Selective internal radiation therapy (SIRT) has emerged as a viable endovascular treatment strategy for hepatocellular carcinoma (HCC). According to the Barcelona Clinic Liver Cancer (BCLC) classification, SIRT is currently recommended for early- and intermediate-stage HCC that is unsuitable for alternative locoregional therapies. Additionally, SIRT remains a recommended treatment for patients with advanced-stage HCC and portal vein thrombosis (PVT) without extrahepatic metastasis. Several studies have shown that SIRT is a versatile and promising treatment with a wide range of applications. Consequently, given its favourable characteristics in various scenarios, SIRT could be an encouraging treatment option for patients with HCC across different BCLC stages. Over the past decade, an increasing number of studies have focused on better understanding the prognostic factors associated with SIRT to identify patients who derive the most benefit from this treatment or to refine the optimal technical procedures of SIRT. Several variables can influence treatment decisions, with a growing emphasis on a personalised approach. This review, based on the literature, will focus on the prognostic factors associated with the effectiveness of radioembolization and related complications. By comprehensively analysing these factors, we aimed to provide a clearer understanding of how to optimise the use of SIRT in managing HCC patients, thereby enhancing outcomes across various clinical scenarios.
Selective internal radiation therapy (SIRT) is a locoregional radiological treatment involving the transarterial infusion of radioactive substances or microspheres loaded with a ß-emitting isotope, such as yttrium-90 (90Y), into the tumour-bearing area through branches of the hepatic artery. This procedure, also known as transarterial radioembolization (TARE), is part of the broad spectrum of hepatocellular carcinoma (HCC) treatments [1]. According to the latest strategy proposed by the Barcelona Clinic Liver Cancer (BCLC) guidelines, SIRT can be considered an effective treatment option for patients in the early stages of HCC with a tumour size of 8 cm or less to prevent tumour progression in liver transplantation (LT) waiting list patients or when ablation/resection is not suitable. Furthermore, SIRT continues to be recommended for the treatment of patients with advanced-stage HCC (BCLC C) and portal vein thrombosis (PVT) without extrahepatic metastasis [2]. Several studies have demonstrated that SIRT could be a promising treatment option across all BCLC stages due to its favourable safety profile and local tumour control [3,4]. However, understanding how certain factors can predict treatment effectiveness is essential for identifying the HCC patients most likely to benefit from radioembolization as a therapeutic approach. This concept is significant for developing a new multiparametric therapeutic hierarchical strategy for the multidisciplinary management of HCC patients, in which all variables influencing treatment decisions are considered to adopt a specific personalised approach [5]. Therefore, we provide an update on the prognostic factors of SIRT and its promising future for treating patients with unresectable HCC.
2Selecting HCC patients for radioembolization: how can suitable candidates be identified for treatment?Several factors need to be considered before treating HCC patients with SIRT, including the tumour burden, patient performance status (PS), liver function, and goal of treatment (curative or palliative intent) (Fig. 1). All patients scheduled for SIRT should undergo a thorough medical history, physical examinations, laboratory tests, diagnostic imaging, and arteriography/macroaggregated albumin (MAA) lung shunt analysis. Thus, it is crucial to understand both the clinical and technical contraindications associated with this treatment. Contraindications for SIRT include a Child–Pugh (CP) score exceeding B7, bilirubin levels above 35 µmol/L, compromised renal function, the presence of clinical ascites, main portal vein tumour invasion (VP4), pregnancy, breastfeeding, hepatopulmonary shunting exceeding 20 %, or a digestive shunt that cannot be embolised. Additionally, while a high liver tumour burden (>50 %) or significant extrahepatic disease are not direct contraindications to SIRT, they are often associated with poorer treatment outcomes [6–8].
Therefore, the correct selection of HCC patients who can benefit from SIRT is a fundamental step. In addition to specific contraindications, certain clinical considerations must be made to stratify HCC patients at low or high risk for disease progression or reduced survival after radioembolization. Several factors capable of influencing the prognosis of HCC patients undergoing SIRT have been identified (Fig. 2).
Schematic illustration of prognostic factors contributing to outcomes in patients with HCC undergoing SIRT.
