The lockdown policy introduced in 2020 to minimize the spread of the COVID-19 pandemic, significantly affected the management and care of patients affected by hepatocellular carcinoma (HCC). The aim of this follow-up study was to determine the 12 months impact of the COVID-19 pandemic on the cohort of patients affected by HCC during the lockdown, within six French academic referral centers in the metropolitan area of Paris.
Materials and MethodsWe performed a 12 months follow-up of the cross-sectional study cohort included in 2020 on the management of patients affected by HCC during the first six weeks of the COVID-19 pandemic (exposed), compared to the same period in 2019 (unexposed). Overall survival were compared between the groups. Predictors of mortality were analysed with Cox regression.
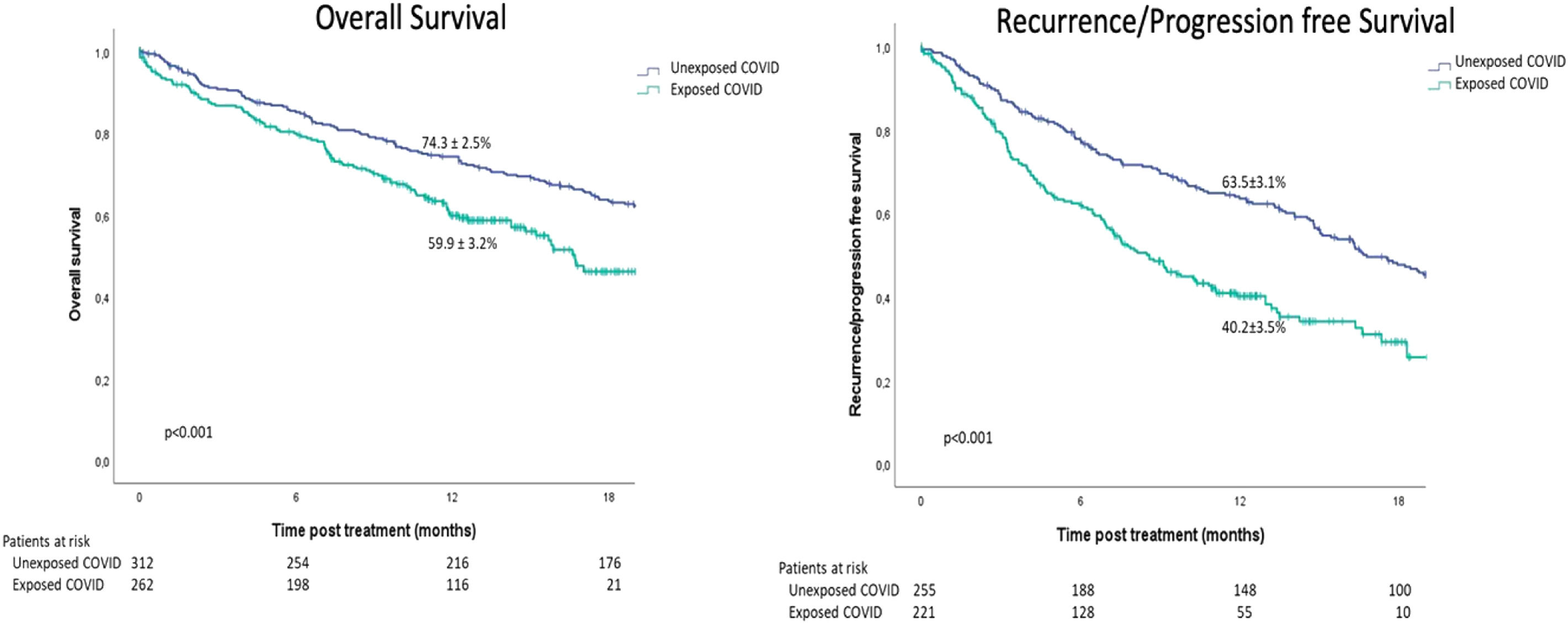
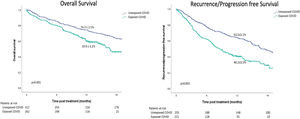
ResultsFrom the initial cohort, 575 patients were included (n = 263 Exposed_COVID, n = 312 Unexposed_COVID). Overall and disease free survival at 12 months were 59.9 ± 3.2% vs. 74.3 ± 2.5% (p<0.001) and 40.2 ± 3.5% vs. 63.5 ± 3.1% (p<0.001) according to the period of exposure (Exposed_COVID vs. Unexposed_COVID, respectively). Adjusted Cox regression revealed that the period of exposure (Exposed_COVID HR: 1.79, 95%CI (1.36, 2.35) p<0.001) and BCLC stage B, C and D (BCLC B HR: 1.82, 95%CI (1.07, 3.08) p = 0.027 - BCLC C HR: 1.96, 95%CI (1.14, 3.38) p = 0.015 - BCLC D HR: 3.21, 95%CI (1.76, 5.85) p<0.001) were predictors of death.
