We initiated this multicenter study to integrate important risk factors to create a nomogram for hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) for clinician decision-making.
Patients and MethodsBetween April 2011 and March 2022, 2281 HCC patients with an HBV-related diagnosis were included. All patients were randomly divided into two groups in a ratio of 7:3 (training cohort, n = 1597; validation cohort, n = 684). The nomogram was built in the training cohort via Cox regression model and validated in the validation cohort.
ResultsMultivariate Cox analyses revealed that the portal vein tumor thrombus, Child–Pugh class, tumor diameter, alanine aminotransferase level, tumor number, extrahepatic metastases, and therapy were independent predictive variables impacting overall survival. We constructed a new nomogram to predict 1-, 2-, and 3-year survival rates based on these factors. The nomogram-related receiver operating characteristics (ROC) curves indicated that the area under the curve (AUC) values were 0.809, 0.806, and 0.764 in predicting 1-, 2-, and 3-year survival rates, respectively. Furthermore, the calibration curves revealed good agreement between real measurements and nomogram predictions. The decision curve analyses (DCA) curves demonstrated excellent therapeutic application potential. In addition, stratified by risk scores, low-risk groups had longer median OS than medium–high-risk groups (p < 0.001).
ConclusionsThe nomogram we constructed showed good performance in predicting the 1-year survival rate for HBV- related HCC.
Hepatocellular carcinoma (HCC) carries a high malignancy and death rate [1]. At the time of diagnosis, the majority of HCC is in an advanced stage with a poor prognosis [2]. Therefore, current research is focused on enhancing the prognosis and efficacy monitoring of HCC.
Today, the development of programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) inhibitors provides HCC patients with new daybreak. With a median overall survival (mOS) of 19.2 months, the combination of atezolizumab and bevacizumab has emerged as the standard of care for HCC [3]. Our prior research also indicated that radiotherapy combined with tislelizumab and lenvatinib could increase the survival of patients with advanced HCC (median progression-free survival, mPFS = 10.2 months; mOS = 20.3 months) [4]. Furthermore, selective internal radiation therapy (SIRT), surgery, transcatheter arterial chemoembolization (TACE), and other treatment techniques have promising therapeutic outcomes in HCC [5–7].
The prognosis of HCC has been improved, but unfortunately, the patients are still prone to short- term recurrence even after undergoing radical surgery. In addition, HBV infection is the major cause of HCC in the Asia-Pacific region, including China, Japan, and South Korea [8,9]. Therefore, it is important to develop new treatment strategies and regular monitoring to improve the long-term survival rate of HBV-related HCC patients.
At present, several staging systems, such as the American Joint Committee on Cancer (AJCC) [10], Okuda staging systems [11], Barcelona Clinic Liver Cancer (BCLC) [12], and albumin–bilirubin (ALBI) [13], are being utilized to forecast the prognosis and direct the treatment of HCC. However, the predictive ability of these methods is restricted due to the fact that individual survival prognosis is dependent on several variables, including age, pathological characteristics, tumor diameter, clinical characteristics, radiotherapy, surgery, and chemotherapy [14,15]. Therefore, these parameters should be examined for improved HCC management.
A nomogram is a clinical prediction model based on multivariate regression analysis, which can integrate multiple predictors to better evaluate prognosis [16]. In addition, the nomogram can be customized to calculate an individual's survival rate, therefore it has potential clinical utility. Currently, HBV-related HCC has generated a tremendous global burden of disease. However, there is a scarcity of large-sample-size research to build nomograms of HBV-related HCC.
We initiated this multicenter study to integrate important risk factors to create a nomogram for HBV-related HCC for clinician decision-making.
2Patients and Methods2.1PatientsFrom April 2011 to March 2022, we consecutively enrolled a total of 2281 HBV-related HCC patients from 4 tertiary hospitals in China. The inclusion criteria were as follows: a) complete clinical data; b) age ≥ 18 years; and c) clinically or pathologically diagnosed HCC. Patients with hepatitis C virus (HCV), liver transplantation, and other types of malignancies were excluded.
