000 by 2030 [2].
Therapeutic options depend on tumour status, and the Barcelona Clinic Liver Cancer (BCLC) classification is the most widely used staging system in Western countries, as well as the one endorsed by the European Association for the Study of the Liver (EASL) [3]. Curative therapy is possible in very early (BCLC 0) and early (BLCL A) stages, achieved through liver transplantation (LT), liver resection (LR) and radiofrequency ablation (RFA). Within the Milan criteria (single tumour ≤ 5 cm or ≤ 3 tumours of ≤ 3 cm in size), LT expected five-year survival is greater than 65% [4], but donor shortage makes it currently impossible to be the mainstay of curative treatment, leaving this place to LR and RFA. Ablation is generally the first-line option for BLCL 0 (≤ 2 cm single nodule) due to its higher cost-effectiveness, but LR has a definitive survival advantage for solitary tumours > 3 cm [5].
If there is a large consensus on LR for HCC within patients without cirrhosis [6], and selection criteria have been proposed in cases of cirrhosis to minimize the risk of liver failure. Some of them include solitary tumours, very well-preserved liver function and the absence of clinically relevant portal hypertension. These approaches can be extended with good outcomes achieved in experienced centres, but no consensus has been reached [3].
A particular subset of tumours > 5 cm excluded from LT (Milan criteria) is considered outside any curative range [7]. The revised BCLC classification nonetheless designated a single large HCC (> 5 cm) as BCLC A [3], rather than BCLC B in the 2012 version [8]. In practice, single large HCC is indicated for LR as early-stage tumours (BCLC A) but appears to have a worse prognosis than HCC within the Milan criteria. It is well established that the five-year overall survival following LR among patients with single large HCC is similar to BCLC B tumours (ranging from 28 to 57%, depending on the study) [9–13]. Nonetheless, several studies have demonstrated improved prognosis after surgical resection vs. transarterial chemoembolization (TACE), which is the recommended treatment for BCLC B [11,14,15]. Taken together, these data advocate for LR as first-line therapy for single large HCC.
Cancer recurrence remains the primary issue of LR, and overall, it complicates up to 70% of cases at five years [16], including “early” recurrence and “de novo” tumours, with two years being the classical threshold. To date, no adjuvant or neoadjuvant therapy has demonstrated clearly proven efficacy for HCC, and there is no standard of care [17]. This failure can partly be credited to the absence of a practical tool for recurrence risk stratification, making it difficult to select patients properly in clinical trials. Different prognostic systems have been proposed to predict recurrence or survival after LR, but most of those were constructed using Asian patients with a broad predominance for hepatitis B [7,18–20].
The aim of this study was to determine the overall and disease-free survival of surgically resected single large HCC and to identify prognostic recurrence factors by analysing pretreatment clinical, radiological and biological features in a multicentre study.
2Material and methodsThis is a retrospective cohort study targeting a population of patients treated by LR first for large HCC within two tertiary academic French centres from 2010 to 2019. The reporting of this study was based on the STROBE recommendations for conservative studies.
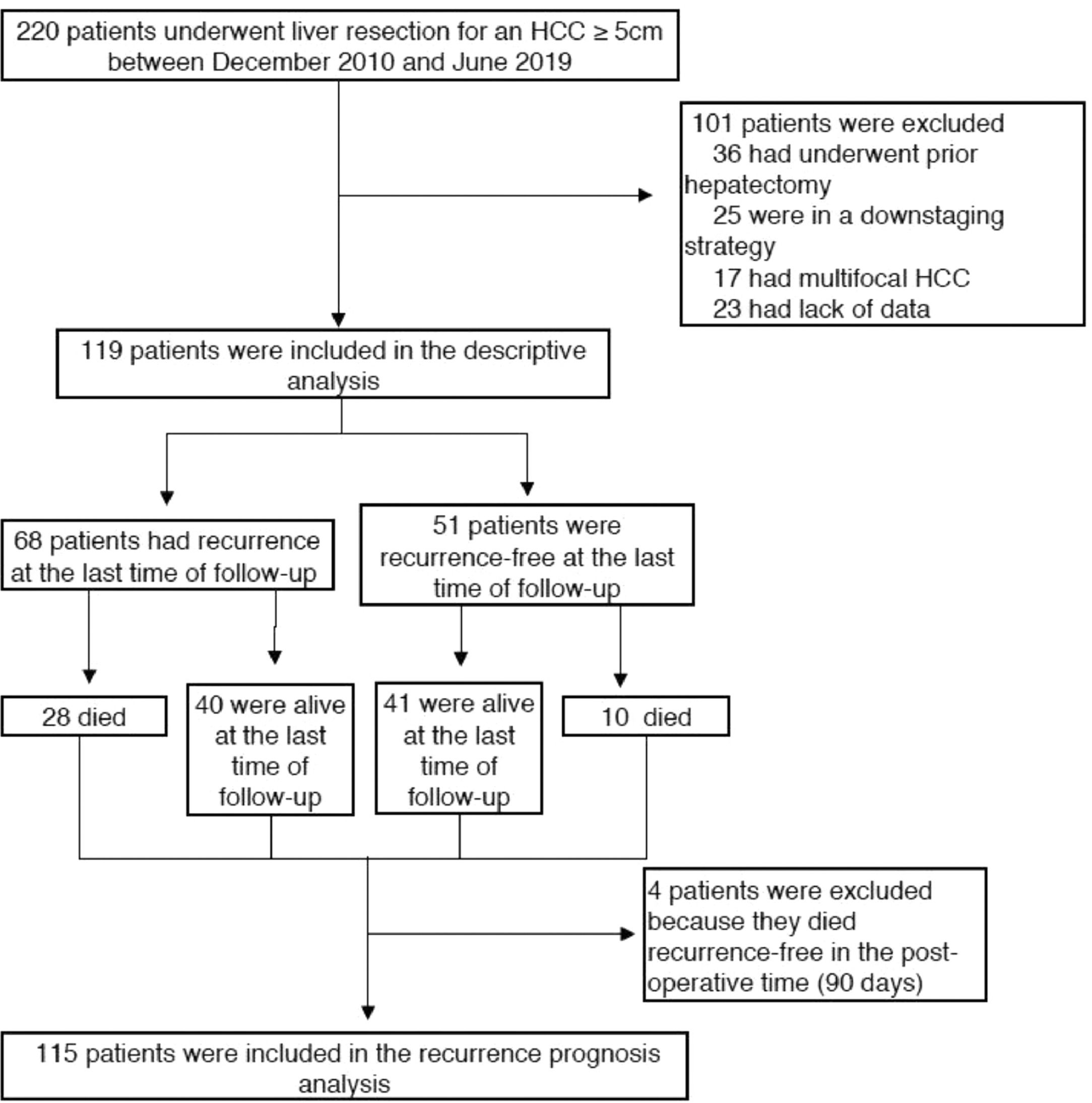
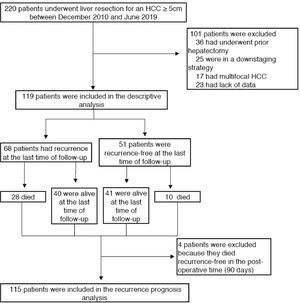
2.1Study population and data collectionPatients who underwent surgical resection of a single large HCC between December 2010 and June 2019 were retrospectively identified using institutional databases (Orbis©, Agfa HealthCare, Mortsel Belgium and FileMaker Pro©, Apple) in two tertiary care French centres, Henri Mondor University Hospital (Paris) and Robert Debré University Hospital (Reims). Single large HCC was defined as a solitary tumour ≥ 50 mm on baseline CT scan or MRI, irrespective of the radiological vascular invasion status. We excluded patients who underwent prior locoregional or systemic treatment, repeated hepatectomy, had multiple HCC nodules, were in a downstaging strategy, and patients with missing data. Recurrence and vital status were prospectively assessed on follow-up using the same databases.
