Both external radiotherapy and sorafenib are promising treatments for hepatocellular carcinoma (HCC). Nevertheless, the combined treatment of external radiotherapy and sorafenib has not been widely applied clinically due to potentially adverse effects. This meta-analysis aimed to evaluate the clinical efficacy and safety of external radiotherapy combined with sorafenib in the treatment of HCC.
MethodsPubmed, MEDLINE, EMBASE, Cochrane Library, and Web of Science databases were searched. The primary and secondary observation endpoints were the end of survival and incidence of adverse events, respectively. 11 studies involving 664 patients were included in this meta-analysis.
ResultsThe results demonstrated that median overall survival (mOS) and median progression-free survival (mPFS) of the external radiotherapy combined with sorafenib (RS) group were 19.45 months and 8.20 months. The one- and two-year survival rates were 0.65 (95%CI: 0.55–0.76) and 0.40 (95%CI: 0.24–0.56). The incidence of adverse events was 0.34 (95%CI: 0.25–0.44).
ConclusionsThe findings demonstrated that the survival of the RS group was significantly improved and few severe adverse events were observed. Hence, it can be concluded that external radiotherapy combined with sorafenib is a safe, effective, and promising therapeutic option for HCC.
Incidence, prevalence, and mortality of hepatocellular carcinoma (HCC) have recently increased in China, due to the endemic high prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) [1]. For early-stage HCC patients, surgical resection and orthotopic liver transplantation (OLT) are recommended [2, 3]. However, since organs are in low supply and lung metastasis is prevalent after surgery, the practical applicability of OLT is restricted [4]. As a result, the majority of patients prefer surgical resection, even though a significant portion of HCC patients is not diagnosed until an advanced stage, making surgical resection unlikely. Radiofrequency ablation, transcatheter arterial chemoembolization, and transcatheter chemotherapy perfusion have also been used, however, none of these have been shown to improve survival.
Currently, external radiotherapy has achieved outstanding results in the treatment of HCC. Meanwhile, sorafenib has been determined as a targeted medicine that can significantly extend the survival of patients with advanced HCC [5]. Nevertheless, the combination of external radiotherapy and sorafenib has been rarely applied clinically, which may be attributed to possible adverse events [6, 7]. Sorafenib is an orally available active targeted cancer medication that reduces tumor proliferation and angiogenesis by inhibiting the interaction of Raf kinase with vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptors [8]. Thus, sorafenib can improve the radiosensitivity of the tumor. Meanwhile, radiotherapy of target lesions can reduce the local tumor burden and improve the overall response rate of patients treated with sorafenib.
Based on these theories, external radiotherapy combined with sorafenib may be an effective strategy for the treatment of HCC. In this study, we comprehensively reviewed relevant literature to evaluate the clinical efficacy and safety of external radiation combined with sorafenib in the treatment of HCC, to help clinicians make more accurate decisions.
2Materials and methods2.1Study protocolThis study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), we comprehensively searched literature published in Pubmed, MEDLINE, EMBASE, Cochrane library, and Web of Science. "HCC", "hepatoma", "external radiation", and "sorafenib" were used as search terms to screen eligible studies. Additionally, references to relevant articles were also searched to identify other eligible studies.