2.2Data collectionWe gathered patient information from the patient's case file. These data included age, sex, HBV, smoking, alcohol, alanine aminotransferase (ALT), diabetes, hypertension, cirrhosis, portal vein tumor thrombus (PVTT), portal hypertension, Child-Pugh class, albumin–bilirubin, AFP, leukocyte, BCLC stage, tumor size, extrahepatic metastasis, tumor number, lymph node metastasis, and treatments. OS was defined as the time from the first day of treatment initiation to the last follow-up or death.
2.3Statistical analysisCategorical variables are expressed as percentages and absolute values and analyzed by the chi-square (χ2) test. We divided all patients into two groups at random in a 7:3 ratio (training cohort, n = 1597; validation cohort, n = 684). We first performed univariate Cox analysis in the validation cohort to identify prognostic indicators (p < 0.05) and then we introduced them into multivariate Cox analysis to identify independent influencing factors. Following that, we developed a new nomogram to predict 1-, 2-, and 3-year survival rates based on independent prognostic factors influencing OS. To validate the nomogram, concordance index (C-index), calibration curves (1000 bootstrap resamples), decision curve analyses (DCA), receiver operating characteristics (ROC) curve, and nomogram risk score were used. Patients were divided into three groups (high risk, moderate risk, and low risk) based on the x- tile risk score with the best cut-off value (Yale University, New Haven, CT). R 3.3.2 software and SPSS (version 26.0) were used for all statistical analyses. Two-sided P < 0.05 were considered statistically significant.
2.4Ethical statementsThis retrospective study was approved by the Ethics Committee of The Affiliated Hospital of Southwest Medical University (approval number KY2020254) and complied with the standards of the Declaration of Helsinki. The Ethics Committee waived the requirement for informed consent because of the retrospective study.