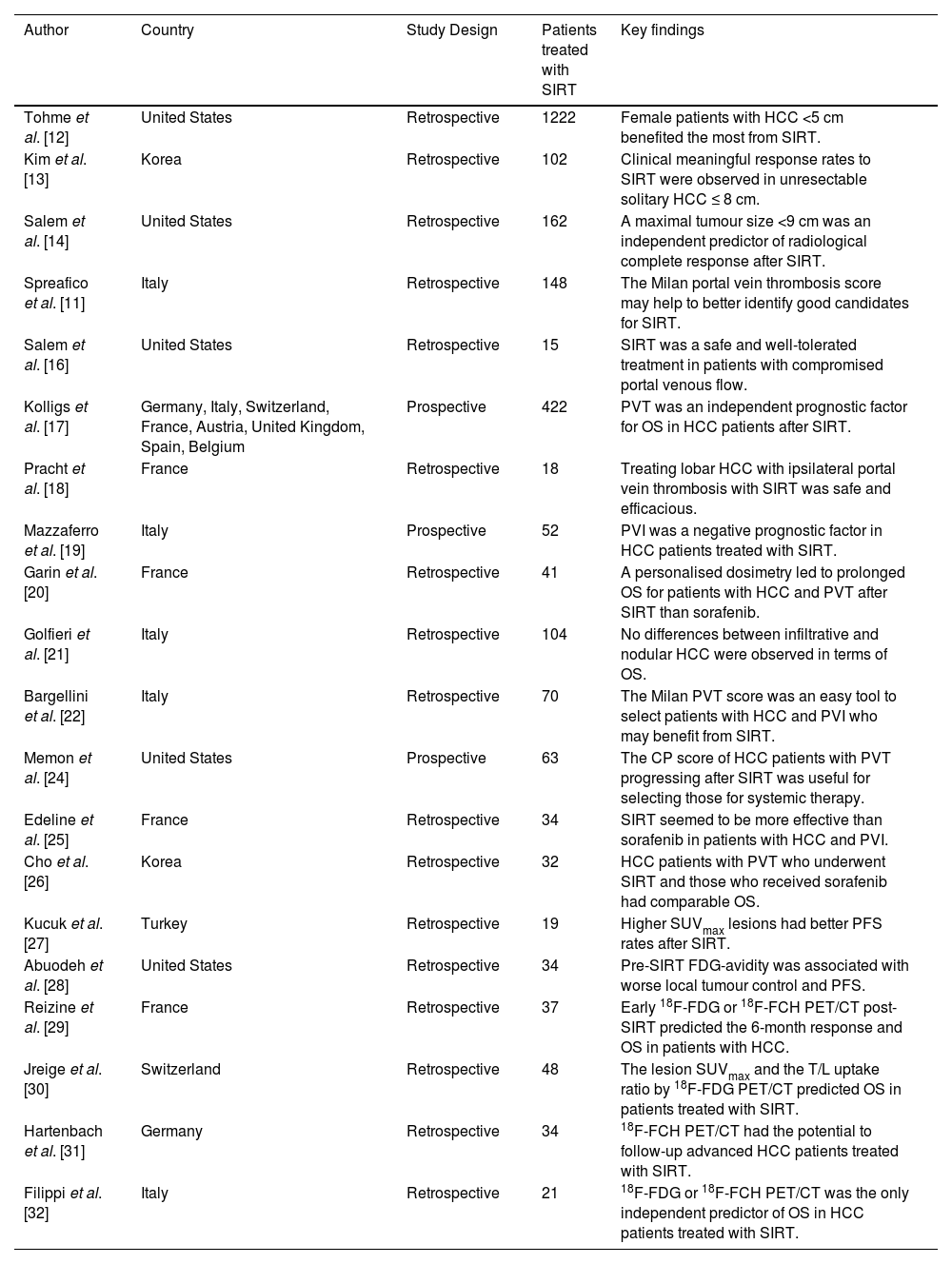
LT, surgical resection, and ablation are considered curative treatment options for patients with solitary HCC. However, these methods may not always be feasible or readily accessible. In such cases, transarterial treatments such as radioembolization can provide alternative therapeutic approaches [9]. Therefore, the adoption of SIRT as a treatment option for patients with unresectable HCC is steadily increasing. Nevertheless, its substantial influence on survival outcomes is subject to several prognostic factors, such as those related to radiological aspects (Table 1) [10].
Studies investigating radiological prognostic factors in unresectable HCC patients undergoing radioembolization.
| Author | Country | Study Design | Patients treated with SIRT | Key findings |
|---|---|---|---|---|
| Tohme et al. [12] | United States | Retrospective | 1222 | Female patients with HCC <5 cm benefited the most from SIRT. |
| Kim et al. [13] | Korea | Retrospective | 102 | Clinical meaningful response rates to SIRT were observed in unresectable solitary HCC ≤ 8 cm. |
| Salem et al. [14] | United States | Retrospective | 162 | A maximal tumour size <9 cm was an independent predictor of radiological complete response after SIRT. |
| Spreafico et al. [11] | Italy | Retrospective | 148 | The Milan portal vein thrombosis score may help to better identify good candidates for SIRT. |
| Salem et al. [16] | United States | Retrospective | 15 | SIRT was a safe and well-tolerated treatment in patients with compromised portal venous flow. |
| Kolligs et al. [17] | Germany, Italy, Switzerland, France, Austria, United Kingdom, Spain, Belgium | Prospective | 422 | PVT was an independent prognostic factor for OS in HCC patients after SIRT. |
| Pracht et al. [18] | France | Retrospective | 18 | Treating lobar HCC with ipsilateral portal vein thrombosis with SIRT was safe and efficacious. |
| Mazzaferro et al. [19] | Italy | Prospective | 52 | PVI was a negative prognostic factor in HCC patients treated with SIRT. |
| Garin et al. [20] | France | Retrospective | 41 | A personalised dosimetry led to prolonged OS for patients with HCC and PVT after SIRT than sorafenib. |
| Golfieri et al. [21] | Italy | Retrospective | 104 | No differences between infiltrative and nodular HCC were observed in terms of OS. |
| Bargellini et al. [22] | Italy | Retrospective | 70 | The Milan PVT score was an easy tool to select patients with HCC and PVI who may benefit from SIRT. |
| Memon et al. [24] | United States | Prospective | 63 | The CP score of HCC patients with PVT progressing after SIRT was useful for selecting those for systemic therapy. |
| Edeline et al. [25] | France | Retrospective | 34 | SIRT seemed to be more effective than sorafenib in patients with HCC and PVI. |
| Cho et al. [26] | Korea | Retrospective | 32 | HCC patients with PVT who underwent SIRT and those who received sorafenib had comparable OS. |
| Kucuk et al. [27] | Turkey | Retrospective | 19 | Higher SUVmax lesions had better PFS rates after SIRT. |
| Abuodeh et al. [28] | United States | Retrospective | 34 | Pre-SIRT FDG-avidity was associated with worse local tumour control and PFS. |
| Reizine et al. [29] | France | Retrospective | 37 | Early 18F-FDG or 18F-FCH PET/CT post-SIRT predicted the 6-month response and OS in patients with HCC. |
| Jreige et al. [30] | Switzerland | Retrospective | 48 | The lesion SUVmax and the T/L uptake ratio by 18F-FDG PET/CT predicted OS in patients treated with SIRT. |
| Hartenbach et al. [31] | Germany | Retrospective | 34 | 18F-FCH PET/CT had the potential to follow-up advanced HCC patients treated with SIRT. |
| Filippi et al. [32] | Italy | Retrospective | 21 | 18F-FDG or 18F-FCH PET/CT was the only independent predictor of OS in HCC patients treated with SIRT. |
HCC, hepatocellular carcinoma; 18F-FCH, 18F-fluorocholine; 18F-FDG, 18F-fluorodeoxyglucose; OS, overall survival; PET/CT, positron emission tomography/computed tomography; PFS, progression-free survival; PVI, portal vein invasion; PVT, portal vein thrombosis; SIRT, selective internal radiation therapy; SUV, standardised uptake value; T/L, tumour-to-liver.