2.2VariablesDemographic and clinical data included age, sex, body mass index (BMI), underlying liver disease (hepatitis B virus (HBV), hepatitis C virus (HCV), metabolic-associated steatohepatitis (MASH), alcohol intake, haemochromatosis), background liver fibrosis (Metavir fibrosis score graded from F0 to F4), classification of the liver disease (Child–Pugh score, MELD score), metabolic risk factors (metabolic syndrome, diabetes), American Society of Anaesthesiologists (ASA) physical status score (graded from 1 to 3), and portal hypertension (presence of oesophageal varices and grade if applicable).
Biological data included aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), bilirubin, albumin, AFP, leukocyte count, lymphocyte count, neutrophil count, haemoglobin, platelet count, prothrombin time (PT), and prognostic scores of HCC (AFP score, albumin-bilirubin score (Albi score), neutrophils on lymphocytes ratio).
Radiological data were considered from a baseline computed tomography scanner (CT scan) or magnetic resonance imaging (MRI). They included tumour size and portal vein invasion, or portal vein tumour thrombus (PVTT), according to the Japanese Vp Staging Classification System: Vp0 (absence of veinous invasion), Vp1 (extension to a third-order branch), Vp2 (extension to a second-order branch), Vp3 (extension to a first-order branch, which is the right or left portal branch) and Vp4 (extension to the main trunk or a contralateral branch) [21].
Histopathological data included microvascular and macrovascular invasion, microsatellite nodule status, tumour necrosis percentage, resection margin status (R0 or R1) and tumour differentiation (as graded by the World Health Organization). These data were all assessed on the final pathological microscopic analysis of the specimen. Surgical data included date and type of surgery (laparoscopy or laparotomy), type of hepatectomy (minor or major, major defined as resection of ≥ 3 segments), number of resected segments, and type of thrombectomy, if applicable.
2.3Outcomes of interestThe primary outcome was overall survival at five years. The secondary objective was recurrence-free survival at five years, and prognostic factors for recurrence and mortality were examined.
2.4Statistical analysisQuantitative data, presented as the median with interquartile range (IQR), were compared using the Mann–Whitney test as appropriate. Qualitative data, presented as percentages, were compared using the chi-square test or Fischer's exact test as appropriate.
Overall survival was measured from the date of resection to the last living visit or loss to follow-up. Recurrence-free survival was measured from the date of resection to recurrence or death without recurrence. Time-to-endpoint analyses were performed using the Kaplan–Meier method.
All variables were analysed using a univariate Cox regression analysis. A multivariate Cox regression analysis was then used, following a forward selection (likelihood ratio), a stepwise selection method with entry testing based on the significance of the score, and removal testing based on the probability of a likelihood ratio based on the maximum partial likelihood estimates. Statistical analyses were performed using SPSS V27 and Stata V13.
2.5Ethical statementGiven the retrospective nature of data mining and analysis, this study was noninterventional, and ethical board approval was not needed. This study was performed according to the Declaration of Helsinki and the local legal requirements of each centre. It was approved by an Institutional Review Board. All necessary written and signed informed consent were obtained from the patients.
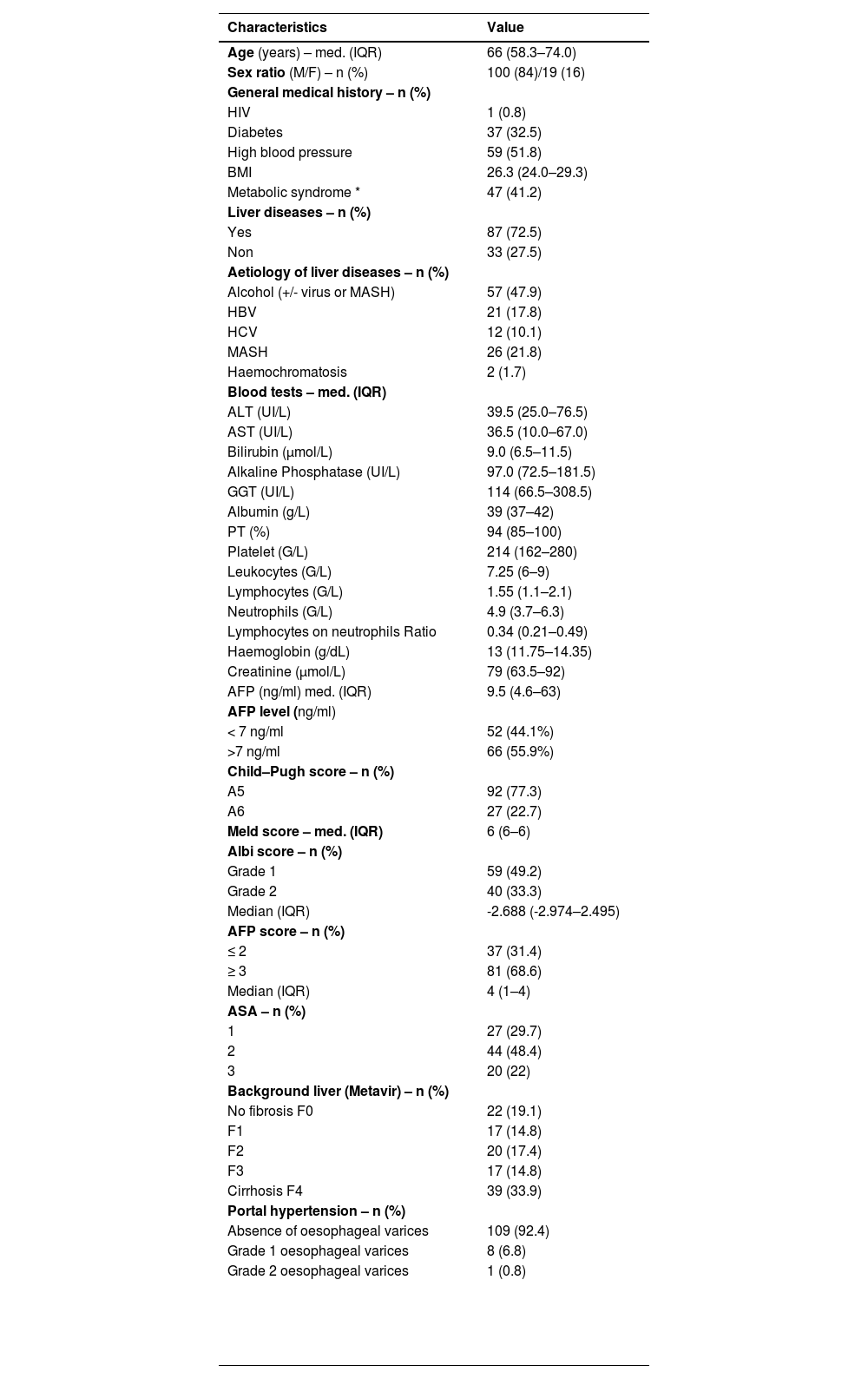
3Results3.1Clinical, radiological and pathological characteristicsDuring the study period, 119 patients were identified and included according to the selection criteria (72 patients from Henri Mondor and 47 from Robert Debré) (see Fig. 1). The median age was 66 years (IQR 58–74), and the majority were male (84%). Thirty-nine (33%) patients presented with cirrhosis (considered as Metavir fibrosis score F4), 17 (15%) had bridging fibrosis (F3) and 59 (49%) had no to low-grade fibrosis (F0-2). All patients were classified as Child–Pugh A, with 93 of them (77.3%) being A5. The median MELD score was 6 (IQR 6–6), and the median Albi score was -2.69 (IQR -2.97 – -2.50). Eighty-two patients (68.3%) presented with an AFP score ≥ 3 (Table 1).