ConclusionsDisruption of routine healthcare services because of the pandemic translated to reduced 1 year overall and disease-free survival among patients affected by HCC, in the metropolitan area of Paris, France.
A lockdown policy was introduced in Europe on March 2020 as a strategy to minimize the spread of the COVID-19 pandemic, thus affecting the management and care of cancer patients [1].
Lockdown measures, including the suspension of routine screenings and limited access to healthcare facilities, led to delayed cancer diagnoses across Europe [2,3]. Patients with symptoms or those requiring routine screenings [4], including those with hepatocellular carcinoma (HCC), experienced delays in diagnosis, potentially resulting in the progression of their condition [2,3].
Many hospitals had to prioritize COVID-19 patients and reduce non-urgent procedures, which caused delays or cancellations for cancer treatments such as surgeries, radiation therapy, and chemotherapy [5,6]. To mitigate the impact of social distancing measures, healthcare providers adopted telemedicine and remote care solutions [7,8]. While these initiatives facilitated patient consultations and follow-ups, they had limited impact on monitoring complex cancer cases and were not suitable for delivering interventional treatments such as surgery or interventional radiology. It is important to note that lockdown policies and access to care varied across European regions [9], leading to disparities in cancer care.
Taken altogether, the delays in diagnosis, screening and treatment might substantially have increased the number of avoidable deaths [3]. Despite the guidelines suggested by the European Association for the Study of the Liver (EASL) [7] and National French authorities [10–12] to maintain the management and care of patients affected by HCC, a significantly longer treatment delay in 2020 was observed in the highly-impacted metropolitan area of Paris compared to 2019 [13].
This follow-up study represents the 12 months update of the multicenter cohort of patients affected by HCC during the COVID-19 lockdown in the metropolitan area of Paris [13].
2Materials and MethodsThis study represents the updated 12 months follow-up (2022) of the multicenter, cross-sectional study [13] on the management of patients affected by HCC during the first six weeks of lockdown due to the COVID-19 pandemic (2020), compared to the same period in 2019, within the metropolitan area of Paris.
Six academic referral centers from the AP-HP network (Pitié Salpêtrière-Paris, Saint-Antoine-Paris, Cochin-Paris, Beaujon-Clichy, Jean-Verdier-Bondy), including the steering committee (Hôpital Henri Mondor, Créteil) were involved in the study.
The study was led in compliance with STROBE guidelines for cross-sectional studies [14].
2.1Inclusion criteriaAny adult patient (>18 y old) affected by HCC (diagnosis according to the EASL criteria [15]), who received during the inclusion period a) proposal of treatment in multi-disciplinary tumor board (MTB) meetings, or b) a programmed surgical or radiological procedure (liver resection – LR, interventional radiology procedure -IR procedure as percutaneous ablation, Trans-arterial-chemo-embolization - TACE, Selective internal radiation therapy - SIRT).
To be noted, the treatment for each patient was decided on the basis of the EASL guidelines [15], ad tailored to the national recommendations for the management of patients affected by liver disease [10] or requiring surgery [11,12,16] during the pandemic. Given the heavy impact of the outbreak on the metropolitan area of Paris and according to the aforementioned recommendations, patients with HCC and asymptomatic COVID-19 could be rescheduled until a negative swab was observed. In case of respiratory symptoms, interventional procedures could be downgraded, or rescheduled after 6–8 weeks.