The international guidelines (BCLC classification) for HCC treatment allocation incorporate prognostic variables that influence therapeutic outcomes. One of these factors is the tumour burden, which allows the classification of patients into stages and determines the most suitable therapeutic choice based on the best outcome [1,2]. A tumour burden replacing >50 % of the total liver volume independently affects survival. Thus, other morphological tumour variables, such as tumour size, number of nodules, and presentation pattern, tend to lose significance concerning the overall tumour bulk, with a reduced functional liver remnant and, consequently, a greater risk of early liver decompensation. An excessive tumour load may impede effective treatment using a single session of SIRT, and in the case of complementary therapy, liver dysfunction may precede this [11]. Indeed, HCC patients with a tumour size <5 cm benefit the most from SIRT, with a significantly greater 5-year overall survival (OS) rate than those with a size >5 cm (p < 0.001) [12]. To improve the selection of suitable candidates for SIRT, a maximal tumour size <9 cm was identified as an independent predictor of radiological complete response (CR) based on the modified Response Evaluation Criteria in Solid Tumours (mRECIST) in a cohort of 102 HCC patients treated with radioembolization (p = 0.020) [13]. Thus, patients with unresectable intrahepatic HCC might benefit from SIRT if they are well selected despite having a large tumour. This concept was confirmed in the LEGACY study, in which clinically meaningful response rates were found in 88.3 % of patients with solitary unresectable HCC ≤8 cm, 62.2 % of whom had a prolonged duration of response (DoR) after SIRT ≥6 months [14].
3.2Portal vein invasionThe presence of portal vein invasion (PVI) is an indication for treating HCC patients with radioembolization, classifying them as local advanced BCLC stage C according to international guidelines [2]. This treatment procedure is designed to minimally occlude the hepatic arteries, thereby proving effective in this setting by preserving blood flow to the liver parenchyma. However, PVI is considered a highly unfavourable prognostic factor because it markedly increases the risk of extrahepatic cancer spread and concurrently reduces OS [15]. The safety of SIRT in unresectable HCC patients with thrombosis of one or both first-order veins and related segmental portal venous branches was reported many years ago [16], but more recent studies have further validated the effectiveness of SIRT for HCC patients who developed PVI. Recently, a European-wide observational study on the clinical application and outcomes of SIRT confirmed that the presence of PVI (segmental PVI: HR 1.46; 95 % CI 1.02–2.10; p = 0.0378; main PVI: HR 2.49; 95 % CI 1.37–4.54; p = 0.0028) is an independent prognostic factor for OS [17]. Indeed, SIRT appears to have a significant antitumour effect when used as a treatment option for patients with lobar HCC and ipsilateral intrahepatic PVI, achieving a disease control rate (DCR) of 88.9 % [18]. However, confirmation that the presence of PVI as a presentation of HCC may benefit most from radioembolization with 90Y-loaded glass microspheres has been highlighted in a study performed in intermediate (no PVI group)/advanced (PVI group) HCC. Although there was no significant difference between the PVI and no PVI groups in terms of time to progression (TTP) or OS, tumour response was identified as the primary factor influencing TTP (p < 0.001) and OS (p = 0.048) [19]. Although these data were confirmed a few years later [20], a different study revealed that the median OS significantly differed between patients with segmental and lobar or main PVI (p = 0.031) but not between patients with infiltrative and nodular HCC, suggesting a further indication for radioembolization in the case of infiltrative HCC [21].
