Primary biliary cholangitis (PBC) is an autoimmune liver disease, with 60% of patients being asymptomatic at diagnosis and 30% progressing rapidly into liver fibrosis. Liver biopsy is standard for staging fibrosis, but performance of non-invasive methods such as transient elastography (TE) have not been evaluated. We conducted a meta-analysis of articles up to May 2022 to evaluate the performance of TE compared with liver biopsy in adult patients with PBC.
Materials and MethodsTwo reviewers performed the search and assessed which articles were included. The quality of each study was evaluated according to QUADAS-2 and NOS. Meta-analysis of sensitivity and specificity was conducted with a bivariate random-effects model. The protocol was registered in PROSPERO, ID CRD42020199915.
ResultsFour studies involving 377 patients were included. Only stages F3 and F4 were computed in the meta-analysis. TE had a pooled sensitivity of 68% and specificity of 92% for stage F3 and a pooled sensitivity of 90% and specificity of 94% for stage F4. The AUROC curves were 0.91 (95% Confidence Interval (CI) 0.88–0.93) and 0.97 (95% CI 0.96–0.98) for stages F3 and F4, respectively. The mean cut-off points of TE for stage F3 were 9.28 kPa (95% CI 4.98–13.57) and for stage F4 were 15.2 kPa (95% CI 7.02–23.37).
ConclusionsTE performance compared with liver biopsy in adult patients with PBC was excellent for staging liver fibrosis and was able to rule out cirrhosis in clinical practice.
Primary biliary cholangitis (PBC) is a chronic autoimmune liver disease characterized by progressive destruction of the bile ducts in the presence of highly specific autoantibodies [1]. In 2018, the Fibrotic Liver Diseases Consortium reported an overall incidence of PBC of 4.3 cases per 100,000 habitants and a prevalence of 23.9 cases per 100,000. Over the last seven years, prevalence has increased from 21.7 to 39.2 per 100,000 population, although the incidence has not changed [2,3]. It is known that females (ratio 3.9:1) and people over 60 years in Europe and Asia have a higher prevalence of PBC [3].
About 60% of cases are asymptomatic and are diagnosed upon work-up of abnormal liver enzymes or cholestasis [1]. Asymptomatic patients have a 10-year survival of 70%, in comparison with symptomatic patients in whom survival ranges from 5 to 8 years after onset of symptoms [1]. PBC is strongly associated with other autoimmune diseases, such as Sjögren's syndrome and Hashimoto's thyroiditis, among others.
Patients with PBC and liver cirrhosis have an estimated survival rate of 30% at 12 years [4]. Therefore, assessment of liver fibrosis in PBC is a mainstay of risk stratification to decide treatment to prevent further complications and improve patient survival [5].
Liver biopsy is considered the gold-standard for staging liver fibrosis. However, the complication rate ranges from 0.8% to 1.7%, and the mortality rate is up to 0.14% [6]. Consequently, non-invasive fibrosis assessment has been proposed as a surrogate for liver biopsy.
The transient elastography allows measurement of the velocity of a sound wave passing through liver tissue and the elastic restoring forces in the tissue that act against shear deformation, and that, correlates with the degree of liver fibrosis and indirectly, with portal hypertension [7,8]. Although this test, have not been evaluated in cholestatic liver diseases, so, in this systematic review and meta-analysis, we evaluated the diagnostic performance of TE using FibroScan® for the evaluation of fibrosis in PBC.
2Materials and Methods2.1Data search and sourcesA comprehensive search of articles indexed in MEDLINE (PubMed), EMBASE, and Cochrane databases was performed to obtain studies that evaluated the diagnostic accuracy of TE for liver fibrosis in PBC and compared its accuracy with liver biopsy from the establishment of the database to May 2022. Search strategies and MESH terms are described in the supplement section (Supplement 1).