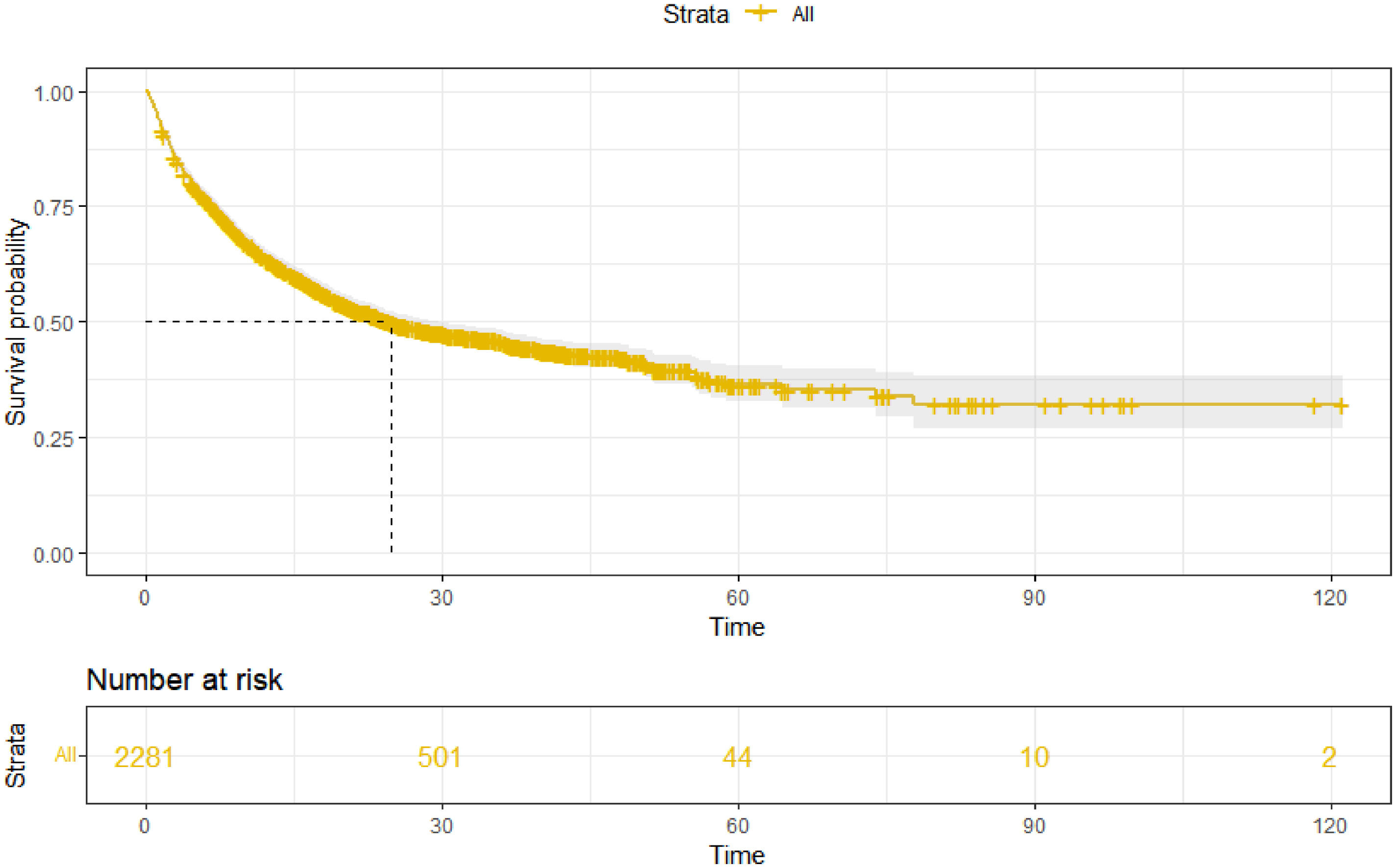
3Results3.1Patient characteristics and overall survivalOur study included 2281 HBV-related HCC patients (training cohort, n = 1597; validation cohort, n = 684). The percentages of age ≥ 65 years, child A, male, AFP < 200 ng/ml, and ALBI-2 grade were 35.1%, 73.2%, 86.7%, 50.3%, and 60.8%, respectively. Moreover, the majority of patients have multiple tumors (69.9%) and are at stage C of BCLC (63.1%). The baseline characteristics of the training and validation cohort are shown in Table 1. As of May 2022, a total of 1145 (50.2%) patients died in this study. The median follow-up time was 28.6 (27.1–30.1) months, and the mOS was 24.9 (22.2–28.9) months (Fig. 1).
Baseline characteristics of the training cohort and validation cohort.
ALBI, albumin-bilirubin; AFP, alpha-fetoprotein; ALT, alanine aminotransferase; BCLC, Barcelona Clinic Liver Cancer; PVTT, portal vein tumor thrombus; TACE, transcatheter arterial chemoembolization; RFA, radiofrequency ablation; ICI, immune checkpoint inhibitor.
In the training cohort, univariate and multivariate Cox analyses revealed that Child–Pugh class, ALT level, tumor number, tumor diameter, PVTT, extrahepatic metastases, and treatment (all p < 0.05) were independent prognostic factors affecting OS (Table 2).
Univariate and multivariate Cox analysis of overall survival in the training cohort Univariable Cox regression Multivariable Cox regression.
ALBI, albumin-bilirubin; AFP, alpha-fetoprotein; ALT, alanine aminotransferase; BCLC, Barcelona Clinic Liver Cancer; PVTT, portal vein tumor thrombus; TACE, transcatheter arterial chemoembolization; RFA, radiofrequency ablation; ICI, immune checkpoint inhibitor.