Despite the encouraging results of SIRT in patients with HCC and PVI, the extent of PVI often impacts a patient's prognosis regardless of the treatment method [22], especially in combination with elevated AFP levels and large tumours [1]. The Milan portal vein tumour thrombosis (PVTT) score was introduced several years ago as a prognostic model to predict the outcomes of patients with advanced HCC and PVI who underwent SIRT. Based on three parameters independently associated with OS (bilirubin levels, the extent of PVI, and tumour burden), 120 patients were stratified into three subgroups with different prognoses: favourable (0 points), intermediate (2–3 points) and dismal (>3 points). Notable differences among these three categories were identified in terms of the median OS (p < 0.001), PFS (p = 0.045), and risk of liver decompensation (p < 0.0001) [16]. This scoring system was subsequently validated in two independent single-centre studies, in which survival results confirmed the reliability of the model [22,23]. Thus, the Milan score represents an easy and helpful tool for selecting candidates who can benefit from radioembolization and those for whom the procedure may be futile by understanding the role of liver function. This concept was highlighted in a cohort of 63 HCC patients with PVI, a CP score ≤7, and no extrahepatic metastasis, in whom liver function decompensation was observed in 55 % of CP A patients and 60 % of CP B patients with disease progression. This information is critical because it allows the potential suitability of these patients for systemic therapy or participation in clinical trials [24].
Nevertheless, SIRT seems to be more effective than sorafenib in patients with HCC and PVI in terms of the median OS (p < 0.001) and response rate (p = 0.003) [25]. A similar significant difference in the response rate (p = 0.01), but not OS (p = 0.22), was observed in another cohort in which patients treated with sorafenib experienced significantly more grade 3/4 adverse effects than did those who underwent radioembolization (p < 0.01) [26].
In conclusion, despite the poor prognosis of unresectable HCC complicated by PVI, SIRT is an effective and well-tolerated treatment that improves health-related quality of life (HRQoL) compared to other locoregional or systemic therapies.
3.318F-FCH or 18F-FDG PET/CT hypermetabolism18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) is a technique with limited sensitivity, demonstrating significant uptake in only 50–65 % of HCC patients. Nevertheless, research has revealed that HCC in patients exhibiting high 18F-FDG uptake displays a more aggressive nature, thus placing patients at a higher risk of progression and recurrence with lower survival rates [27]. SIRT was found to be less effective in this specific population, leading to a significant reduction in local control, PFS, and OS [28,29]. Moreover, metabolic parameters derived from 18F-FDG PET/CT, such as the mean and median standardised uptake value (SUV)max of lesions and the tumour-to-liver uptake ratio, have emerged as independent negative predictors of PFS (both p = 0.01) and OS (p = 0.02) [30]. Conversely, another study showed that patients with higher SUVmax lesions unexpectedly had better PFS after SIRT, suggesting a therapeutic advantage of the procedure over other treatment options [27]. Further extensive studies are required to validate the predictive role of 18F-FDG PET/CT in patients with HCC treated with SIRT. 18F-FDG PET/CT may represent an accurate imaging modality for staging and follow-up of HCC patients before and after SIRT, as well as for assessing tumour viability after intravascular therapy. Thus, early evaluation of the treatment response of HCC patients after SIRT represents a challenge, as conventional imaging criteria may not reveal a response until six months post-treatment. Interestingly, while HCC demonstrates variable affinity for FDG, some patients show affinity for 18F-fluorocholine (18F-FCH). 18F-FCH PET/CT has been demonstrated to be a positive predictor of treatment failure (p < 0.001) [31], but radiological metabolic response with either 18F-FDG or 18F-FCH PET/CT has been deemed a significant independent predictor of OS at six months (p = 0.01) [29] and at eight weeks (p < 0.001) [32] after SIRT. Therefore, it is suitable to emphasise how these results can influence the subsequent clinical management of patients with HCC undergoing radioembolization.
4Clinical prognostic factors4.1Performance statusThe PS of patients is one of the main criteria used to select those who benefit better from SIRT. The Eastern Cooperative Oncology Group (ECOG) scale helps determine the patient's level of functioning in terms of self-care, daily activity, and physical ability, which is crucial for guiding treatment decisions and prognosis in clinical settings. An ECOG score >0 has been shown to be a statistically independent factor for OS in a cohort of HCC patients treated with ⁹⁰Y-loaded resin microspheres (p = 0.002) [17]. Similarly, a lower frequency of an ECOG score of 1 was also significantly associated with better survival outcomes (p = 0.025) [33]. Thus, this aspect could be instrumental in enhancing patient selection to improve survival outcomes after SIRT.