2.2Study selections and participantsWe selected: (1) randomized controlled trials, cohort studies, case-control, and cross-sectional studies with information about the diagnostic accuracy, sensitivity, specificity, and positive and negative predictive values; (2) studies evaluating the diagnostic value of TE compared with biopsy as gold standard performed either by percutaneous puncture, trans jugular catheterization, or diagnostic laparoscopy, that determined the degree of fibrosis with one of the following systems, METAVIR, Nakanuma, Scheuer or Ludwig; (3) studies that established the degree of fibrosis through liver biopsy and a non-invasive method by TE, and (4) studies published in English or Spanish, irrespective of publication status.
2.3ParticipantsWe included studies conducted in the adult population (over 18 years old), males and females, and patients with diagnosis of primary biliary cholangitis (formerly known as primary biliary cirrhosis) using the 2 out of 3 criteria for diagnosis: alkaline phosphatase elevated at least 24 weeks, antimitochondrial antibody positive or liver biopsy that shows non-suppurative destructive cholangitis, and destruction of interlobular bile ducts).
2.4Index testThe index test was TE measured in kilo pascals (KPa) using Fibroscan®, with at least 10 valid measurements, with interquartile range (IQR) of 30% or less (operator variability). The time between TE and liver biopsy was recorded as reported in the original manuscript.
Individual study selection and data extraction were performed independently by two investigators (LAMF and JAM). Any differences in the study selection or data extraction were resolved by consultation with all authors.
2.5Data extraction and managementInitially, we reviewed the titles, selected those for abstract review and if the study was considered relevant, the full text was obtained for data collection. Any discrepancies with respect to the relevance of the studies were resolved between all the authors.
We extracted demographic information such as mean or median age of the population, mean or median age by gender, patients who received treatment or not, study characteristics such as first author and publication year, type of study, center or hospital where the study was conducted, diagnostic criteria, the time between biopsy and TE, length of liver biopsy, total patients, biopsy system classification, patients per fibrosis stage, and the information of the index test and gold standard (sensitivity, specificity, and area under the receiver characteristic curve [AUROC]). If the data in the individual study was insufficient, we contacted the first and corresponding authors by e-mail to obtain missing data. If no response was obtained, the study was excluded. A summary of the included studies is presented in the supplement section (Supplementary Material 1 and Table 1).
Characteristics of the studies included.
Floreani and Corpechot includes F0 and F1 in Stage 1.
SD, standard deviation; kPa, kilo pascals.
The quality of each study was evaluated according to the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool [10]. The assessment of methodological quality (internal validation) was evaluated according to the Newcastle-Ottawa Scale (NOS) [11]. The tools described above are presented in Supplement 1.
2.7Statistical analysis and data synthesisA meta-analysis of sensitivity and specificity of TE for the evaluation and detection of fibrosis at the four stages was conducted with a bivariate random-effects model allowing for heterogeneity among studies. We obtained summary statistics for sensitivity and specificity with 95% confidence intervals (CIs). Forest plots were constructed to demonstrate study sensitivity and specificity. Pooled summary statistics for sensitivities and specificities for the individual studies and stages were reported by means of a random-effect model. A hierarchical summary receiver operating characteristic (ROC) curve was plotted with a 95% confidence contour and area under the curve (AUC).
Results were presented in AUROC curves to estimate the performance of the test. Only stages 3 and 4 were computed for meta-analysis since there was not enough information for stages 1 and 2. An I2 statistic > 50% was considered substantial for heterogeneity [12]. All statistical analyses were performed using Stata 17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX) and the statistical package MIDAS (Stata module for meta-analytical integration of diagnostic test accuracy studies, Statistical Software Components S456880, Boston College Department of Economics, Boston, MASS) [13].