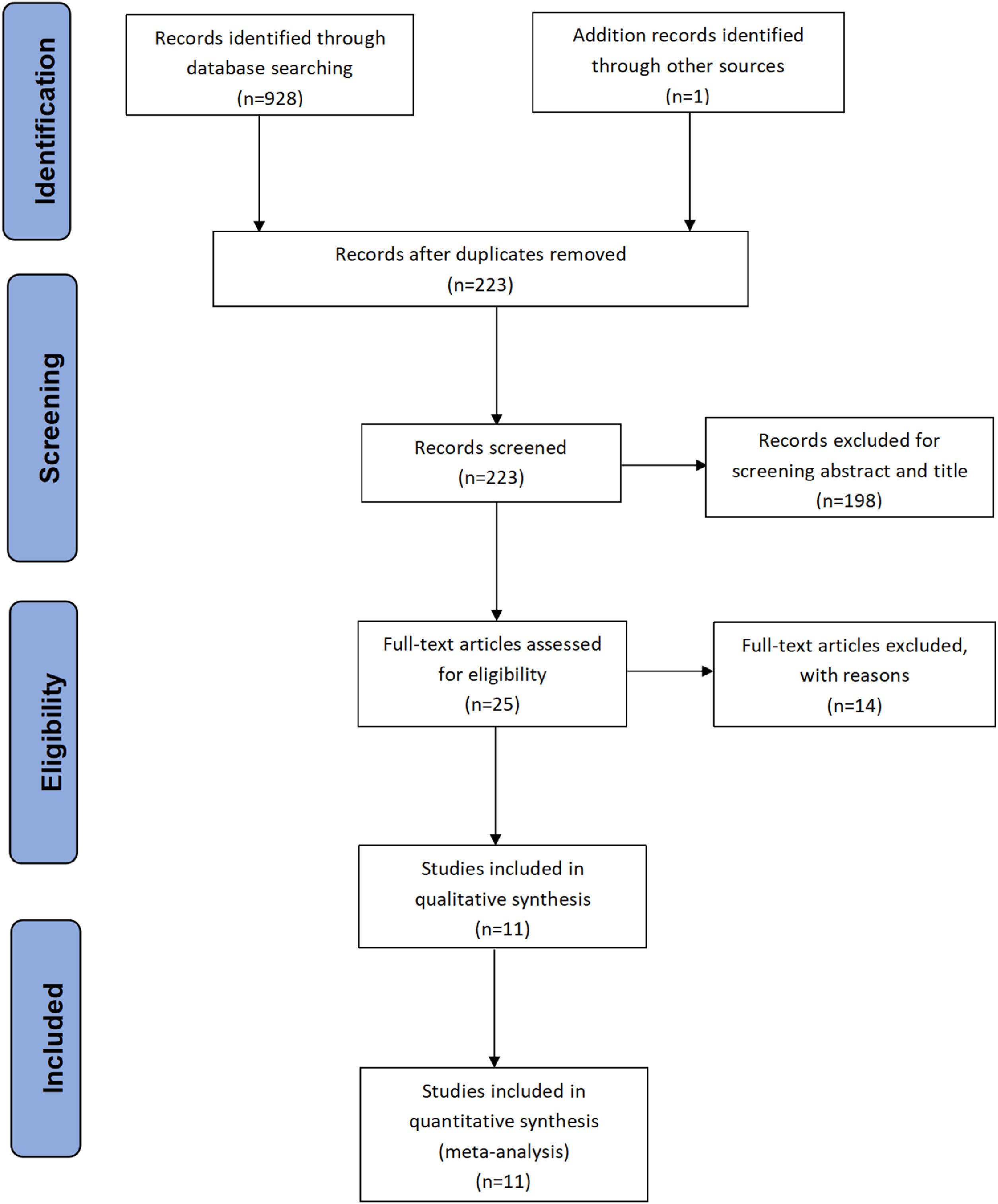
2.2Selection criteriaThe inclusion criteria for eligible studies used are as follows: (1) Clinical trials of external radiation combined with sorafenib; (2) prospective and retrospective studies; (3) at least one survival data (OS or PFS) of the HCC patients provided. Endnote document management software was applied to delete duplicate documents. Abstracts, reviews, comments, letters, conference papers, case reports, and animal studies were excluded by screening the titles and abstracts. If several studies in the same institution meet the inclusion criteria, the study with the largest sample was included. Finally, the remaining studies were reviewed to determine whether they were related to the subjects and fully met the inclusion criteria. All study selection processes were completed by two independent reviewers, and the final inclusion decision was based on the consent of both reviewers. A detailed inclusion scheme was shown in the PRISMA flowchart (Fig. 1).