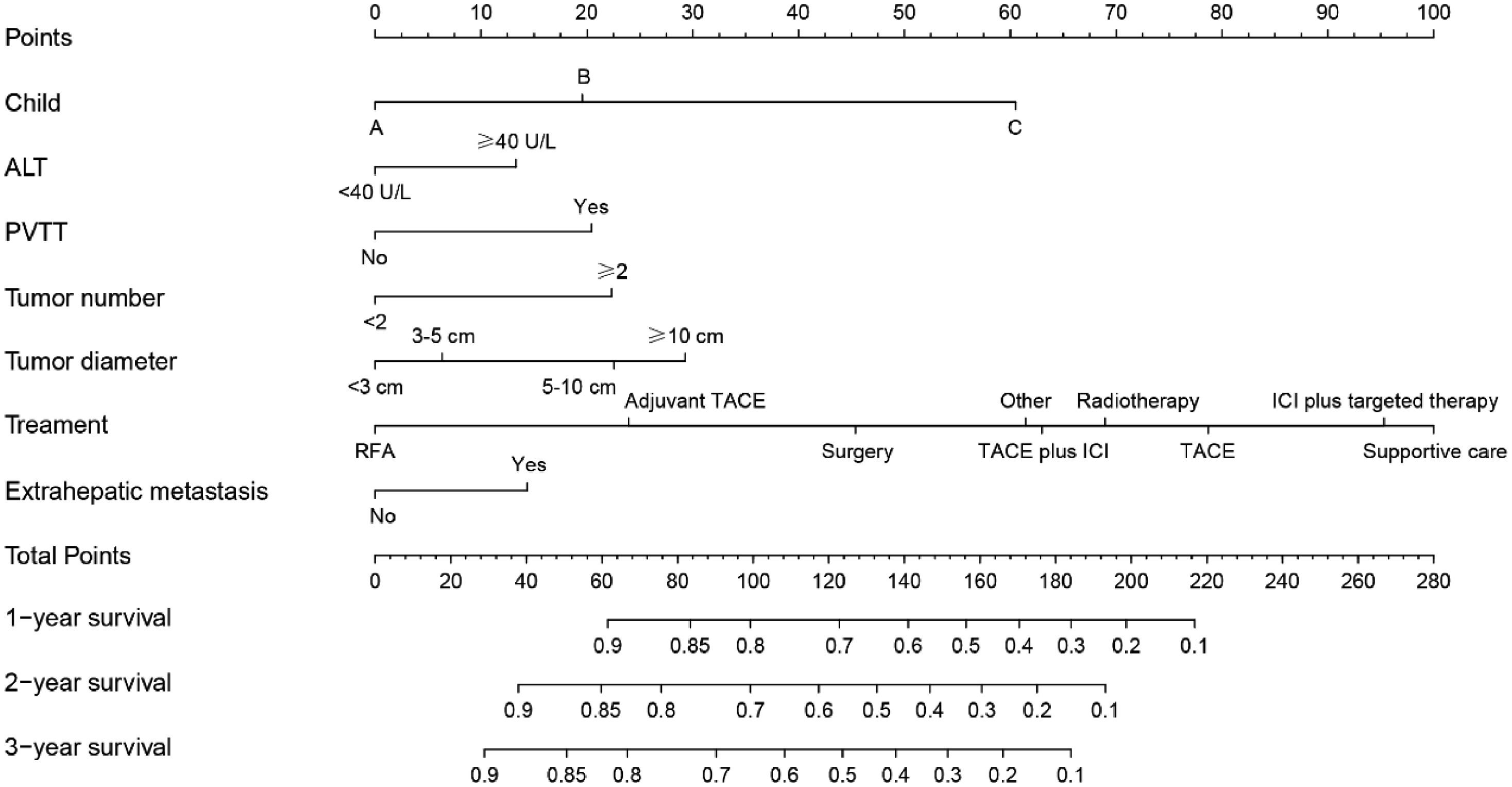
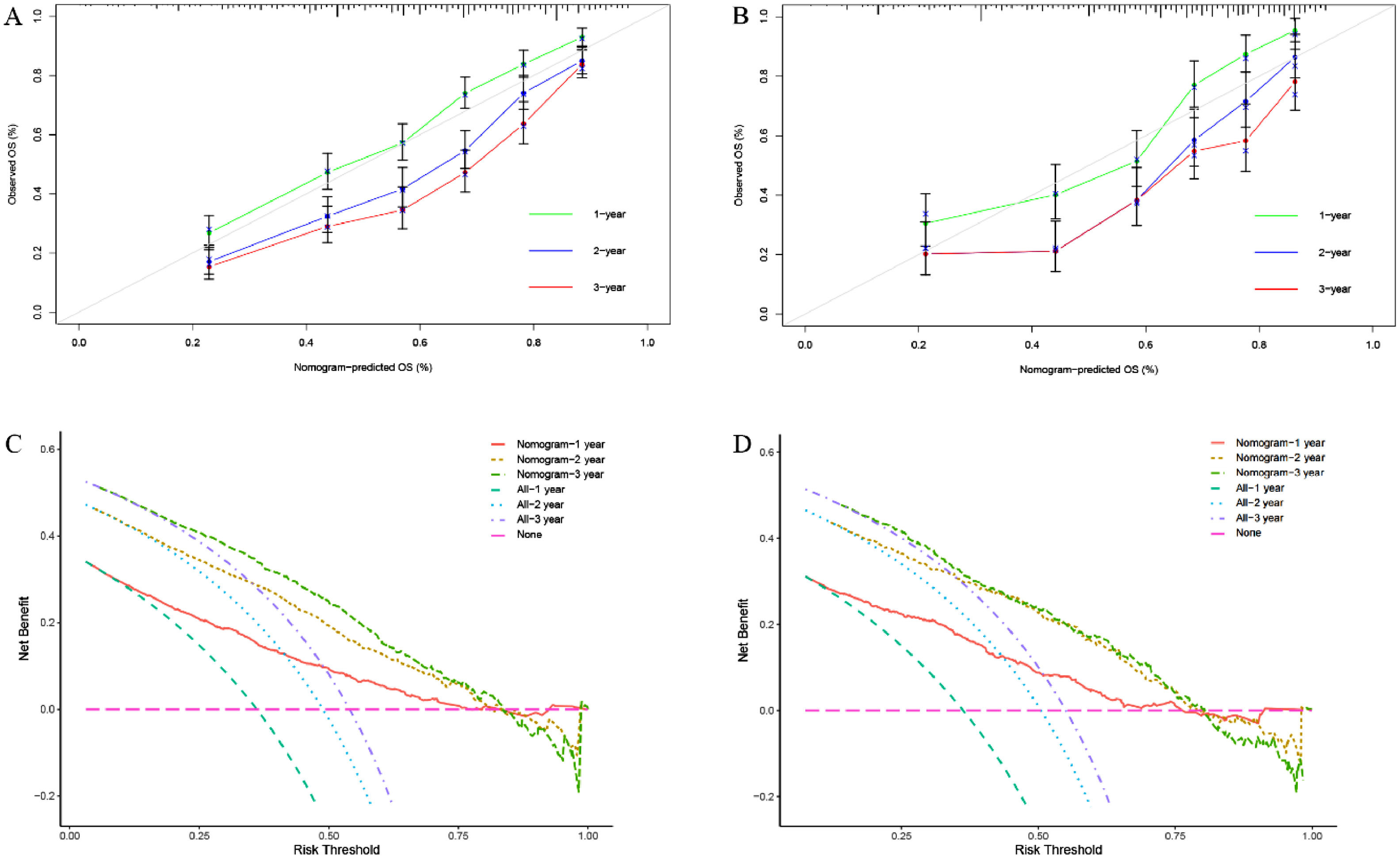
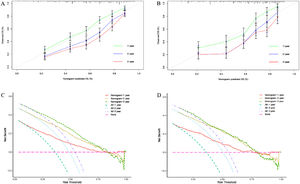
We constructed a nomogram based on independent influencing factors identified in the multivariate analysis to predict 1-, 2-, and 3-year survival rates (Fig. 2). The C-index is 0.743 and 0.754 in the training and validation cohort, respectively. The calibration curves indicated good consistency in predicting 1-, 2-, and 3-year survival rates between actual observations and nomogram predictions in the training (Fig. 3A) and validation cohorts (Fig. 3B). The DCA curves of 1, 2, and 3-year survival rates also expressed promising potential for clinical application in the training (Fig. 3C) and validation cohorts (Fig. 3D). As shown by the DCA [17], the "all" and "none" represented the assumption that all patients were alive and dead, respectively. The net benefit of the nomogram was higher than that of treat none or treat all strategies for the threshold probability within a range of 0.1–0.7.