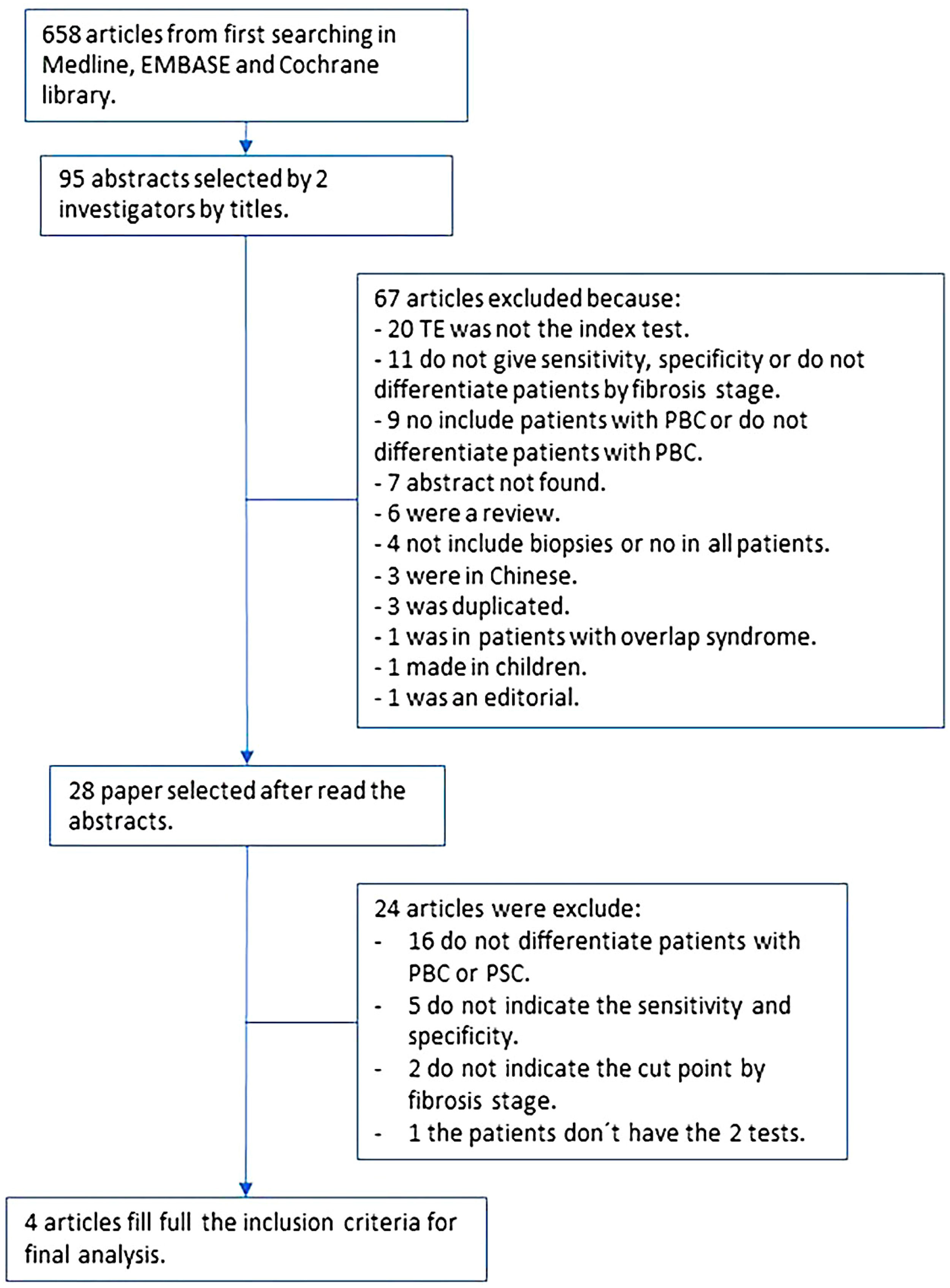
2.8Ethical statementWe conducted a meta-analysis according to the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Fig. 1) [9]. The protocol was registered in the PROSPERO database with ID: CRD42020199915.