2.3Data extractionThe data were extracted by two independent researchers. In case of disagreement, it can be solved through a re-evaluation of the literature and discussion. The following information was collected: (1) general information included the author's name, time of publication, institution, time of patient recruitment, and the number of patients; (2) clinical data included incidence of Child-Pugh Grade A, radiotherapy pattern and dosage, and sorafenib dose; (3) prognosis included gastrointestinal, hepatologic, hematologic, dermatologic adverse events (≥ grade 3), as well as survival rate. In most of the included studies, the efficacy was comprehensively evaluated by using Response Evaluation Criteria in Solid Tumors (RECIST) [9], modified RECIST [10], or WHO standards, as well as MRI T2 weighted imaging and diffusion-weighted imaging. In the absence of digital data, the survival rate was calculated by the software (GetData Graph Digitizer 2.26). To evaluate the toxicity, most studies applied the toxicity grading standards proposed by the Radiation Therapy Oncology Group (RTOG) and European Organization for Research and Treatment of Cancer (EORTC) [11] or Common Terminology Criteria for Adverse Events (CTCAE).
2.4Quality assessmentGiven that most included research was retrospective, the Nottingham Ottawa scale (NOS) [12] was used to evaluate the quality of each study, showing that most of them had medium quality. Two independent researchers scored and discussed until consistent results were obtained. The NOS score of 7–9 represented a high-quality report and 4–6 represented medium quality. Studies with a score of less than 4 were classified as low-quality studies.
2.5Statistical analysisPrimary observation endpoints were mOS, mPFS, and one- and two-year survival rates. Secondary observation endpoints treatment were toxicities (≥ grade 3). Stata/SE 15.0 software was used for meta-analysis. Meanwhile, publication bias was assessed by assessing the symmetry of the funnel plot. The intercept was quantitatively assessed by Egger's test [13]. If Egger's test p was less than 0.1, the Duval and Tweedie clipping results would have been presented [14]. The difference in median survival and incidence of adverse events between the RS group and the control group were evaluated by median survival ratio (MSR) and odd ratio (OR), respectively.
2.6Register name and registration numberNone
3ResultsAmong the 929 initally included studies, 375 were excluded due to duplication between databases, 331 were excluded due to irrelevant format, 223 were screened for titles and abstracts, 25 were reviewed, and 14 unrelated studies were excluded. Through the full-text review, 11 studies that fully met the inclusion criteria were finally included [15, 6, 16, 17, 18, 19, 20, 21, 22, 23, 24]. The selection process is shown in Fig. 1
All the 11 studies included were retrospective. These studies began recruiting patients as early as 2007 and as late as 2015. Six studies were from China, one from Canada, two from Japan, and two from South Korea. Eight studies were double-arm trials and three were single-arm trials. The median incidence of Child-Pugh A in all studies was 96%. In terms of radiotherapy, 5 studies used IMRT or tomotherapy, 3 studies used SBRT, and 3 studies used 3D-CRT. Most trials used 400 mg sorafenib bid, which was adjusted dependent on adverse effects. In this study, we conducted subgroup analyses, and the results showed that there was no significant difference in the influence of different radiotherapy types and doses on the survival time and toxicity of patients. The significant influence of heterogeneity between different research designs on the display of results was excluded. Table 1 summarizes the general characteristics of the included studies.
Study characteristics.
Abbreviations: HCC, hepatocellular carcinoma; 3D-CRT, 3-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; SBRT, stereotactic body radiotherapy; RS, external rdiotherapy combined with sorafenib.