Patient characteristics at baseline (n = 119).
| Characteristics | Value |
|---|---|
| Age (years) – med. (IQR) | 66 (58.3–74.0) |
| Sex ratio (M/F) – n (%) | 100 (84)/19 (16) |
| General medical history – n (%) | |
| HIV | 1 (0.8) |
| Diabetes | 37 (32.5) |
| High blood pressure | 59 (51.8) |
| BMI | 26.3 (24.0–29.3) |
| Metabolic syndrome * | 47 (41.2) |
| Liver diseases – n (%) | |
| Yes | 87 (72.5) |
| Non | 33 (27.5) |
| Aetiology of liver diseases – n (%) | |
| Alcohol (+/- virus or MASH) | 57 (47.9) |
| HBV | 21 (17.8) |
| HCV | 12 (10.1) |
| MASH | 26 (21.8) |
| Haemochromatosis | 2 (1.7) |
| Blood tests – med. (IQR) | |
| ALT (UI/L) | 39.5 (25.0–76.5) |
| AST (UI/L) | 36.5 (10.0–67.0) |
| Bilirubin (μmol/L) | 9.0 (6.5–11.5) |
| Alkaline Phosphatase (UI/L) | 97.0 (72.5–181.5) |
| GGT (UI/L) | 114 (66.5–308.5) |
| Albumin (g/L) | 39 (37–42) |
| PT (%) | 94 (85–100) |
| Platelet (G/L) | 214 (162–280) |
| Leukocytes (G/L) | 7.25 (6–9) |
| Lymphocytes (G/L) | 1.55 (1.1–2.1) |
| Neutrophils (G/L) | 4.9 (3.7–6.3) |
| Lymphocytes on neutrophils Ratio | 0.34 (0.21–0.49) |
| Haemoglobin (g/dL) | 13 (11.75–14.35) |
| Creatinine (μmol/L) | 79 (63.5–92) |
| AFP (ng/ml) med. (IQR) | 9.5 (4.6–63) |
| AFP level (ng/ml) | |
| < 7 ng/ml | 52 (44.1%) |
| >7 ng/ml | 66 (55.9%) |
| Child–Pugh score – n (%) | |
| A5 | 92 (77.3) |
| A6 | 27 (22.7) |
| Meld score – med. (IQR) | 6 (6–6) |
| Albi score – n (%) | |
| Grade 1 | 59 (49.2) |
| Grade 2 | 40 (33.3) |
| Median (IQR) | -2.688 (-2.974–2.495) |
| AFP score – n (%) | |
| ≤ 2 | 37 (31.4) |
| ≥ 3 | 81 (68.6) |
| Median (IQR) | 4 (1–4) |
| ASA – n (%) | |
| 1 | 27 (29.7) |
| 2 | 44 (48.4) |
| 3 | 20 (22) |
| Background liver (Metavir) – n (%) | |
| No fibrosis F0 | 22 (19.1) |
| F1 | 17 (14.8) |
| F2 | 20 (17.4) |
| F3 | 17 (14.8) |
| Cirrhosis F4 | 39 (33.9) |
| Portal hypertension – n (%) | |
| Absence of oesophageal varices | 109 (92.4) |
| Grade 1 oesophageal varices | 8 (6.8) |
| Grade 2 oesophageal varices | 1 (0.8) |
In terms of aetiology, the majority had alcohol-related liver disease (47.9%), followed by 26 patients with MASH (21.8%), 21 patients positive for hepatitis B (17.8%) and 12 for hepatitis C (10.1%). Forty-seven patients (41.2%) had metabolic syndrome, which we defined as the combination of high blood pressure, diabetes and/or a body mass index > 25 kg/m2. Two patients (1.7%) presented with haemochromatosis. Thirty-three patients (27.5%) had no history of liver disease.
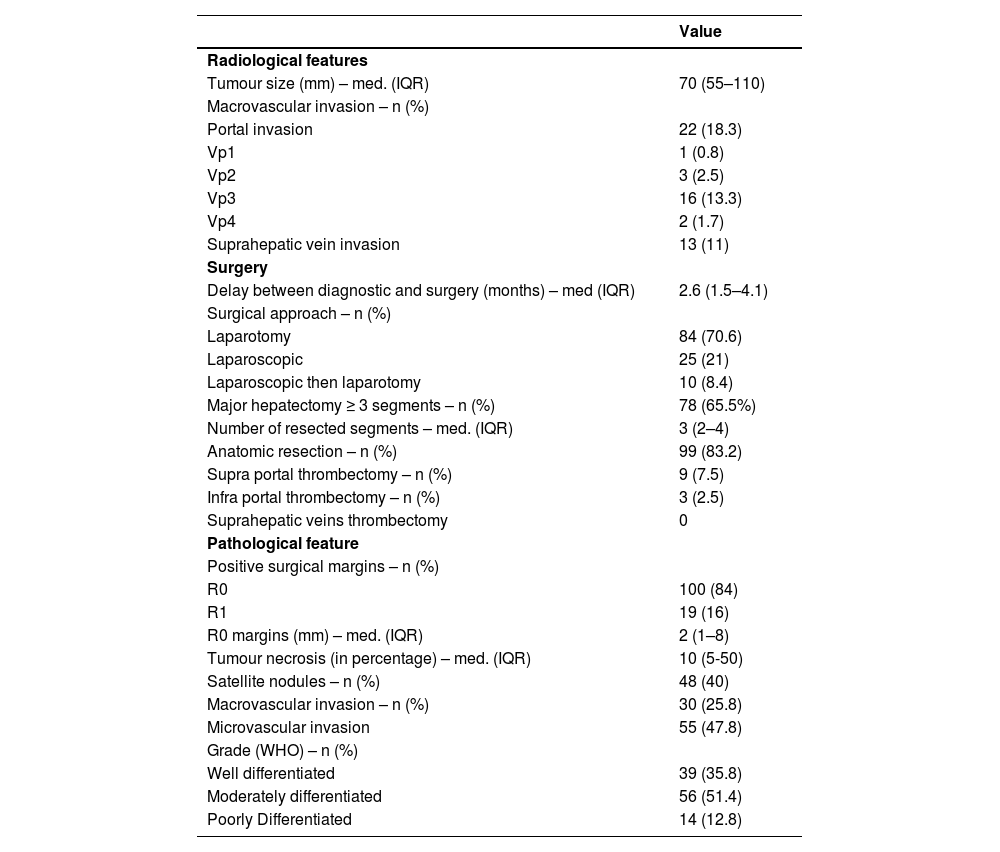
The median tumour size was 70 mm (IQR 55–110, range 50–220). Radiological imaging revealed portal vein invasion in 22 patients (18.5%), including 16 tumoral invasions of the right or left portal branch (Vp3), 3 of a second-order branch (Vp2), 2 of a third-order branch (Vp1) and 1 of the main trunk (Vp4). There were 13 cases (10.8%) of invasion of a hepatic vein (Table 2).