3ResultsAfter the search was conducted, we found a total of 658 articles. From those, we selected 95 abstracts, and after applying the inclusion and exclusion criteria, 28 articles were selected for complete reading (Fig. 1). We excluded 16 articles that did not differentiate patients with PBC from primary sclerosing cholangitis. We found nine articles with missing information; only one author answered the request for missing data and was included in the final analysis. The rest of the studies with missing data were excluded either because they did not report sensitivity or specificity, did not report a cut-off point for each state, or did not compare both tests. Finally, four articles were included in the final analysis with a total of 377 patients [14–17].
We found a higher proportion of women versus men (81% vs 9%), with a mean age of 50 years (SD ± 13). The studies were performed in Germany, Spain, France, and Italy. The time between liver biopsy and TE ranged from 3 to 16 months, and the length of the biopsy ranged from 8 to 22 mm (Tables 1 and 3).
Regarding bias assessment, patient selection in all the centers was based on the diagnostic criteria of the disease and had a low risk of bias. The interpretation of the index test had low risk of bias, although there was no pre-established threshold, and it was not specified whether the clinician was aware of the result of the reference standard. The interpretation was valid if the test met the quality criteria. Concerning the reference standard, all patients had a liver biopsy, the pathologists were blinded and used a system that had been validated to classify liver fibrosis. Therefore, the articles included had a low risk of bias (Supplement 1, Table 2).
Results by stage of fibrosis.
CI, confidence interval; AUROC, area under the ROC curve; kPa, kilo pascals; ND, no data.
Using NOS, we granted 3 points to the studies included in the analysis. According to our results, the included studies had a very high risk of bias, particularly in the section on selection, assessment, and comparability (which has 0 points) (Supplement 1, Table 3).
Characteristics of the biopsy.
TE, transient elastography; mm, millimeters; ND, no data.
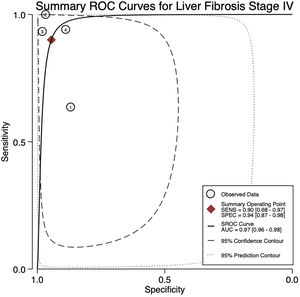
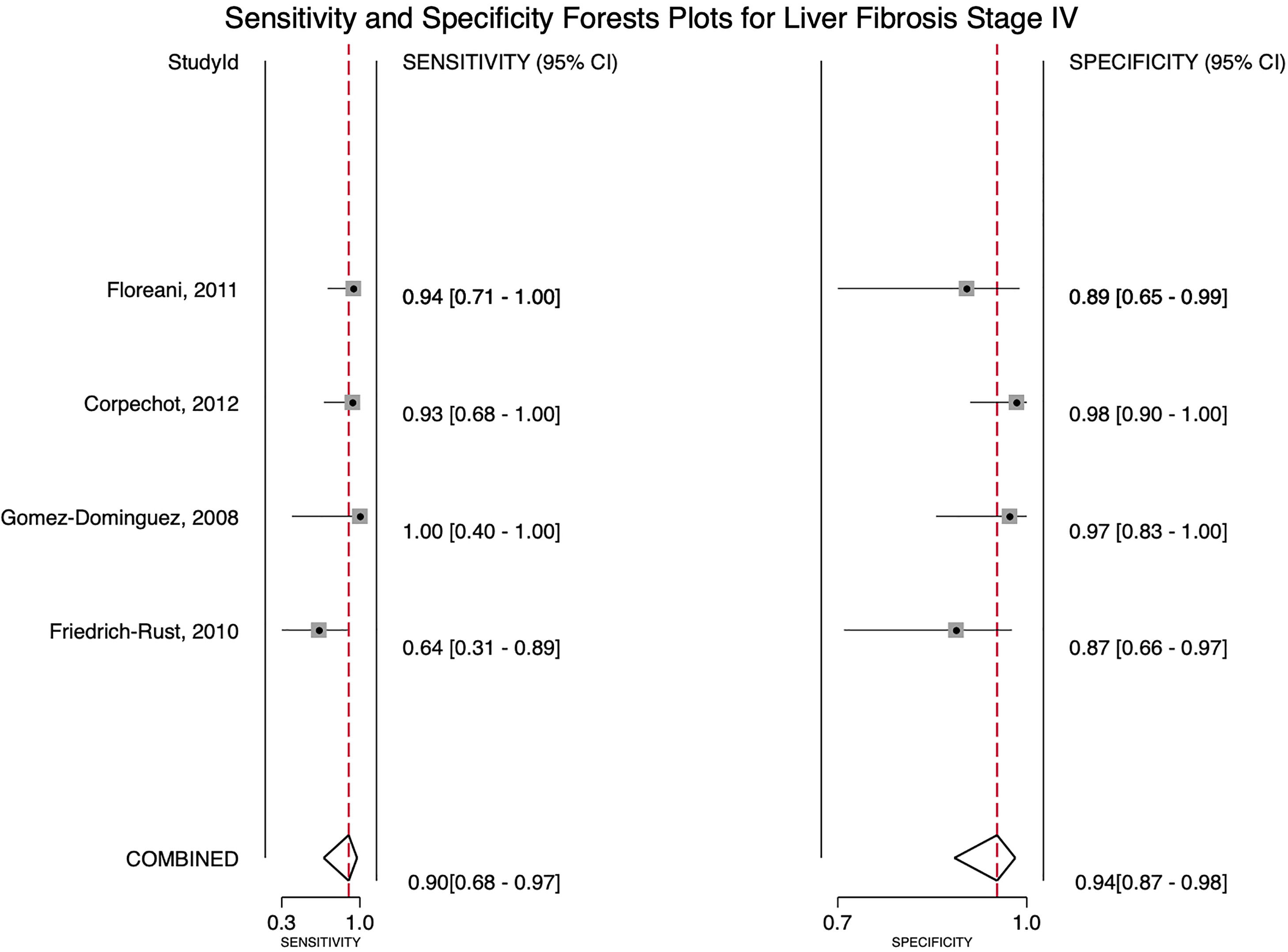
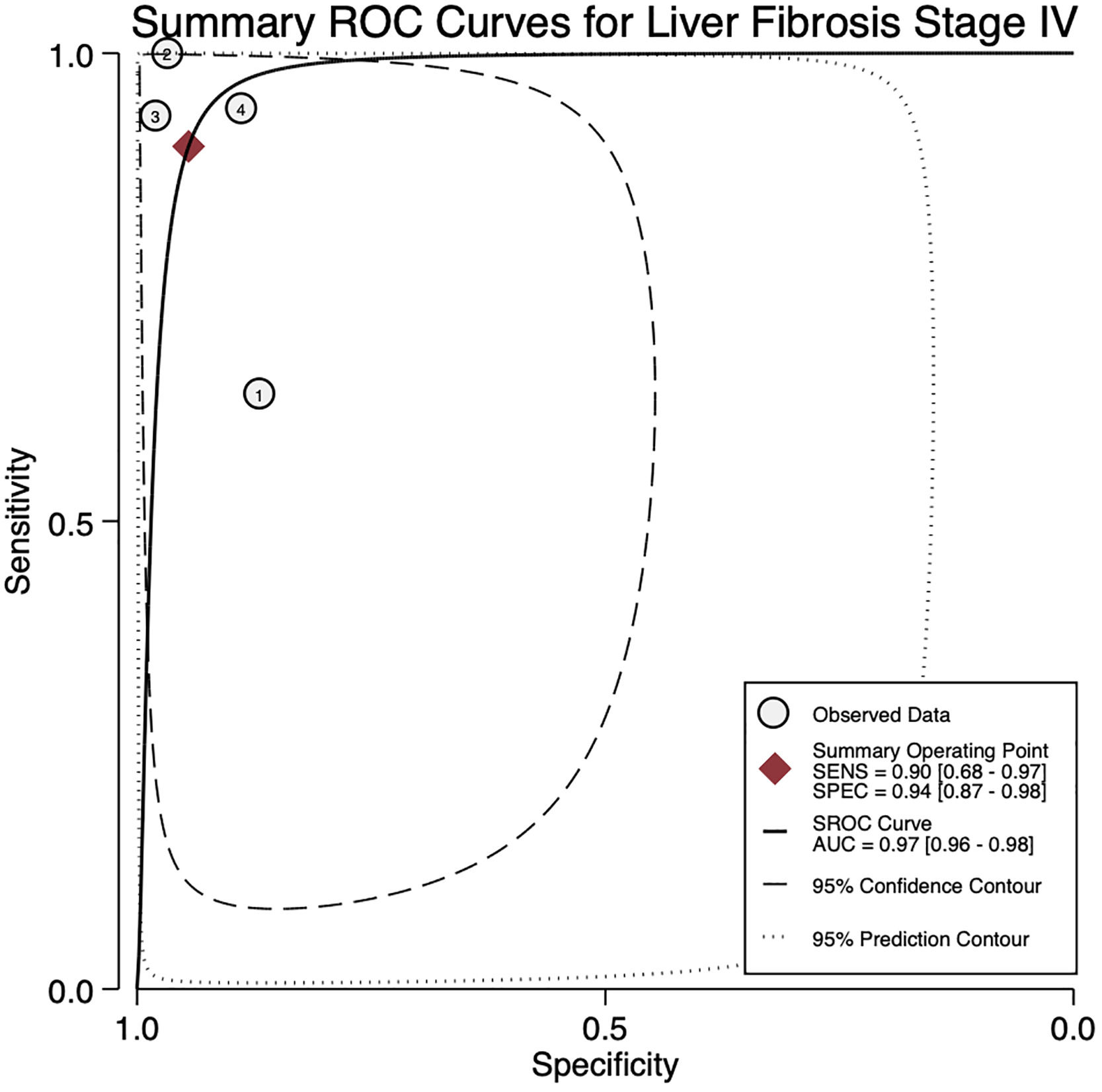
For stage F4, three studies were included with a pooled sensitivity and specificity of 90% (95% CI 68%–97%) and 94% (95% CI 87%–98%), respectively, with no significant heterogeneity, I²= 0 (Fig. 2).
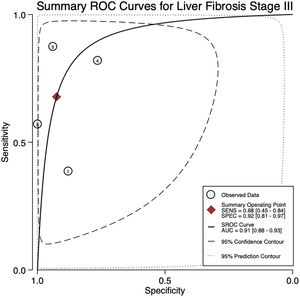