4.2SarcopeniaSarcopenia is increasingly recognised as a predictive marker for assessing outcomes in HCC patients, as it is associated with a lack of response to liver tumour treatments, new episodes of liver decompensation, and increased mortality in cirrhotic patients. However, data on the effects of sarcopenia in HCC patients treated with SIRT are scarce, and conflicting results have been reported on this topic. One reason may be the significant heterogeneity in the methods used to assess sarcopenia in these studies [34].
The fat-free muscle area (FFMA), a measure of sarcopenia, predicts OS and might represent a promising new biomarker for survival prognosis in patients undergoing radioembolization for the treatment of unresectable HCC. In this context, patients with a low FFMA measured by magnetic resonance imaging (MRI) before SIRT had a significantly shorter OS (p = 0.024) and tended to have a shorter PFS (p = 0.068) than patients with a high FFMA [35]. Notably, sarcopenia has been proven to be an independent predictor of mortality (p = 0.048), even after adjusting for BCLC stage, and appears to independently predict increased mortality for HCC patients with an intermediate BCLC stage (p < 0.001) [36].
In addition to assessing the FFMA as an indicator of sarcopenia, the psoas muscle index (PMI) has also proven to be a useful measure. In this context, one research study investigated the sarcopenia status using the PMI in 86 patients with HCC at the time of treatment with radioembolization and one month later. A worsening of sarcopenia status after SIRT has been proven to be an independent predictor of disease progression (p = 0.009), and no sarcopenic patients had higher 6- or 12-month TTP rates than did the sarcopenic patients (p = 0.005) [37]. However, further studies are still needed to better understand the prognostic value of sarcopenia for the SIRT response in patients with HCC.
5Biological prognostic factors5.1Alpha-fetoprotein and des-gamma-carboxy prothrombinTumour markers, such as AFP or des-gamma-carboxy prothrombin (DCP), could be useful predictors of prognosis after SIRT (Table 2). Higher AFP levels before treatment significantly correlate with decreased survival (p = 0.04), and post-treatment AFP levels have a significant effect on mortality rates (p = 0.025) [38]. In particular, an AFP > 200 ng/mL and a DCP > 15,000 mAU/mL independently correlate with an increased risk of mortality (p < 0.05), but an AFP of ≤200 ng/mL predicts an increased probability of achieving curative treatment after SIRT (p = 0.017), providing a survival benefit in patients with intrahepatic HCC [39]. However, the prognostic role of tumour markers was also evaluated with the AFP or DCP responses (defined as a more than 50 % decrease after SIRT from baseline). AFP- and DCP-responsive patients had significantly longer survival than non-responders (p < 0.05), with the DCP response also defined as an independent predictor of OS 3 months after SIRT (p = 0.003) [40].
Studies investigating biological prognostic factors in unresectable HCC patients undergoing radioembolization.
| Author | Country | Study Design | Patients treated with SIRT | Key findings |
|---|---|---|---|---|
| Ekmekcioglu et al. [38] | Turkey | Retrospective | 24 | Pre- and post-SIRT AFP levels predicted survival rates in HCC patients. |
| Kim et al. [39] | Korea | Retrospective | 143 | An AFP level ≤200 ng/mL independently predicted the increased probability of achieving curative treatment after SIRT. |
| Lim et al. [40] | Korea | Retrospective | 112 | Tumour marker responses (AFP, DCP) may be useful to predict post-SIRT survival. |
| Van Doorn et al. [41] | The Netherlands | Retrospective | 69 | The ALBI score predicted liver decompensation and OS, aiding in patient selection for SIRT. |
| Lescure et al. [42] | France | Retrospective | 222 | The baseline ALBI score and variations of the ALBI were prognostic predictors of OS after SIRT. |
| Hickey et al. [43] | United States | Prospective | 205 | The ALBI grade outperformed the CP class at discriminating OS in patients receiving SIRT. |
| Antkowiak et al. [44] | United States | Prospective | 1000 | The ALBI outperformed CP in survival prognosis in patients with HCC treated with SIRT. |
| Gui et al. [45] | United States | Retrospective | 117 | The ALBI grade better predicted OS than the CP class amongst HCC patients treated with SIRT. |
| Mohammadi et al. [46] | United States | Retrospective | 124 | The ALBI was a more sensitive marker than CP for predicting better outcomes after SIRT. |
| Ali et al. [47] | United States | Retrospective | 401 | Post-SIRT AFP and ALBI scores were significant OS prognosticators. |
| Moctezuma-Velazquez et al. [48] | Canada | Retrospective | 132 | ALBI-grade 3 was associated with a higher risk of adverse events after SIRT. |
| Reincke et al. [49] | Germany | Retrospective | 61 | The pre-treatment ALBI score was predictive of hepatic decompensation after SIRT. |
| Taussig et al. [50] | United States | Retrospective | 86 | NLR >3 was confirmed as a predictor of early progression after radioembolization. |
| Soydal et al. [51] | Turkey | Retrospective | 86 | Patients with a low basal ALBI grade and NLR survived longer after SIRT in unresectable HCC patients. |
| Sukato et al. [52] | United States | Retrospective | 176 | Elevated NLR discriminated patients with HCC BCLC C who may not derive benefit from SIRT. |