Hepatocellular carcinoma characteristics at baseline.
| Value | |
|---|---|
| Radiological features | |
| Tumour size (mm) – med. (IQR) | 70 (55–110) |
| Macrovascular invasion – n (%) | |
| Portal invasion | 22 (18.3) |
| Vp1 | 1 (0.8) |
| Vp2 | 3 (2.5) |
| Vp3 | 16 (13.3) |
| Vp4 | 2 (1.7) |
| Suprahepatic vein invasion | 13 (11) |
| Surgery | |
| Delay between diagnostic and surgery (months) – med (IQR) | 2.6 (1.5–4.1) |
| Surgical approach – n (%) | |
| Laparotomy | 84 (70.6) |
| Laparoscopic | 25 (21) |
| Laparoscopic then laparotomy | 10 (8.4) |
| Major hepatectomy ≥ 3 segments – n (%) | 78 (65.5%) |
| Number of resected segments – med. (IQR) | 3 (2–4) |
| Anatomic resection – n (%) | 99 (83.2) |
| Supra portal thrombectomy – n (%) | 9 (7.5) |
| Infra portal thrombectomy – n (%) | 3 (2.5) |
| Suprahepatic veins thrombectomy | 0 |
| Pathological feature | |
| Positive surgical margins – n (%) | |
| R0 | 100 (84) |
| R1 | 19 (16) |
| R0 margins (mm) – med. (IQR) | 2 (1–8) |
| Tumour necrosis (in percentage) – med. (IQR) | 10 (5-50) |
| Satellite nodules – n (%) | 48 (40) |
| Macrovascular invasion – n (%) | 30 (25.8) |
| Microvascular invasion | 55 (47.8) |
| Grade (WHO) – n (%) | |
| Well differentiated | 39 (35.8) |
| Moderately differentiated | 56 (51.4) |
| Poorly Differentiated | 14 (12.8) |
Final pathological evaluation of the specimens revealed satellite nodules in 48 cases (40%) and microvascular invasion in 55 (45.8%). There were 30 cases (25%) of macrovascular invasion, 7 of which were not preoperatively diagnosed on baseline CT scan or MRI.
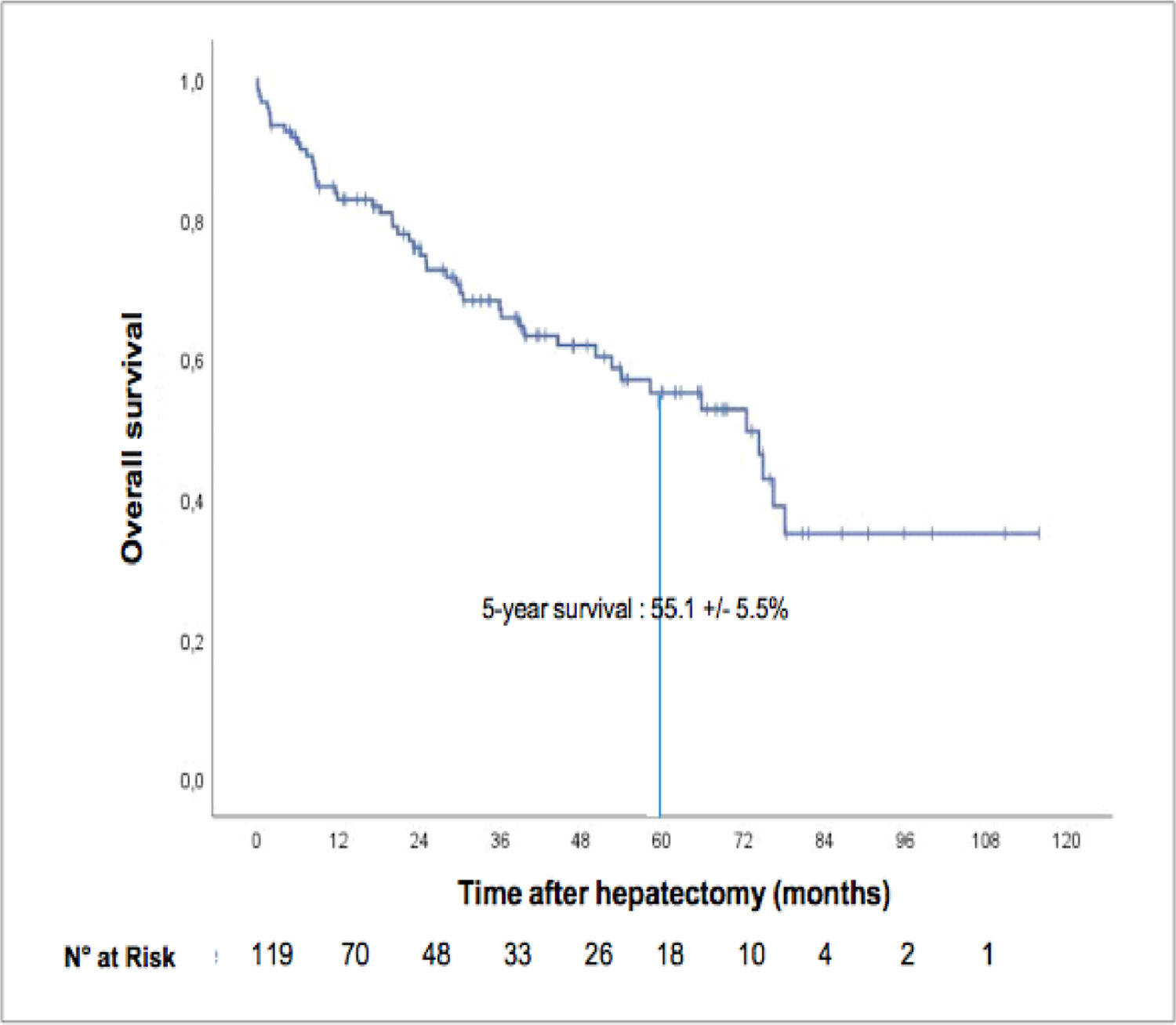
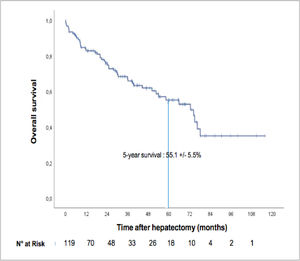
3.2Overall survival of the entire cohortThe median overall survival was 72.5 months (IC 95%: 56.2–88.7), and the five-year overall survival was 55.1 ± 5.5% (see Fig. 2). The median follow-up period since surgery was 28.88 months (IQR 8.69–53.53). At the time of the last follow-up, 38 patients (31.9%) had died, and 81 (68%) were alive.