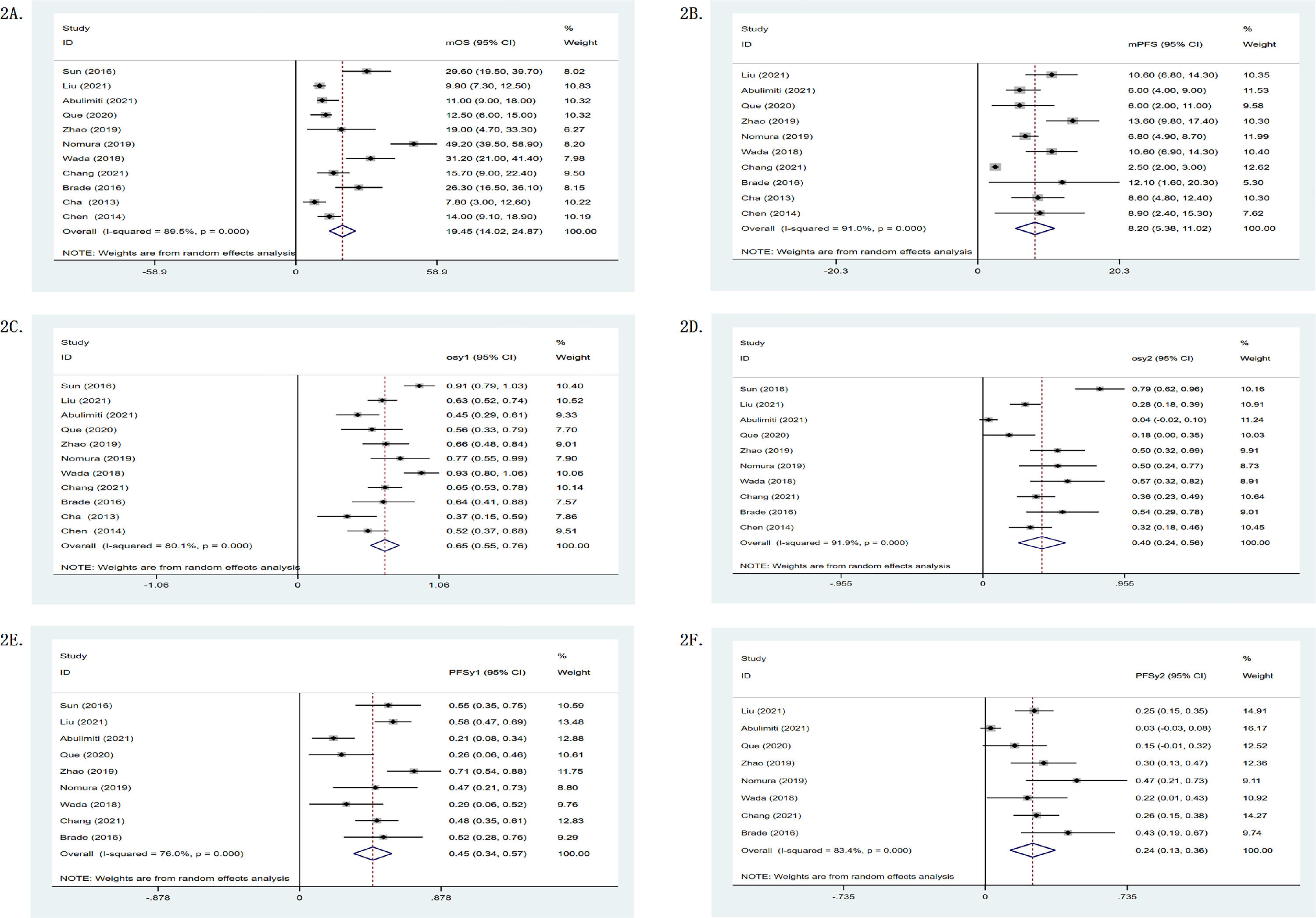
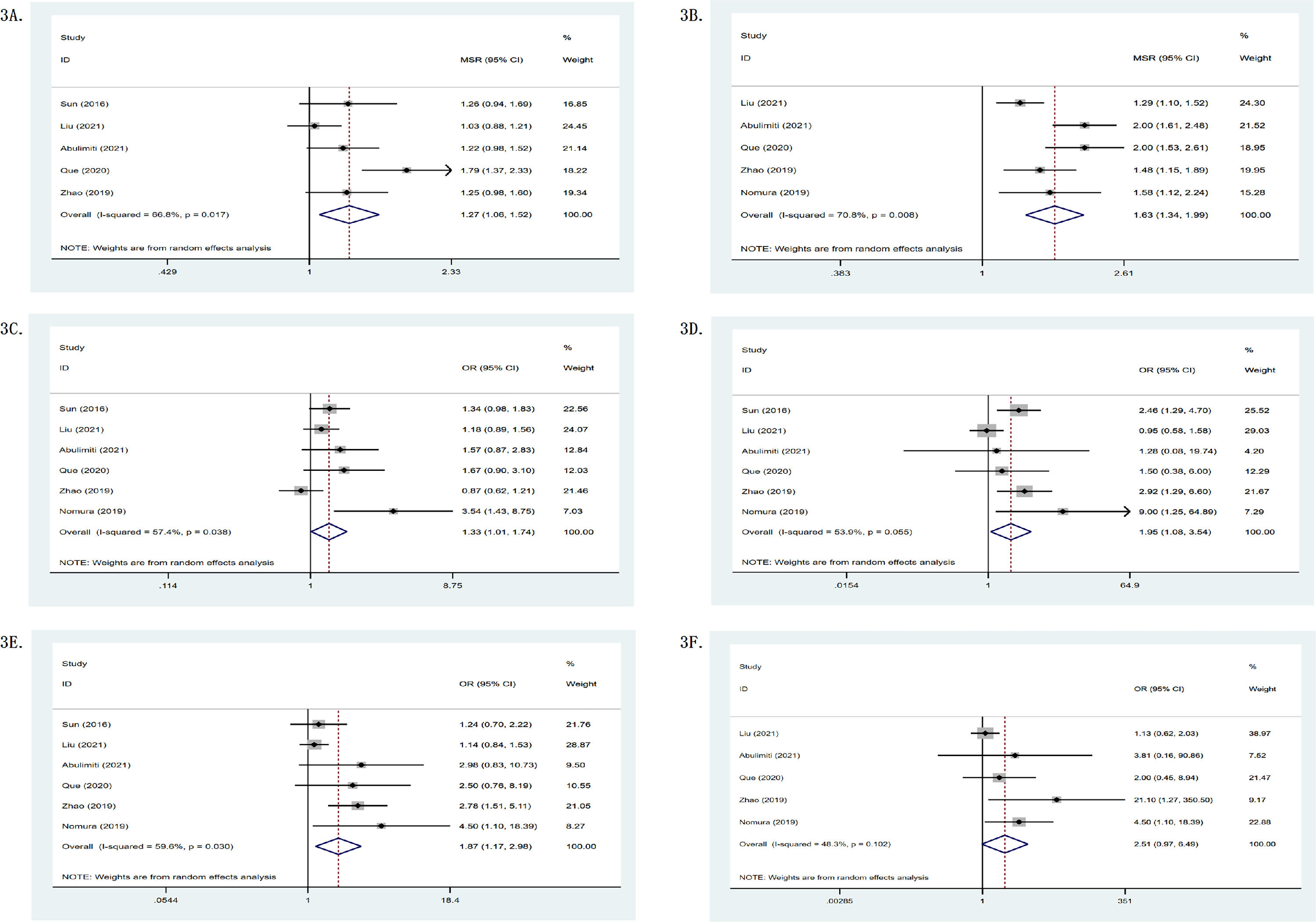
In single-arm researches, the mOS of the RS group was 19.45 months, while its one- and two-year survival rates were 0.65 (95%CI: 0.55–0.76) and 0.40 (95%CI: 0.24–0.56), as shown in Table 2. Meanwhile, Fig. 2 depicts a forest plot. The mOS of the R group was 11.86 months, while its one- and two-year survival rates were 0.47 (95%CI: 0.30–0.63) and 0.17 (95%CI: 0.06–0.27); the mOS of the S group was 10.54 months, while its one- and two-year survival rates were 0.48 (95%CI: 0.39–0.58) and 0.14 (95%CI: 0.07–0.21). In double-arm researches, in terms of one- and two-year survival rates, the RS group was found to be superior to the R group or the S group. The mOS of the RS group was 1.27 (95%CI: 1.06–1.52, p = .017, I2 = 66.8%) and 2.04 (95%CI: 1.31–3.18, p = .004, I2 = 87.7%) compared with the R group and the S group, respectively (p < .05). It can be seen that the survival of the RS group was significantly higher than that of the R group or the S group. Fig. 3 shows the forest plot.