| Young et al. [53] | United States | Retrospective | 145 | Pre- and post-treatment NLR may be associated with OS in HCC patients undergoing SIRT. |
| Estrade et al. [54] | France | Retrospective | 164 | An increased NLR at 3 months after SIRT was associated with poor survival. |
| Li et al. [55] | China | Retrospective | 236 | NLR and delta NLR predicted OS in patients with HCC BCLC C treated with SIRT. |
| D'Emic et al. [56] | United States | Retrospective | 116 | The pre- and/or post-SIRT NLR and/or PLR were predictive of clinical outcomes. |
| Öcal et al. [57] | Germany/Spain | Post hoc analysis | 275 | There was no significant difference in OS between patients with a low PLR treated with SIRT/sorafenib or sorafenib alone. |
| Yoneoka et al. [58] | United States | Retrospective | 42 | NLR may be superior to PLR for predicting response to SIRT as primary treatment for HCC. |
| Seidensticker et al. [62] | Germany | Retrospective | 34 | Baseline IL-6 and IL-8 were associated with later liver dysfunction and OS in patients treated with SIRT. |
| Öcal et al. [63] | Germany | Post hoc analysis | 86 | IL-6 values were a prognostic factor in advanced HCC patients receiving SIRT+sorafenib. |
| Lewandowski et al. [64] | United States | Prospective | 23 | SIRT was associated with a mild increase in angiogenic markers. |
Abbreviations: AFP, alpha-fetoprotein; ALBI, albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer; CP, Child‒Pugh; HCC, hepatocellular carcinoma; DCP, des-gamma-carboxy prothrombin; IL-6, interleukin-6; IL-8, interleukin-8; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; OS, overall survival; SIRT, selective internal radiation therapy.
The albumin-bilirubin (ALBI) grade has been used to assess liver function in patients with HCC (Table 2). Recent studies have revealed that the baseline ALBI grade has significant predictive power for radioembolization-induced liver disease (REILD) and survival [41,42]. Interestingly, patients with ALBI grade 1 exhibit a significantly lower incidence of REILD after the first SIRT than those with ALBI grade 2 or 3 (p = 0.002). Furthermore, both the baseline ALBI grade and variations in the ALBI score after SIRT demonstrated a significant association with OS (p = 0.001 and p ≤ 0.001, respectively) [42]. The ALBI grade outperforms the CP class as the most effective discriminator of OS in HCC patients undergoing transarterial chemoembolization (TACE) or SIRT (c-indices of 0.792 and 0.763, respectively) [43], as well as in those treated with SIRT alone (c-indices of 0.623 and 0.616, respectively) [44]. This remarkable discriminatory capacity of the ALBI grade was found mainly in CP-A patients, where lower ALBI grades before radioembolization were consistently associated with significantly improved survival outcomes (p = 0.002) [45,46]. A laboratory score system combining the ALBI grade and AFP level confirmed a correlation between a decrease in the score post-SIRT and an improvement in OS (p < 0.001) [47]. However, although many studies have highlighted that a higher ALBI grade has the potential to serve as a prognostic factor for OS, it may also be associated with a greater risk of developing adverse events such as hepatic decompensation after radioembolization [17,48]. Indeed, in this context, the ALBI score at baseline proved to be an independent risk factor for developing liver failure (p < 0.005) [49].
5.3Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratioIn recent studies, markers of inflammation, such as the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), have emerged as valuable prognostic indicators in HCC (Table 2). Notably, the NLR may predict shorter OS for HCC patients undergoing surgical or intra-arterial therapies, and it could also serve as a serological biomarker linked to early disease progression. The DCR (assessed by mRECIST) was significantly compromised (p = 0.014) with an NLR greater than 3, which proved to be the best predictor of early progression (p < 0.0001) [50]. However, the NLR is significantly correlated with OS (p = 0.007), and patients with a low basal ALBI grade and a low NLR have a more prolonged survival after SIRT [51]. Similarly, an elevated NLR has been confirmed to be an independent predictor of worse survival, especially in patients with BCLC stage C disease [52]. This close, directly proportional correlation between a higher NLR and increased mortality has been recently recognised with evaluations of pre- and post-radioembolization NLR values (p ≤ 0.016 and p ≤ 0.001, respectively) [53]. An increase in the NLR that persists even three months after SIRT treatment may be independently associated with a poor outcome (p < 0.001). The NLR cut-off is 7.2, and patients with an NLR >7.2 at three months post-SIRT have a significantly shorter median OS than those with an NLR <7.2 (p = 0.003) [54]. Nevertheless, despite a specific time point, it is always necessary to consider any changes in the NLR before and after the therapeutic procedure because these changes could provide valuable information on the dynamic nature of the white blood cell state for the host immune response to cancer. Indeed, the post-SIRT/pre-SIRT NLR (NLR post-procedure/NLR pre-procedure) and change in NLR (delta NLR = NLR post-procedure – NLR pre-procedure) are associated with worse OS, even in BCLC C patients (p = 0.024 and p = 0.006, respectively) [55].