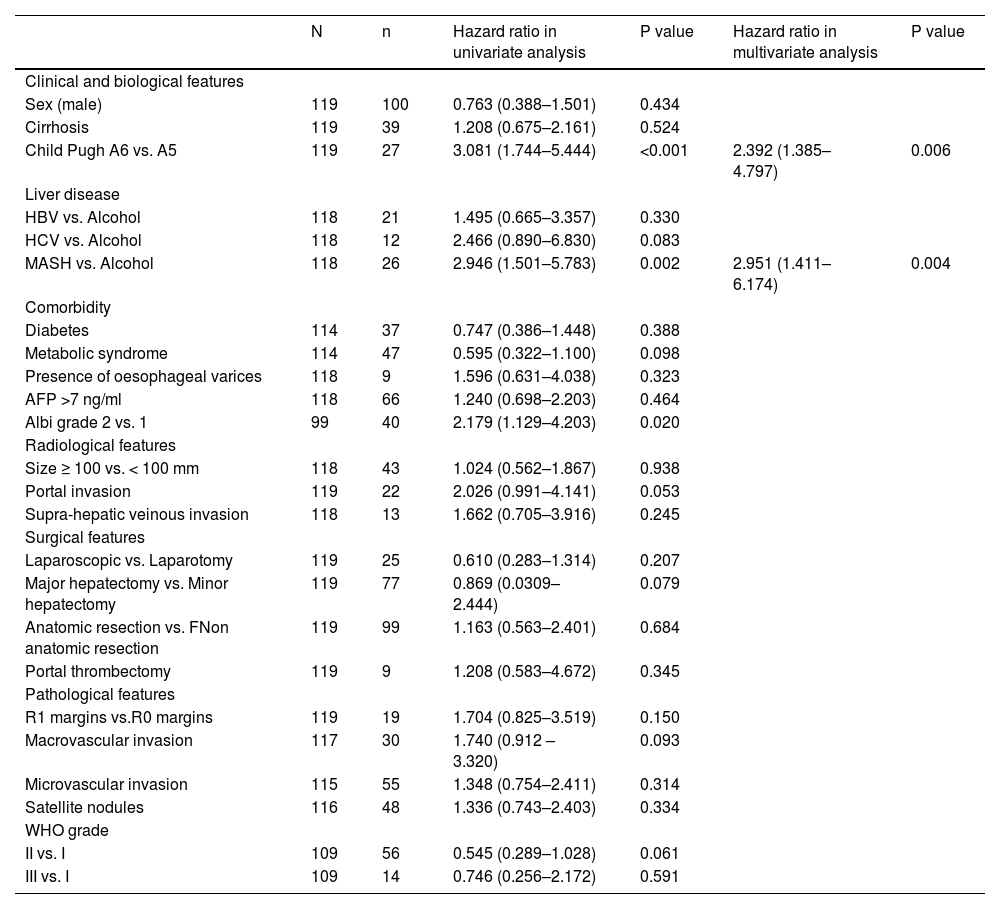
3.3Factors associated with overall survivalIn a univariate analysis (Table 3), a Child–Pugh score of A6 vs. A5 was found to be predictive of mortality (HR 3.081, p<0.001). Regarding aetiology, there was no difference between alcohol- and HBV- or HCV-related liver disease, but patients with MASH experienced more adverse outcomes than those with alcohol-related liver disease (HR 2.946, p=0.002). Overall recurrence was not associated with survival (HR = 1.436, p=0.224), but time-to-recurrence was (p=0.037); patients with early recurrence (< 24 months) had the worst prognosis (HR 2.625, p=0.004). Multivariate analysis confirmed that Child–Pugh score A6 vs. A5 (2.578 (1.385–4.797); p=0.003) and MASH versus alcohol-related liver disease (3.025 (1.438–6.363); p =0.004) was a predictor of death Table 4, Table 5.
Risk factors for mortality in univariate and multivariate analyses.
| N | n | Hazard ratio in univariate analysis | P value | Hazard ratio in multivariate analysis | P value | |
|---|---|---|---|---|---|---|
| Clinical and biological features | ||||||
| Sex (male) | 119 | 100 | 0.763 (0.388–1.501) | 0.434 | ||
| Cirrhosis | 119 | 39 | 1.208 (0.675–2.161) | 0.524 | ||
| Child Pugh A6 vs. A5 | 119 | 27 | 3.081 (1.744–5.444) | <0.001 | 2.392 (1.385–4.797) | 0.006 |
| Liver disease | ||||||
| HBV vs. Alcohol | 118 | 21 | 1.495 (0.665–3.357) | 0.330 | ||
| HCV vs. Alcohol | 118 | 12 | 2.466 (0.890–6.830) | 0.083 | ||
| MASH vs. Alcohol | 118 | 26 | 2.946 (1.501–5.783) | 0.002 | 2.951 (1.411–6.174) | 0.004 |
| Comorbidity | ||||||
| Diabetes | 114 | 37 | 0.747 (0.386–1.448) | 0.388 | ||
| Metabolic syndrome | 114 | 47 | 0.595 (0.322–1.100) | 0.098 | ||
| Presence of oesophageal varices | 118 | 9 | 1.596 (0.631–4.038) | 0.323 | ||
| AFP >7 ng/ml | 118 | 66 | 1.240 (0.698–2.203) | 0.464 | ||
| Albi grade 2 vs. 1 | 99 | 40 | 2.179 (1.129–4.203) | 0.020 | ||
| Radiological features | ||||||
| Size ≥ 100 vs. < 100 mm | 118 | 43 | 1.024 (0.562–1.867) | 0.938 | ||
| Portal invasion | 119 | 22 | 2.026 (0.991–4.141) | 0.053 | ||
| Supra-hepatic veinous invasion | 118 | 13 | 1.662 (0.705–3.916) | 0.245 | ||
| Surgical features | ||||||
| Laparoscopic vs. Laparotomy | 119 | 25 | 0.610 (0.283–1.314) | 0.207 | ||
| Major hepatectomy vs. Minor hepatectomy | 119 | 77 | 0.869 (0.0309–2.444) | 0.079 | ||
| Anatomic resection vs. FNon anatomic resection | 119 | 99 | 1.163 (0.563–2.401) | 0.684 | ||
| Portal thrombectomy | 119 | 9 | 1.208 (0.583–4.672) | 0.345 | ||
| Pathological features | ||||||
| R1 margins vs.R0 margins | 119 | 19 | 1.704 (0.825–3.519) | 0.150 | ||
| Macrovascular invasion | 117 | 30 | 1.740 (0.912 – 3.320) | 0.093 | ||
| Microvascular invasion | 115 | 55 | 1.348 (0.754–2.411) | 0.314 | ||
| Satellite nodules | 116 | 48 | 1.336 (0.743–2.403) | 0.334 | ||
| WHO grade | ||||||
| II vs. I | 109 | 56 | 0.545 (0.289–1.028) | 0.061 | ||
| III vs. I | 109 | 14 | 0.746 (0.256–2.172) | 0.591 | ||
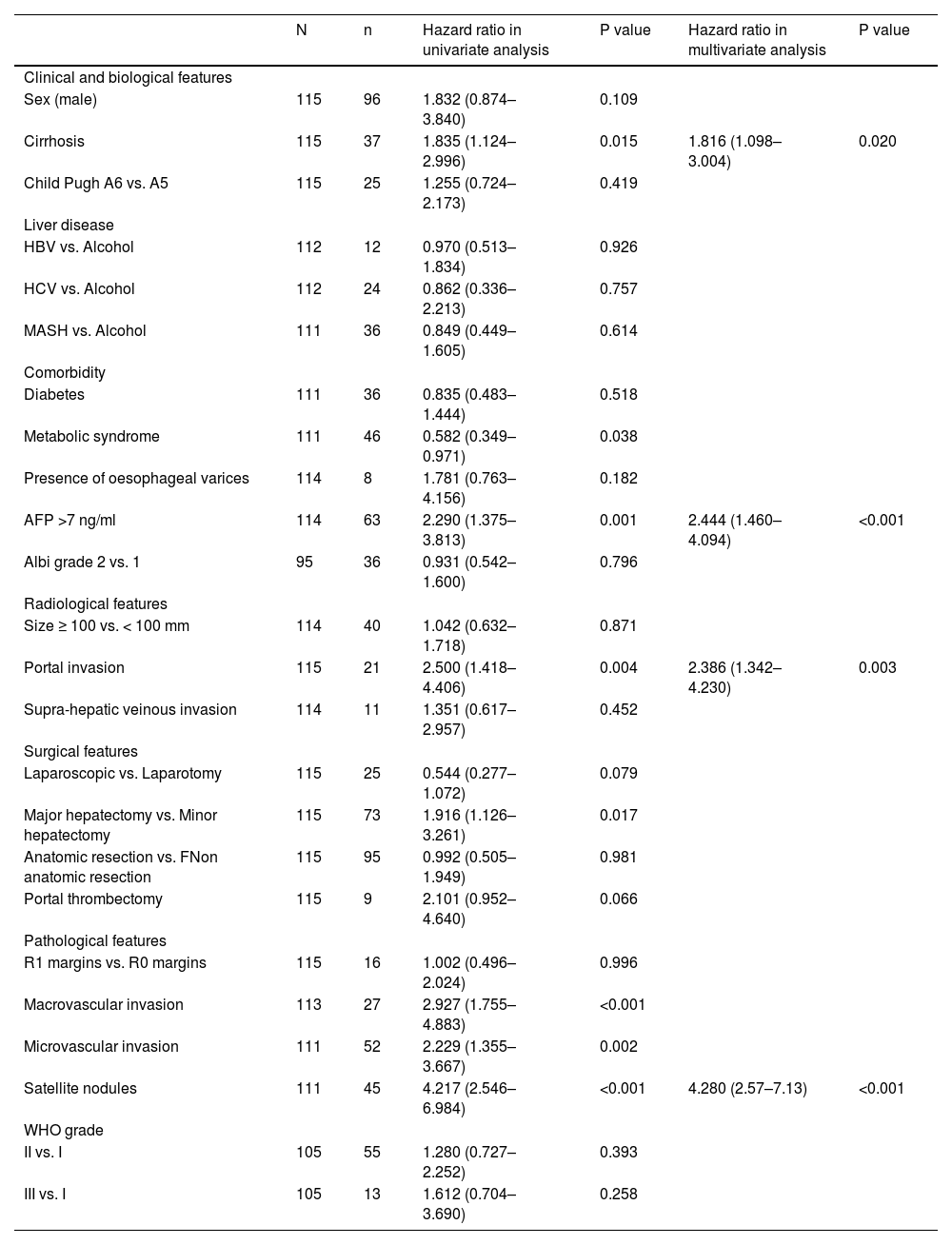
Risk factors for HCC recurrence in univariate and multivariate analyses.