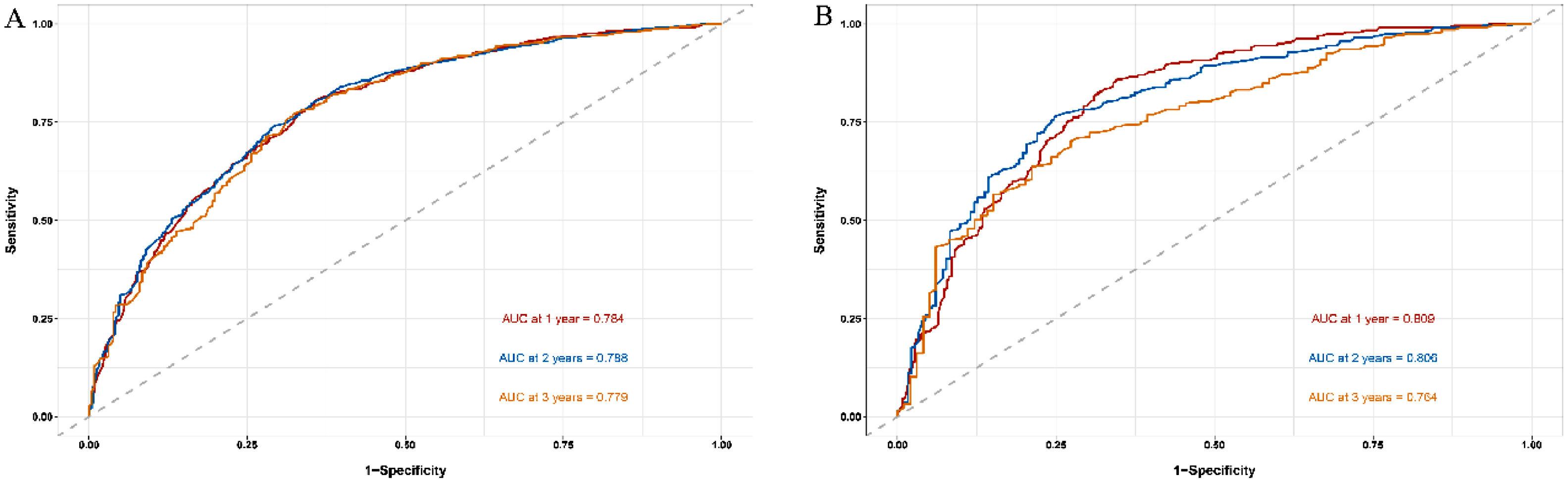
The time ROC package of R software is used to draw ROC curve and calculate AUC value [15]. The nomogram-related ROC curves of the training cohort indicated that the area under the curve (AUC) values were 0.784, 0.788, and 0.779 in predicting 1-, 2-, and 3-year survival rates, respectively (Fig. 4A). In the validation cohort, the AUC values of 1-, 2-, and 3-year survival rates were 0.809 (0.719–0.901), 0.806 (0.706–0.855), and 0.764 (0.629–0.836), respectively, which were higher than the single significant factor affecting the prognosis of HBV-related HCC (Fig. 4B, Supplementary Fig. 1A–G).
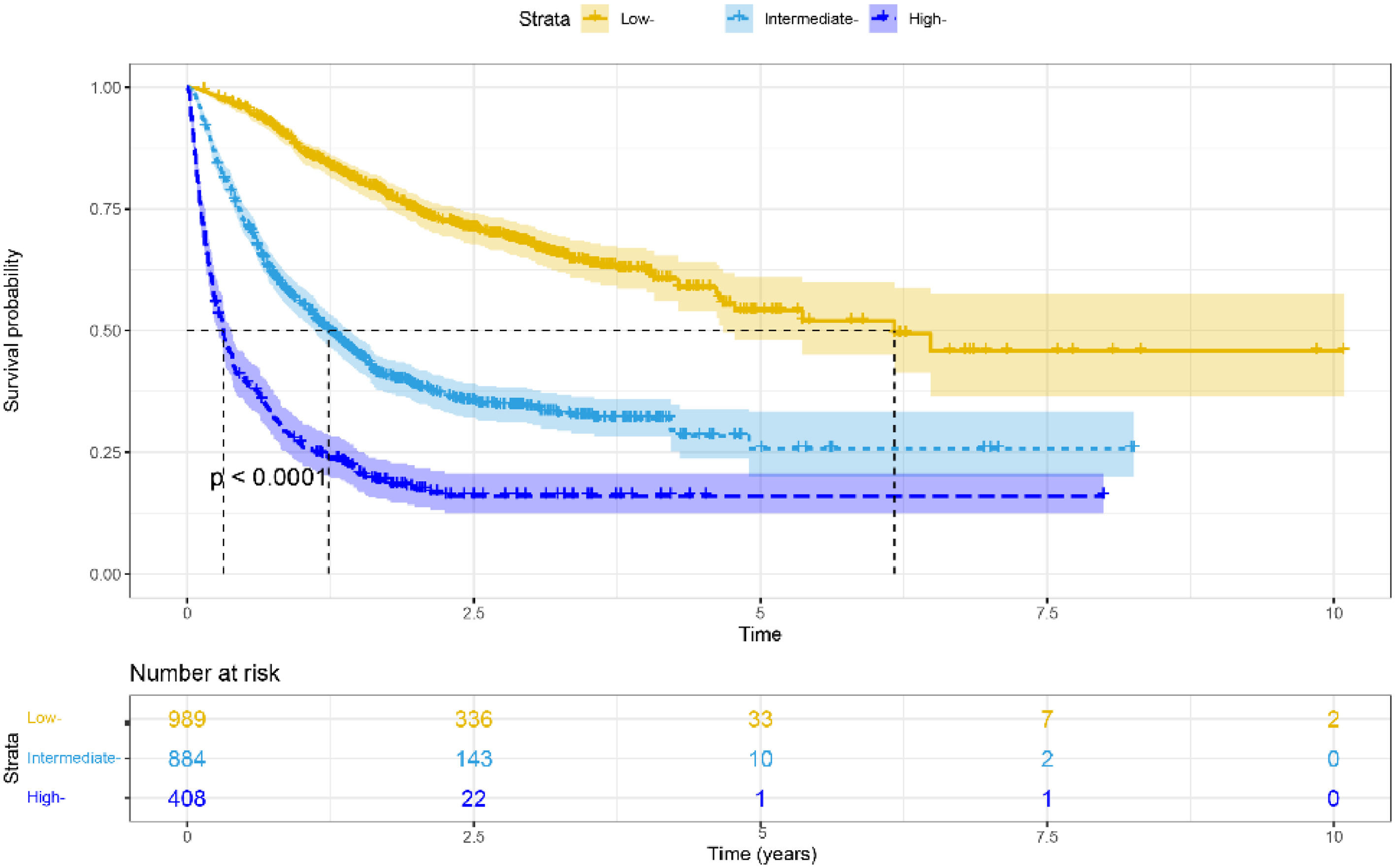
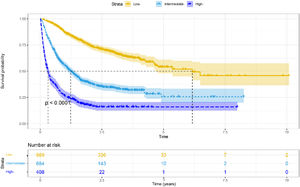
3.4Risk stratificationWe used nomograms to calculate the total point for each patient and then used x-tile software to stratify the data. We divided all HBV-related HCC patients into high-risk (> 211.68), moderate-risk (141.19–211.68), and low-risk (< 141.19) groups based on the optimal cut-off score determined by x- tile. Furthermore, in all cohorts, low-risk groups stratified by risk scores exhibited longer mOS than medium–high-risk groups (6.167 vs. 1.233 vs. 0.317 years, p < 0.001, Fig. 5). Furthermore, similar results were achieved in the training and validation cohorts (Supplementary Fig. 2A-B).
4DiscussionCurrently, HBV is the leading cause of HCC in the Asia-Pacific region [8,9]. We created and validated a prediction model for HBV-related HCC in this large, multicenter, retrospective investigation. The model, which included tumor burden, laboratory testing, and treatment, performed well in terms of prediction. This assists doctors in making clinical decisions on the management of HBV-related HCC.