2.2Study periodDuring the previous published [13] cross-sectional study, patients exposed to the pandemic between March 6th to April 17th, 2020 were considered as cases (Exposed_COVID), and those fulfilling the same inclusion criteria between March 6th to April 17th, 2019 (Unexposed_COVID) were considered as controls.
Both the groups were regularly followed for 18 months and censored at 12 months for the purpose of the present study.
2.3Study endpointsThe primary objective was to compare the overall survival (OS) rates between the two groups (Exposed_COVID and Unexposed_COVID) at 12 months. Variables required to measure the primary endpoint were the event (death) and time until the event (OS).
Secondary objectives were to compare survival at 12 and 18 months according to BCLC stage and treatment strategy between the two groups, as well as predictors for mortality.
2.4VariablesThe same variables analyzed during the cross-sectional study [13] were considered, including general demographic, underlying liver disease, HCC characteristics, BCLC staging, and clinical management proposed within MTB meetings, type of curative treatment realized (surgery or percutaneous ablation by IR) as well as those with palliative intent (systemic therapies, external radiotherapy, TACE, SIRT), best supportive care, date of latest news and survival status. The quality of data management was compliant with the reference methodology on personal data processing and protection (MR004), as stated by the French data protection authority (Commission Nationale de l'Informatique et des Libertés, CNIL n 2,209,983 v 0).
2.5Sample sizeFrom the original cohort of 670 patients previously published [13], 95 (14%) were excluded because lost at follow-up (n = 30 Exposed_COVID and n = 65 Unexposed_COVID): 575 patients were included (n = 263 Exposed_COVID, n = 312 Unexposed_COVID), and followed during 18 months.
2.6Statistical analysisCategorical variables were reported as percentages, while continuous variables were summarized as means and standard deviation (SD) or median and range for discrete variables, as appropriate. The Student's t-test or Mann-Whitney U test were used for comparisons of quantitative variables as appropriate, whereas a χ² test or Fisher's exact test was used to compare categorical data.
Kaplan–Meier curves for OS were created, with two strata corresponding to both groups (Exposed_COVID and Unexposed_COVID).
Unadjusted hazard ratios (HRs) and 95% confidence intervals (95% CI) were calculated by Cox proportional hazards regression analysis for variables associated with death. Variables with a p-value <0.1 (as well as those considered clinically relevant) were entered into a multivariate Cox model to identify factors independently associated with death. The final model expressed the adjusted HRs and 95%CI. No multiple imputations were used. A p-value ≤0.05 was considered significant.
All analyses were performed using IBM SPSS V26 and Stata V13.0 (StataCorp, College Station, Texas).
2.7Ethical statementWritten informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethics Committee of Henri-Mondor Institutional Review Board (Ethics number committee 00,011,558, Approval Number 2020–071), and led in compliance with Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cross-sectional studies.
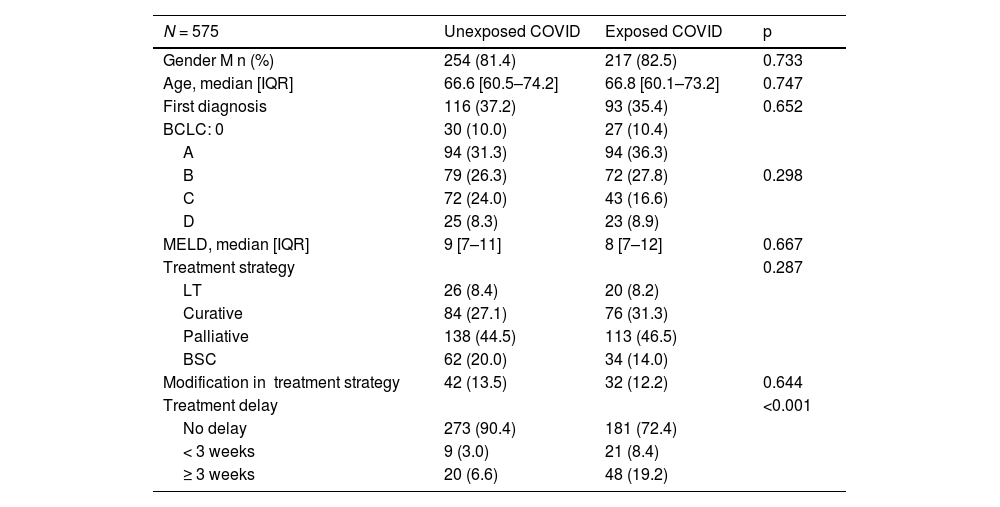
3Results3.1General characteristicsIn terms of gender distribution, males accounted for 81.4% of the population studied. No significant differences were observed between the two periods in terms of patients' characteristics (age, BCLC, MELD), and the therapeutic strategy proposed and realized. However, a significantly higher percentage of patients who were exposed to the pandemic experienced a treatment delay of more than three weeks (19.2%, n = 48 in the Exposed_COVID group compared to 6.6%, n = 20 in the Unexposed_COVID group, respectively; p<0.001) (Table 1).