| N | n | Hazard ratio in univariate analysis | P value | Hazard ratio in multivariate analysis | P value | |
|---|---|---|---|---|---|---|
| Clinical and biological features | ||||||
| Sex (male) | 115 | 96 | 1.832 (0.874–3.840) | 0.109 | ||
| Cirrhosis | 115 | 37 | 1.835 (1.124–2.996) | 0.015 | 1.816 (1.098–3.004) | 0.020 |
| Child Pugh A6 vs. A5 | 115 | 25 | 1.255 (0.724–2.173) | 0.419 | ||
| Liver disease | ||||||
| HBV vs. Alcohol | 112 | 12 | 0.970 (0.513–1.834) | 0.926 | ||
| HCV vs. Alcohol | 112 | 24 | 0.862 (0.336–2.213) | 0.757 | ||
| MASH vs. Alcohol | 111 | 36 | 0.849 (0.449–1.605) | 0.614 | ||
| Comorbidity | ||||||
| Diabetes | 111 | 36 | 0.835 (0.483–1.444) | 0.518 | ||
| Metabolic syndrome | 111 | 46 | 0.582 (0.349–0.971) | 0.038 | ||
| Presence of oesophageal varices | 114 | 8 | 1.781 (0.763–4.156) | 0.182 | ||
| AFP >7 ng/ml | 114 | 63 | 2.290 (1.375–3.813) | 0.001 | 2.444 (1.460–4.094) | <0.001 |
| Albi grade 2 vs. 1 | 95 | 36 | 0.931 (0.542–1.600) | 0.796 | ||
| Radiological features | ||||||
| Size ≥ 100 vs. < 100 mm | 114 | 40 | 1.042 (0.632–1.718) | 0.871 | ||
| Portal invasion | 115 | 21 | 2.500 (1.418–4.406) | 0.004 | 2.386 (1.342–4.230) | 0.003 |
| Supra-hepatic veinous invasion | 114 | 11 | 1.351 (0.617–2.957) | 0.452 | ||
| Surgical features | ||||||
| Laparoscopic vs. Laparotomy | 115 | 25 | 0.544 (0.277–1.072) | 0.079 | ||
| Major hepatectomy vs. Minor hepatectomy | 115 | 73 | 1.916 (1.126–3.261) | 0.017 | ||
| Anatomic resection vs. FNon anatomic resection | 115 | 95 | 0.992 (0.505–1.949) | 0.981 | ||
| Portal thrombectomy | 115 | 9 | 2.101 (0.952–4.640) | 0.066 | ||
| Pathological features | ||||||
| R1 margins vs. R0 margins | 115 | 16 | 1.002 (0.496–2.024) | 0.996 | ||
| Macrovascular invasion | 113 | 27 | 2.927 (1.755–4.883) | <0.001 | ||
| Microvascular invasion | 111 | 52 | 2.229 (1.355–3.667) | 0.002 | ||
| Satellite nodules | 111 | 45 | 4.217 (2.546–6.984) | <0.001 | 4.280 (2.57–7.13) | <0.001 |
| WHO grade | ||||||
| II vs. I | 105 | 55 | 1.280 (0.727–2.252) | 0.393 | ||
| III vs. I | 105 | 13 | 1.612 (0.704–3.690) | 0.258 | ||
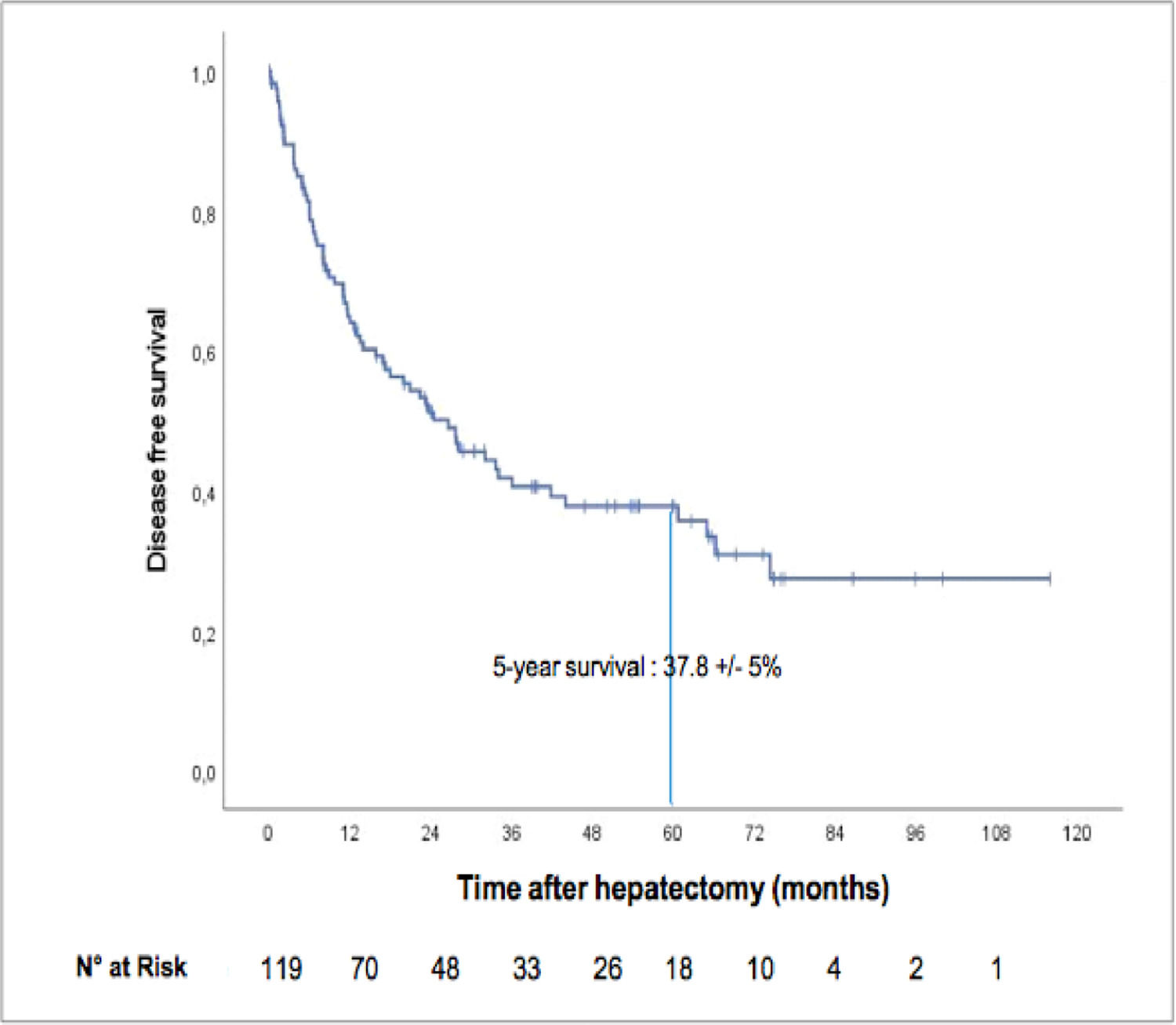
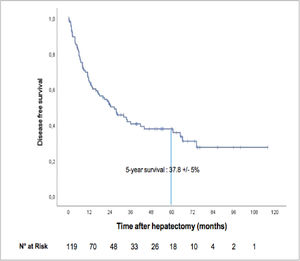
The median recurrence-free survival was 26.6 months (95% CI: 16.0–37.1), and the five-year recurrence-free survival rate was 37.8± 5% ( Fig. 3). A total of 68 patients (57.1%) relapsed, among whom 28 died Fig. 4.