At the moment, the introduction of immune checkpoint inhibitors has created new opportunities for cancer patients. Various immunotherapy regimens, including atezolizumab + bevacizumab [3], lenvatinib + pembrolizumab [18], camrelizumab + apatinib [19], and TACE + camrelizumab [20], were found to prolong the life of advanced HCC patients. However, predicting the efficacy of HCC still faces great challenges.
Unlike earlier prognostic indicators such as BCLC, ALBI, and Child–Pugh grade, for example, the model we developed estimates each patient's survival rate rather than merely separating patients into different risk categories, so better decreases the impacts of heterogeneity [13,21]. In the nomogram of HBV-related HCC established by Mo [22], C-index was 0.680 (0.645–0.715). And in Zheng's [23] nomogram for HBV-related HCC, the AUC values for predicting 1-, 2-, and 3-year disease-free survival were 0.761, 0.716, and 0.715, respectively. In addition, Huang et al. [24] reported that in the model of advanced HCC receiving stereotactic body radiation therapy, the AUC value for predicting a 1-year survival rate was 0.77. Furthermore, in the HCC nomogram of Zhang et al. [25], the AUC values for predicting 1-, 3-, and 5-year postoperative survival rates were 0.645, 0.671, and 0.635, respectively. In our study, the model we developed includes a wider range of treatment regimens and a bigger number of data, reducing sampling bias. The C-index was 0.754 and the AUC values for the 1-, 2-, and 3-year survival rates were 0.809, 0.806, and 0.764, respectively, which were superior to previous studies [22–25].