Survival statistics. mOS = median overall survival; mPFS = median progression-free survival; osy1 = 1-year survival rate; osy2 = 2-year survival rate; PFSy1 = 1-year PFS; PFSy2 = 2-year PFS; CR = complete remission; PR = partial remission; SD = stable disease; PD = progressive disease; ORR = objective remission rate; DCR = disease control rate.
(A) mOS comparison between the RS and the R groups; (B) mPFS comparison between the RS and the R groups; (C) osy1 comparison between the RS and the R groups; (D) osy2 comparison between the RS and the R groups; (E) PFSy1 comparison between the RS and the R groups; (F) PFSy2 comparison between the RS and the R groups.
Seven of the 11 studies provided response rates. In terms of objective remission rate (ORR) and disease control rate (DCR), the RS group was superior to the R group or the S group, as shown in Table 2. In single-arm researches, the mPFS of the RS group was 8.20 months. Fig. 2 shows the forest plot. The mPFS of the R group was 4.90 months, the mPFS of the S group was 2.30 months. In double-arm researches, one- and two-year PFS of the RS group were better than those of the R group or the S group. The mPFS of the RS group was 1.63 (95%CI: 1.34–1.99) and 1.95 (95%CI: 0.74–5.15) compared with the R group and the S group. Fig. 3 shows the forest plot.
3.3Treatment toxicitiesThe adverse events (≥ grade 3) were classified and analyzed, including the gastrointestinal (e.g., gastrointestinal ulcer or perforation, abdominal pain, severe nausea, and vomiting), hepatologic (e.g., aminotransferase rise, decompensation of liver function), hematologic (e.g., thrombocytopenia, leucopenia, anemia) and dermatologic adverse events. Among all patients included in the study, the RS group had gastrointestinal, hepatologic, hematologic, dermatologic adverse events in 11, 14, 29, and 8 patients, respectively, as shown in Table 3. The incidence of adverse events of the RS group was 0.34 (95%CI: 0.25–0.44). However, the incidences of adverse events of the RS group were 1.55 (95%CI: 1.02–2.36) and 1.04 (95%CI: 0.32–3.37) compared with the R group and the S group. The results demonstrated that the incidence of adverse events in the RS group was higher than those in the R group or the S group. Herein, hematologic adverse events were common in the RS group, the incidence of the RS group was 2.20 (95%CI: 0.58–8.38) compared with the R group.
Clinical results of included trials.
Potential publication bias in this meta-analysis was examined by evaluating the symmetry of the funnel diagram. The funnel diagram was roughly symmetrical, indicating that the comprehensive analysis results were unlikely to be wrong due to publication bias.
4DiscussionAs the primary endpoints of this meta-analysis, the mOS of the RS group was 19.45 months, the mPFS was 8.20 months, and the combined one- and two-year survival rates were 65% and 40%, respectively. The therapeutic benefit was significantly higher than that of the control group. Meanwhile, the ORR and DCR of the RS group were also better than those of the control group. The findings revealed that external radiation combined with sorafenib may dramatically improve survival in patients with HCC, making it an effective therapeutic option.