3.5Factors associated with recurrence-free survivalPostoperative mortality, occurring in the first 90 days as described in the Dindo-Clavien classification (30), affected four patients. Those were excluded from the recurrence analysis.
In a univariate analysis, several factors were predictive of relapse: cirrhosis (p=0.015), metabolic syndrome (p=0.038), portal vein tumoral thrombus (p= 0.004), histological macrovascular (p<0.001) and microvascular invasion (p=0.002), presence of satellite nodules (p<0.001), and major hepatectomy (p=0.017) (Table 3). A baseline AFP > 7 ng/mL (p=0.001) was strongly associated with recurrence (p=0.001). The threshold of 7 ng/mL was chosen according to the best Youden index (Se = 69.1%, Sp = 57.4%, AUC = 0.629 (IC95%: 0.525–0.732), p=0.019). Tumour size was not predictive of relapse, including tumours >10 cm.
In a multivariate analysis, three preoperative factors remained associated with recurrence: baseline AFP > 7 ng/mL (HR 2.44, p<0.001), portal veinous invasion on baseline CT scan or MRI (HR 2.39, p=0.003) and cirrhosis (HR 1.86, p=0.020).
Only one postoperative factor was associated with recurrence: the presence of satellite nodules (HR 4.280 (2.57–7.13), p <0.001).
3.6Relationship between preoperative and postoperative predictive factorsA baseline AFP > 7 ng/mL was associated with microvascular invasion (p<0.001), satellite nodules (p<0.001), and WHO tumour grade (p<0.001). Portal veinous invasion was associated with a major hepatectomy (p<0.001), macrovascular invasion (p<0.001), microvascular invasion (p<0.001) and satellite nodules (p<0.001). Cirrhosis had no association with any postoperative predictive factor.
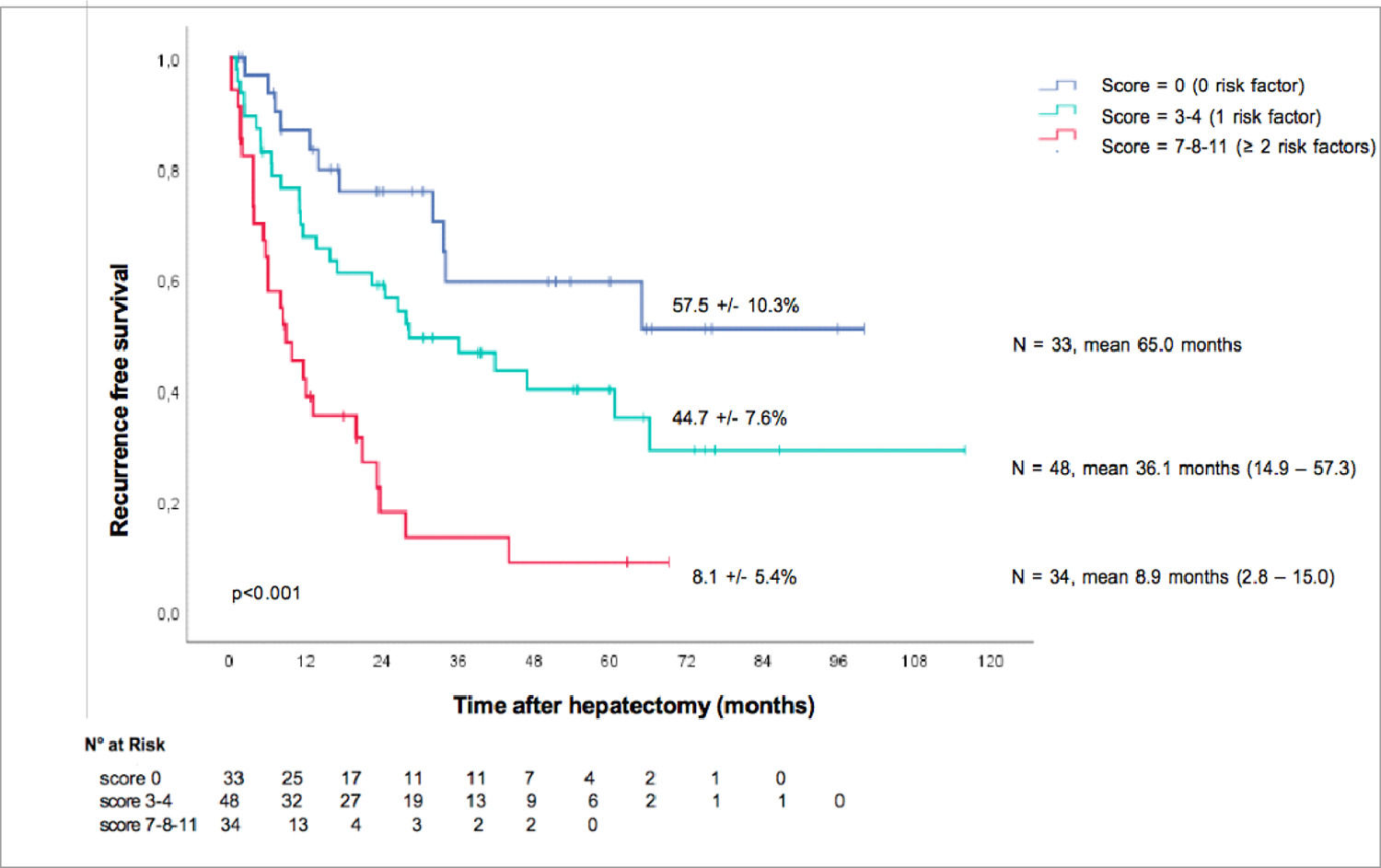
3.7Preoperative prognosis score for recurrenceOnce we identified those three preoperative risk factors for relapse, their beta coefficient was multiplied by four to obtain a simple three-item recurrence risk scoring system that was able to classify three groups with significantly different disease-free survival medians (p<0.001).