Clinical and demographic characteristics of patients included in the study.
M: male; IQR: Inter quartile range; LT: Liver transplantation; BSC: best supportive care.
In the entire cohort and based on the period of exposure (Exposed_COVID vs. Unexposed_COVID), the Kaplan-Meier estimation of overall survival (OS) at 12 and 18 months was 59.9 ± 3.2% and 46.2 ± 4.3% compared to 74.3 ± 2.5% and 63.4 ± 2.8%, respectively (p < 0.001; Fig. 1). Similarly, the Kaplan-Meier estimation of progression-free survival (PFS) at 12 and 18 months was 40.2 ± 3.5% and 29.2 ± 4.2% versus 63.5 ± 3.1% and 47.6 ± 3.3% (p < 0.001) for the same period of exposure (Fig. 1).
A difference in the 12-month OS estimation was observed between patients undergoing treatment with a curative intent (66.4 ± 5.0% vs. 82.3 ± 3.7%; p = 0.005) and those receiving palliative care (58.9 ± 4.8% vs. 72.7 ± 3.9%; p = 0.017), according to the period of exposure (Exposed_COVID vs. Unexposed_COVID, respectively).
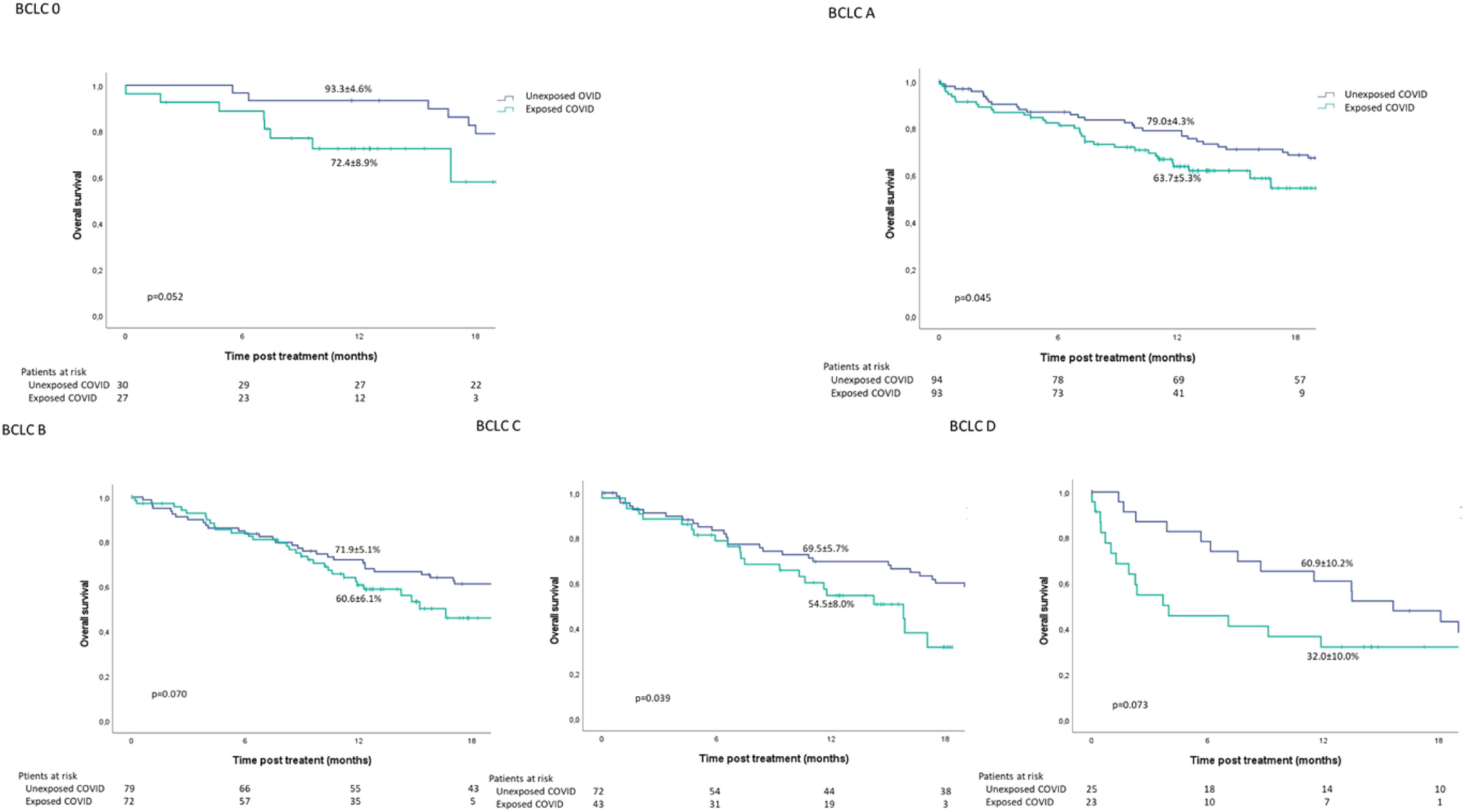
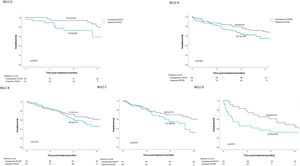
Based on the BCLC stage and period of exposure (Exposed_COVID vs. Unexposed_COVID), a difference in the Kaplan-Meier estimation of OS at 12 months was observed in the BCLC A (63.7 ± 5.3% vs. 79.0 ± 4.3%; p = 0.045) and BCLC C (54.5 ± 8.0% vs. 69.5 ± 5.7%; p = 0.039) subgroups (Fig. 2).
No significant difference in survival was observed among the BCLC 0, BCLC B, and BCLC D subgroups.
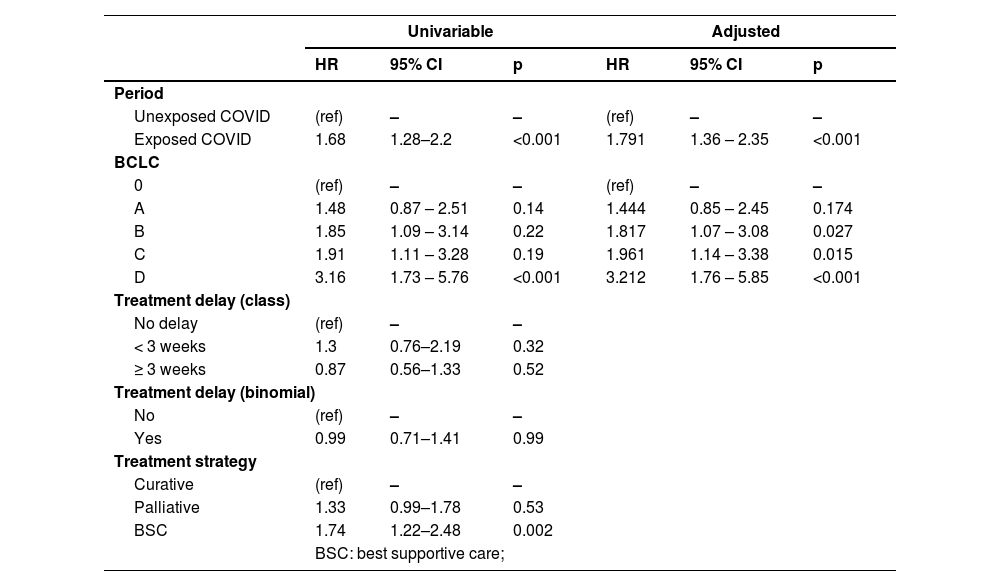
3.3Predictors of mortalityCox model was used to analyze the variables "period of exposure" (Exposed_COVID vs. Unexposed_COVID), "delay in treatment" (binomial Y/N or factorial based on the classes of delay), and "BCLC stage" (factor) to identify predictors of death at 12 months.