The influence of SIRT on blood count changes extends beyond the NLR, with alterations in the PLR as well. An elevated PLR before and after SIRT could be a significant determinant of reduced OS and PFS. An increase in the post-treatment PLR of more than threefold has emerged as a predictor of increased mortality risk, while a pre-treatment PLR > 78 is the most predictive serum marker associated with improved OS [56]. Furthermore, the baseline PLR has been shown to be a predictive factor for better survival in patients treated with SIRT/sorafenib compared to patients treated with SIRT alone (p = 0.03) [57]. Although the NLR and PLR are systemic inflammatory prognostic factors for OS in patients treated with SIRT, the NLR may outperform the PLR in evaluating the response rate to SIRT as the primary treatment for HCC. A pre-treatment NLR ≥2.83 has been proven to be the only significant predictor of response failure to radioembolization (p = 0.036) [58].
Nevertheless, future studies are needed to validate these findings in a larger cohort and, notably, to better test biological prognostic factors such as the PLR [59,60].
5.4Interleukins: IL-6 and IL-8Interleukin-6 (IL-6) plays a role in hepatocarcinogenesis and is correlated with poor outcomes in HCC patients (Table 2). Additionally, IL-8 has been shown to reflect the extent of tumour burden across various tumour types, including HCC. It also correlates with the tumour stage in HCC patients, indicating its potential as a biomarker for assessing disease progression [61]. Some studies have suggested that IL-6 and IL-8 can predict treatment response and survival after TACE in patients with primary and metastatic liver tumours. Moreover, a prospective exploratory study revealed that specific threshold levels — 6.53 pg/mL for IL-6 and 60.8 pg/mL for IL-8 — are linked to OS regardless of the tumour type following treatment with SIRT in patients with HCC or metastatic conditions [62]. These prognostic baseline values were confirmed in a post hoc analysis of 83 HCC patients treated with 90Y-SIRT combined with sorafenib as part of the prospective multicentre phase II trial SORAMIC. Patients with high baseline levels of IL-6 and IL-8 experienced a significantly shorter OS (7.8 vs. 19.0 months for IL-6 and 8.4 vs. 16.0 months for IL-8). Furthermore, elevated baseline IL-6 levels have emerged as an independent prognostic factor for OS (HR 2.35, 95 % CI 1.35–4.1, p = 0.002) and liver dysfunction (HR 2.67, 95 % CI 1.21–5.94, p = 0.016) [63]. Radioembolization has also been linked to a modest increase in angiogenic markers, as demonstrated by a statistically significant increase in IL-6 levels observed four weeks following SIRT (p = 0.05) [64]. Thus, IL-6 might be helpful in selecting patients or stratifying those undergoing 90Y-radioembolization in clinical practice and for future clinical trials.
6Technical prognostic factors6.1Personalised dosimetry: a key factor of efficacyCurrently, two 90Y devices are commercially available and use different dosimetric models. The administration of resin microspheres (SIR-Spheres® Sirtex, AU) relies on an activity-based empirical calculation method, mainly considering the patient's body surface area. Instead, glass microspheres (TheraSphere®, BTG Biocompatibles, UK) have been approved using a standardised method that delivers a uniform absorbed dose to the perfused tissue volume within a predetermined dose range. Due to variations in the size and specific activity of glass and resin microspheres, it is essential to distinguish between these two types of 90Y devices with different recommendations [65]. For HCC, a tumour-absorbed dose equal to or greater than 100–120 Gy with resin microspheres and equal to or greater than 205–300 Gy with glass microspheres is recommended. Some studies have shown that selected patients (such as those with CP-A, unilobar disease, and adequate hepatic reserve) can tolerate an absorbed dose exceeding 150 Gy in the treated lobe, but the average dose delivered to the entire liver (both treated and untreated parts) should remain below 150 Gy [20,66].
The success of radioembolization relies on optimizing the dose delivered to the tumour while minimizing the dose absorbed into adjacent non-tumoural liver tissue and limiting exposure to the lung parenchyma. A macroaggregated albumin scan can be conducted before SIRT to assess the absorbed dose in the tumour, as it has been shown to be a predictive tool for evaluating treatment response and OS [67]. Patients treated with glass microspheres receiving a suboptimal tumoural dose (<205 Gy) have a median OS of 4.3 months, while those receiving an effective personalised tumoural dose (≥205 Gy) have a median OS of 18.2 months (p = 0.005) [20]. Similar findings were reported from a retrospective study of 230 patients with unresectable HCC treated with ⁹⁰Y-loaded resin microspheres, in which those receiving at least 100 Gy had a longer OS than those receiving <100 Gy (p < 0.001) [68]. Furthermore, compared with the standard approach, the personalised dosimetry approach of SIRT with ⁹⁰Y-loaded glass microspheres significantly improved the objective response rate in patients with locally advanced HCC (p = 0.007) [69]. Even in the case of SIRT with ⁹⁰Y-loaded resin microspheres, optimizing treatment application and patient selection remains challenging. A more complex but personalised method to calculate the applied radiation activity results in improved OS compared to generic body surface area calculations (p = 0.0144) [17]. In general, two dosimetric factors predict the efficacy of SIRT: a high tumour-absorbed dose and a homogeneous distribution of microspheres in the tumour. Nevertheless, due to the distinct dose calculation methods used for resin and glass microspheres, it is recommended to adopt a personalised approach using dosimetry, which may become particularly important when planning for whole or selective (non-ablative or ablative) liver SIRT. In this context, the recently published guidelines on dosimetry for glass and resin microspheres may also be helpful [65,70]. Therefore, the results of previous and future studies on SIRT that do not apply personalised dosimetry should be interpreted with caution.