Based on certain double-arm studies, there was no significant difference in the occurrence of adverse events between the two groups in terms of adverse events. Abulimiti [15] reported that the RS group only slightly increased the incidence of nausea, anorexia, abdominal pain, fatigue, and skin reactions (grade 1-2) compared with the R group, and there was no significant difference in other adverse events. Grade 3 Adverse effects occurred in both groups, but the treatment could continue after symptomatic treatment. Nevertheless, several cases suffered severe adverse events in the RS group. Brade reported that one patient in the RS group had lower gastrointestinal bleeding of grade 3 diagnosed by colonoscopy during treatment [6]. The reuse of sorafenib resulted in the recurrence of gastrointestinal bleeding, so sorafenib was forced to stop. One patient developed an acute exacerbation of chronic intestinal obstruction (grade 4) and finally developed into clinical intestinal obstruction. One patient developed acute upper gastrointestinal bleeding with black stool as well as bloody stool and eventually died. Relevant literature reported that the total incidence of acute upper gastrointestinal bleeding after hepatoma radiotherapy was 2–38%, which was related to the coverage of hollow organs in radiotherapy, high dose of radiotherapy, and decline of liver function [25, 26, 27]. The adverse events of the RS group were generally mild. Adverse events of grades 3-4 were rare and most of the symptoms can be relieved by symptomatic treatment or after treatment.
Sorafenib, reduces tumor-mesenchymal interactions, tumor metastasis, and carcinogenesis, as a targeted therapy targeting the Raf/MEK/ERK pathway [28, 29]. Meanwhile, sorafenib can inhibit DNA damage repair of cancer cells in the tumor microenvironment, and enhance the oxygen effect by normalizing the surviving tumor vascular system [30, 31]. The BCLC guidelines recommend sorafenib as a first-line treatment for advanced HCC, but its efficacy is limited [32]. However, sorafenib combined with radiotherapy can achieve unexpected results in the treatment of HCC. External radiation can induce mitotic death and affect tumor metabolism through DNA damage [33]. Radiotherapy not only mediates DNA damage and leads to cancer cell death, but also produces immunogenicity by triggering the release of pro-inflammatory mediators, and increases immunostimulation to inhibit tumor cell infiltration and enhance the expression of new antigens [34, 35]. It has been demonstrated sorafenib-induced blockage of Raf/MAPK and VEGF receptor pathways may enhance the efficacy of radiotherapy. The combination of the two can significantly improve the prognosis of HCC patients.
This study has several limitations. First, a meta-analysis of observational studies is controversial [36]. Because researchers have some subjectivity in collecting and analyzing data. Then, the lack of further adjustment for baseline characteristics, such as age, gender, or comorbidity conditions, may affect the reliability of our results. Additionally, grey literature and those that have not been officially published in peer-reviewed journals were not included. These kinds of literature are usually incomplete and it is barely possible to extract all necessary data or evaluate its quality through the NOS scale. As the number of included studies is limited, although the funnel diagram is generally symmetrical, it should be recognized that publication bias may still affect the reliability of the comprehensive analysis of the results. Nonetheless, for diseases for which there is neither a standard treatment plan in the clinic nor a sufficient amount of relevant literature, a meta-analysis of observational research may be one of the sole possibilities for therapeutic suggestion [37, 38]. Meanwhile, efforts should be made to improve the quality of meta-analyses, such as heterogeneity analysis, formal quality assessment, and sensitivity analysis [37, 39]. More studies focusing on external radiation combined with sorafenib for the treatment of HCC are anticipated to be conducted in the future to provide the best evidence for clinical decision-making.
5ConclusionsThis meta-analysis demonstrated that external radiotherapy combined with sorafenib is a rational strategy for the clinical treatment of HCC. It has a low risk of adverse effects and provides large survival benefits. This finding will be supported by research with larger sample sizes in other populations. Furthermore, experimental studies must be necessary to reveal the precise mechanism of synergistic effect between external radiotherapy and sorafenib.
FundingThis work was supported by a grant from the Project of Science and Technology Department of Sichuan Province (2020JDTD0036).
Author's contributionsJiali Chen, responsible for the integrity of the whole article, the analysis of the article data and the writing of manuscripts. Kun He, responsible for statistical analysis. Yunwei Han, responsible for writing manuscripts. Lu Guo, responsible for screening literature and extracting data. Ke Su, responsible for screening literature and extracting data. Zhenying Wu, responsible for extracting data.
Declarations of interestNone