The multivariable analysis identified the period of exposure (Exposed_COVID HR: 1.79, 95%CI 1.36, 2.35; p < 0.001) and BCLC stage B, C, and D (BCLC B HR: 1.82, 95%CI 1.07, 3.08; p = 0.027 - BCLC C HR: 1.96, 95%CI 1.14, 3.38; p = 0.015 - BCLC D HR: 3.21, 95%CI 1.76, 5.85; p < 0.001) as predictors of mortality (Table 2).
Results of univariable and multivariable Cox analysis of predictors of mortality.
The worldwide impact of the COVID-19 pandemic on the management and care of patients with liver cancer has been observed, affecting screening, diagnosis, and treatment processes [17,18]. In Paris, France, similar findings were reported, with a higher proportion of HCC patients experiencing larger tumor burden and longer delays between MTB (Multidisciplinary Tumor Board) and treatment during the pandemic compared to 2019 [13]. The primary reason cited was the shortage of surgical theaters and staff, including nurses and physicians, due to their redeployment to intensive care units [18].
In this follow-up study, it was found that HCC patients exposed to the COVID-19 pandemic had significantly lower rates of overall survival and disease-free survival at 12 months compared to control groups who were unexposed to COVID-19, regardless of the treatment proposed.
When considering the BCLC stage, a lower survival rate at 12 months was observed among patients in BCLC A and BCLC C. The reasons for this were explored in a previous report [13] which revealed that patients exposed to the COVID-19 pandemic experienced a significantly longer interval between MTB and treatment. In the case of BCLC C patients, they had a larger tumor burden, impaired clinical condition, and compromised liver function, leading to a shift towards palliative treatment.
The COX multivariable analysis suggested that BCLC stages B, C and D, and exposure to the COVID-19 pandemic were significantly associated with mortality. The association between BCLC stage and mortality is well documented, and this observation is therefore not surprising. However, the association between pandemic exposure and mortality is likely due to increased delays in MTB-to-treatment and the downgrading of "severe" patients (those with significant disease progression or requiring intensive care) to palliative treatment or best supportive care due to restricted access to ICU during the pandemic.
This study has limitations, including the short-term follow-up period of 12 months and the localized geographical area of the study. Nationwide studies could reveal heterogeneity in survival rates based on regional differences (metropolitan vs. rural areas), hospital characteristics (general hospitals, tertiary or academic centers), and access to care.
5ConclusionsThe reduced early survival (both overall and disease-free) among a homogeneous population of HCC patients within a network of six academic French centers in the metropolitan area of Paris during the 2020 pandemic highlights the need for better prioritization of HCC treatment services in future pandemics. Considering the observed increase in 12-month mortality across the patient cohort, irrespective of treatment delays, long-term follow-up of this cohort would be valuable.
Author contributionsGA and RB conceived and organized the study, planned the data management, organized data collection for the steering center and contributed to the statistical analyses, wrote the manuscript and figures, gave their final approval of the manuscript. JCN, MA, ML, CH, HR, MB organized data collection for each participating center, significantly contributed to the manuscript and gave the final approval before submission. FRT planned and realized the statistical analyses, significantly contributed to the manuscript and gave their final approval before submission. All the remaining Authors revised the manuscript and gave their final approval before submission.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Paris Liver Cancer Group Olivier Sutter, Pierre Nahon, Marianne Ziol, Julien Calderaro, Alexis Laurent, Ariane Mallat, Julien Calderaro, Christophe Duvoux, Vania Tacher, Vincent Mallet, Sebastien Gaujoux, Philippe Soyer, Benoit Terris, Olivier Soubrane, Maxime Ronot, Valérie Paradis, Lionel Arrivé, Dominique Wandum, Mathilde Wagner, Claire Goumard, Eric Savier.