6.2Radiation segmentectomy: a cure in selected patientsRadiation segmentectomy involves administering a calculated lobar dose of 90Y into a segmental vessel, reducing radiation exposure to healthy liver parenchyma [71]. This approach has demonstrated great safety, an excellent tumour response rate, and improved patient survival compared to lobar treatment [72,73]. Notably, the higher TTP and OS reported after SIRT in patients with a solitary HCC lesion smaller than 5 cm confirmed this concept [74,75]. Overall response rates and PFS were also greater for radiation segmentectomy than for TACE (p < 0.001 and p = 0.002, respectively) [76]. This treatment approach is safe and effective in early-stage HCC, and the recent LEGACY study also supports the concept of radioembolization as a curative-intent treatment or bridge to transplantation [14].
6.3Radiation lobectomy: efficacy and safetyRadiation lobectomy is a specialised technique that involves the administration of 90Y-loaded microspheres via trans-arterial infusion to induce changes in the volume of liver lobes [3,77]. This method thus has the potential to exploit the atrophy of the ipsilateral lobe and the hypertrophy of the contralateral lobe, making it useful in the case of unresectable HCC due to a small future liver remnant and providing an advantage in tumour growth control [78,79]. Furthermore, compared to portal vein embolization (PVE), radiation lobectomy is safe and effective, with specific antitumour activity and a longer TTP on the waiting list in selected patients [80]. In patients with right unilobar HCC and without prior locoregional therapies, radiation lobectomy results in significant volumetric changes and future liver remnant hypertrophy (1 % and 45 % at 1 and 9 months post-treatment, respectively; p < 0.001). Although there are comparable results for lobe hypertrophy with PVE, radiation lobectomy remains a promising technique, especially as a bridge to resection, with fewer risks than those related to PVE [75].
6.4Optimization of tumour targeting and MAA imaging: new tools in interventional radiologyPretherapy 99mTc-macroaggregated albumin (MAA) distribution in HCC and healthy liver compartments in single-photon emission computed tomography (SPECT) with CT (SPECT/CT) scans and post-therapy 90Y PET/CT absorbed doses are strongly correlated with the tumour response and patient outcome (high dose absorbed by the tumour) or with liver toxicity (high dose absorbed by healthy liver) [81]. Despite the low accuracy of MAA SPECT/CT in predicting tumour-absorbed doses, numerous studies have demonstrated a strong relationship between the MAA tumour-absorbed dose and the efficacy of SIRT [82]. In particular, when a specific MAA tumour absorbed dose threshold was reached, the radiological response and patient outcome significantly improved [83]. New interventional imaging tools are emerging to optimise tumour targeting. Virtual parenchymal perfusion (VPP) software is an accurate and reliable technique for predicting MAA SPECT volumetric and targeting results in HCC patients during SIRT. It is also used preoperatively to optimise the microcatheter position for 90Y infusion, allowing precise tumour targeting while preserving non-tumoural parenchyma, and postoperatively to allow an accurate estimation of the perfused volume by each arterial branch, thus ensuring precise 90Y dosimetry for SIRT procedures [84,85].
7ConclusionsRadioembolization is now considered a safe and effective treatment for selected patients with early, intermediate, or local advanced BCLC stage HCC. As a result, the use of this treatment in HCC patients has increased, sparking more significant interest in clinical research. Many studies have attempted to identify prognostic factors associated with better or worse outcomes in HCC patients treated with SIRT. This approach has consequently revolutionised the clinical management of HCC patients, allowing for better selection of those who will benefit from this intra-arterial treatment, thereby improving survival rates. However, future studies are needed to define new prognostic factors for clinical outcomes or survival to improve the management and treatment of patients with HCC.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
Author contributionsConceptualization and article design (Giuliana Amaddeo, Maria Stella Franzè), acquisition of data (Maria Stella Franzè, Paul Vigneron, Anna Sessa, Carlo Saitta, Julia Chalaye, Hélène Regnault, Ancuta Bejan, Rami Rhaiem, Raffaele Brustia), analysis and interpretation of data (Maria Stella Franzè, Paul Vigneron, Vania Tacher, Alain Luciani, Daniele Sommacale, Vincent Leroy, Giovanni Raimondo), drafting of the manuscript (Maria Stella Franzè, Paul Vigneron, Giuliana Amaddeo), critical review and editing (Carlo Saitta, Julia Chalaye, Vania Tacher, Alain Luciani, Hélène Regnault, Ancuta Bejan, Rami Rhaiem, Daniele Sommacale, Vincent Leroy, Raffaele Brustia, Giovanni Raimondo), supervision (Giuliana Amaddeo, Giovanni Raimondo), final approval (All authors).