For stage F3, four articles were included with a pooled sensitivity and specificity of 68% (95% CI 45%–84%) and 92% (95% CI 81%–97%), respectively, with significant heterogeneity, I²= 65% (Fig. 3).
The AUROC curves for stages F3 and F4 were 0.91 (95% CI 0.88–0.93) and 0.97 (95% CI 0.96–0.98), respectively (Fig.s 4 and 5).
The mean cut-off points for fibrosis stage F3 were 9.28 kPa (CI 4.98–13.57) and for F4 were 15.2 kPa (CI 7.02–23.37) (Table 2).
Due to insufficient data for stages F1 and F2 in the included articles, we were not able to explore the performance of the non-invasive test.
4DiscussionWe aimed to evaluate the diagnostic performance of TE with FibroScan® for the evaluation of fibrosis in PBC, and in addition, obtain cutoff points for the fibrosis stages. We were only able to calculate results for stages F3 and F4 due to sample size. For stage F4, with a cutoff of 15.2 kPa, we found a sensitivity of 90% and specificity of 94% with no significant heterogeneity and an almost perfect AUROC of 0.97. This suggests, that TE with a cutoff of 15.2 kPa could identify and rule out cirrhosis with a high degree of certainty in clinical practice and could replace liver biopsy. Although the TE cutoff of 9.28 kPa had a low sensitivity, it had high specificity for stage F3, and if the patient has a value of TE below the cutoff point, could rule out an advanced stage of fibrosis (≥ F3).