In addition, the calibration curves revealed excellent consistency in forecasting 1-, 2-, and 3-year survival rates based on real observations and nomogram predictions. Further risk classification based on the individual's overall score revealed that the low-risk group had a superior mOS than the moderate-to-high-risk group (p < 0.001). Based on these findings, this comprehensive model has potential prediction performance and can be employed in clinical research as a stratification criterion for HBV-related HCC.
In our study, we gathered information such as laboratory tests, past medical history, tumor burden, liver function, staging, demographic information, and treatment, among other things. The Child–Pugh class, ALT, PVTT, tumor number, tumor diameter, extrahepatic metastasis, and treatment were all found to be independent predictors of survival, prompting the development of a new nomogram. Previous research has also found that these indicators are related to prognosis [5,13,21,26,27]. Moreover, the AUC values of models constructed by combining tumor burden, laboratory tests, and treatment were greater than those of the individual predictors in our study. This further demonstrates that the model we developed is an intuitive clinical tool with good predictive performance to assist physicians in making rational HBV-related HCC treatment decisions. The constructed nomogram also illustrated the impact of various treatments on prognosis. Patients receiving only supportive care had the highest risk scores, whereas patients receiving postoperative adjuvant TACE had a higher survival rate than those receiving only surgery. Similar phenomena have been described in prior research [28,29]. Patients receiving radiofrequency ablation (RFA) had the lowest risk scores, which was a striking anomaly. Numerous studies have reported comparable efficacy between RFA and surgery [6,30,31]. This may be due to the small number of training cohort patients who received RFA (n = 53). In addition, risk scores for various treatments are not recommended as a direct guide for physicians to make treatment decisions because different treatments have different indications. Clinical decisions should be based on tumor burden and patient comorbidities, among other factors.
Although some nomograms for predicting the prognosis of HBV-related HCC have been developed [22,32], the model we developed has a larger sample size and more comprehensive data. Furthermore, this is the first multicenter, large-sample study to include multiple treatment modalities in predicting the prognosis of HBV-related HCC. It provides a theoretical foundation for the current comprehensive HCC treatment. This helps clinicians make clinical decisions about individualized HBV-related HCC treatment and lays the groundwork for the clinical management of high-risk patients.
In spite of the aforementioned benefits, the limitations of our study cannot be overlooked. First, because this is a retrospective study, we cannot disregard the possibility of selective bias. Nonetheless, the large number of samples increases the model's reliability. The heterogeneity generated by different immune checkpoint inhibitors (ICIs) also affects the predictive performance of our model, despite the inclusion of immunotherapy in our study. Besides, the lack of external validation also affects our interpretation of the results. In addition, we cannot provide data on the causes of death and perform competing risks analysis. Finally, we cannot directly compare the different available nomograms with their prediction rule in the same cohort of patients due to the lack of relevant data. Future experiments will require prospective studies and more specific drugs to further validate the model we constructed.
5ConclusionsThe nomogram we constructed showed good performance in predicting a 1-year survival rate for HBV- related HCC. For HBV-related HCC patients with a high risk of death, a close surveillance program and adjuvant therapy should be considered.
FundingThis work was supported by Sichuan Science and Technology Program (2022YFS0622).
Author ContributionsKe Su, Han Li, Lu Guo, Ke Xu, Tao Gu, Hao Chi, Yanlin Liu, Xueting Li, Lianbin Wen, Yanqiong Song, Kun He, Qulian Guo, Jiali Chen, Zhenying Wu, Yi Jiang, and Yunwei Han collected the data. Yunwei Han and Kun He designed the research study. Ke Su, Lu Guo, Jian Tong, Tao Gu, and Ke Xu wrote the manuscript and analyzed the data. Qiuni Shen participated in the revision of the manuscript. All authors approved the final version of the manuscript.