A baseline AFP > 7 ng/mL counted for four points, portal veinous invasion counted for four points, and cirrhosis counted for three points. The three groups were divided by score as follows: score = 0, score =1, and score ≥ 2.
This allowed us to propose an easy scoring system: the absence of risk factors corresponded to the low-risk group (median 65.0 months), one risk factor corresponded to the intermediate-risk group (median 36.1 months) and ≥ 2 risk factors corresponded to the high-risk group (median 8.9 months).
4DiscussionWe retrospectively analysed data from patients with single large HCC who underwent liver resection in 2 tertiary academic French centres. The median overall survival was 72.5 months, and the five-year overall survival was 55.1%, which is on the high end of typical figures [9–13].
This study, to our knowledge, is one of the largest European cohorts of HCC > 5 cm undergoing surgery and confirms in a Western population that liver resection can have acceptable results in large HCC. The majority of the literature on this subject relates to Asian countries, where liver disease is vastly associated with HBV infection [7,18,19,22]. Our cohort matches a more European repartition of causes, with a predominance of alcohol-related liver disease and an increasing fraction of MASH [23].
One-third of patients had no history of liver disease, and 49% had no to low-grade fibrosis (F0-2). This may be expected in a surgical cohort because large tumours might require major hepatectomy, which is mostly impossible in cirrhosis. It is well known that in those patients, liver resection is the treatment of choice because there is little to no risk of liver failure [8]. Furthermore, as those patients did not benefit from HCC screening, tumours are more likely to be diagnosed at a symptomatic stage, hence a more advanced stage.
Recurrence is the primary complication of liver resection for large HCC, and 57% of our cohort experienced recurrence, which is slightly less than typical estimates [16]. Almost 80% occurred during the first 24 months. Overall recurrence was not associated with overall survival. This might be explained by smaller recurrences leading to the use of other curative therapies, such as RFA or LT. Early (< 2 years) and late (> 2 years) recurrences are most likely different issues with different mechanisms. Indeed, it has been previously shown that early recurrence is associated with what could be called metastatic factors (microvascular invasion and elevated AFP), whereas “de novo” tumour recurrence is more related to the persistence of carcinogenic factors (hepatitis activity, cirrhosis) [20]. In our study, the only factor associated with early phase recurrence was indeed abnormal AFP levels, which were also associated with microvascular invasion and satellite nodules, supporting the plausible reflection of metastatic aggressiveness.
High blood pressure and metabolic syndrome were associated with recurrence. They did not reach significance in the multivariate analysis; nonetheless, this result supports the growing role of the nonalcoholic fatty liver disease (NAFLD) spectrum in HCC development [24].
Cirrhosis, AFP > 7 ng/mL and portal invasion were the three preoperative factors associated with recurrence in the multivariate analysis. Cirrhosis is well established to influence prognosis after liver resection [20]. In contrast, serum AFP levels are a controversial topic regarding their prognostic value, and some authors have suggested that their predictive ability notably depends on treatment strategy and tumour size; it has been proposed that if it was a weak prognostic tool for small tumours and nonsurgical strategy, its best use would rely on surgery and large tumours [25]. In our study, 22 (18.3%) patients presented with portal invasion, also called portal vein tumour thrombus (PVTT), with a majority of Vp3 (13.3%). Surgical thrombectomy was performed in 12 cases. PVTT intrinsically is a metastatic process and thereby has an obvious relation to recurrence. HCC with major PVTT is classified as BCLC C and hence considered intractable with respect to cure according to European recommendations [3]. Surgical resection, in addition to multiple interdisciplinary treatments, such as transarterial chemoembolization, hepatic arterial infusion of chemotherapy, and radiation, has been proposed on a trial-and-error basis, but the optimal therapy has yet to be defined [26]. The natural history of untreated HCC with portal invasion is very grim, with a reported survival median of fewer than three months [27]. In the latest Japanese nationwide follow-up survey of primary liver cancer, among 30 300 patients surgically treated for HCC with portal invasion graded Vp0, Vp1, Vp2, Vp3 and Vp4, the five-year cumulative survival rates were 67.5, 50.5, 35.7, 30.5 and 17.1%, respectively [28]. Recently, Govalan et al. reported the superiority of surgical resection over other treatments for patients with vascular invasion based on data from the National Cancer Database (NCDB) in the US. Surgical resection was associated with improved survival compared to systemic therapies (adjusted hazard ratio: 0.496, 95% confidence interval: 0.426–0.578), with a median survival of 21 months for the former versus 8.1 months for the latter.
Interestingly, size was not predictive of relapse or mortality, including tumours > 10 cm. This result is consistent with previous studies, where sizes above the threshold of 5 cm no longer appeared to affect postsurgical outcomes [29]. This can seem paradoxical, as microvascular invasion and satellite nodules are widely admitted to be prognostic markers of negative outcomes [29–32], and their frequency tends to increase with tumour size [29]. This might suggest that tumour size does not independently influence the survival of patients with large HCC but is among the important cofounding factors, as it has been reported that microvascular invasion and satellite nodules increase above the threshold of 2 cm [33].
There was no negative impact of R1 resection on overall or recurrence-free survival. This additional counterintuitive result is not new to the literature; anatomical resection, negative margins and its required width are controversial. Authors have even suggested that tumour exposure does not impact prognosis [34] and that it should even be preferred when the tumour is in contact with a major vessel [35], as it is quite established that minimizing liver excision and therefore conserving maximal residual liver volume is much more important for prognosis than surgical margin width [36].
Because the patients benefited from large tumour surgery (the mean number of resected segments was 3), a vast majority were Child–Pugh A5. Additionally, the median bilirubin and platelet counts were normal (9 μmol/L and 214 G/L, respectively). This might partially explain the satisfying outcomes, since it is widely accepted that liver function is the mainstay of short- and long-term postoperative results in HCC surgery [32,37].
We developed a preoperative recurrence prognosis score that is truly user-friendly since it leverages only three easy-to-access clinical (cirrhosis), radiological (portal vein invasion) and biological (AFP > 7 ng/mL) data, with no need for calculation. It can be used by the clinician to conveniently evaluate recurrence risk at the patient's bedside and therefore adapt care and follow-up accordingly. In addition, since immunotherapy is becoming a proper part of the therapeutic arsenal for HCC, this simple score could help identify patients who could benefit from future (neo)adjuvant therapies [38].
Our study has several limitations. Due to its retrospective nature, selection bias exists in this work. We tried to preclude this issue with a good sample size and the use of 2 centres. Due to patients being lost to follow-up, we might have underestimated recurrence, especially in the late phase. In that sense, we excluded the 4 patients who died relapse-free during the first 3 months from the recurrence analysis. Finally, our prognostic score needs further external validation to confirm its reliability.
5ConclusionLiver resection is the only curative option for single large HCC, and we confirmed that it could be associated with satisfying long-term outcomes in experienced centres.
Recurrence is the primary complication of surgery, and we proposed a simple preoperative score that efficiently predicts relapse risk, helping to identify candidates for closer follow-up or combined therapy. In this era of advanced systemic therapies using molecularly targeted agents and immuno-check point inhibitors, a combination of promising systemic therapy and surgery may be a future path to improve survival in selected patients.
Author contributionsGA and DS: the conception and design of the study. VNK, RB, RR, AL: acquisition of data. VNK, GA, DS, RB and FRT: analysis and interpretation of data. VNK, GA and DS: drafting the article. HR, AS, SM, CD, VL, JC, AL: revising the article critically for important intellectual content. All authors: final approval of the version to be submitted.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
None.