Three studies were prospective, the fourth was retrospective. All were performed in Europe. The time between the liver biopsy and TE ranged from 3 to 16 months, and this time gap may confer a risk of bias. The total number of patients included was 377, with not enough data for stages F0, F1, and F2.
According to the NOS, studies had a low risk of bias but overall low-quality regarding selection, comparability, and outcomes. Factors such as variability in biopsy characteristics and time between the tests could influence quality since it is unknown if complications of the disease were present at the time of diagnosis.
To our knowledge, this is the first meta-analysis of TE in PBC, and four studies met the inclusion criteria. There are other studies reporting the diagnostic accuracy of TE but have limitations, first, those included patients with other liver diseases such as chronic hepatitis B or C infection, non-alcoholic fatty liver disease (NAFLD), or a combination of etiologies of liver diseases, so, the population was high. For example, the performance of TE in chronic hepatitis B virus infection was reported in 2016 in a meta-analysis of 27 articles including 4,386 patients, with a pooled sensitivity and specificity of up to 80%, and the AUROC near 0.9 for fibrosis stages F2, F3, and F4 [18]. Another meta-analysis which included 50 studies with many liver diseases (chronic infection with HBV and HCV, NAFLD, alcoholic liver cirrhosis, metabolic, hereditary, and autoimmune liver diseases) reported an AUROC for diagnosis of F4 cirrhosis of 0.94, for severe fibrosis (F3) of 0.89, and for significant fibrosis (F2) of 0.84 [19], and the most recent study evaluating TE only in PBC was published in 2012 and reported a sensitivity, specificity, and AUROC in stage F4 of 93%, 99%, and 0.99, respectively. Nonetheless, from the total of 103 patients, only 15 were in stage F4 [15].
The performance reported in the previous articles that had a high number of patients cannot be generalized to a specific liver disease because those articles included all liver diseases since there are some data that suggests that extrahepatic cholestasis may increase the liver stiffness, and intrahepatic cholestasis as well [20].
This study used the data extracted from the final results of 4 papers, since we ask for the complete data from the articles and we have no answers from the authors, for more accurate cutoff points for every fibrosis stage, an individual data meta-analysis should be performed. Some actual studies were not included in the final analysis because they do not give a cutoff for a specific fibrosis stage or not all the patients have both tests [21,22].
We acknowledge some limitations of this study. First, a small number of studies were included in the final analysis with few patients in the early stages of liver fibrosis or no data reported in the articles. Second, there was missing data in some studies including gender, size of the biopsy, and number of patients by stage of fibrosis. Third, included studies had a high risk of bias in selection, assessment, and comparability. Fourth, only European patients were included in the studies; therefore, our results cannot be generalized. There is evidence that in Latin populations some characteristics of PBC are different, such as the diagnostic performance of anti-mitochondrial antibodies and the response to treatment with ursodeoxycholic acid [23,24]. Fifth, the time elapsed between the liver biopsy and TE was highly variable across the studies (from 3 to 16 months), hence the results of the TE may not reflect the true stage of liver fibrosis at the time. Finally, in two studies almost all the patients had received treatment for the disease, and the population was highly heterogeneous.
The major strength of our study is the application of strict inclusion criteria whereby we only selected studies describing outcomes in patients with PBC.
5ConclusionsDespite the limited number of studies, TE measured by Fibroscan® has an excellent performance in its ability to identify and rule out patients with advanced fibrosis and cirrhosis, and may help physicians to stratify patients with PBC, particularly in the European population. These results should be interpreted with caution in stage F3 since a high heterogeneity among studies was found, and high-quality research in other populations should be performed.
More studies with a high number of patients included in the early stages of fibrosis are needed to understand the performance of the test in stages of fibrosis F1 and F2. In addition, information on a Latin-American population is lacking. It is important to know if the test shows similar performance in this population and whether the diagnostic accuracy can be generalized to all populations.
Author contributionsGuarantor of the article: Norberto Chavez-Tapia. Specific author contributions: Manzo-Francisco LA, Aquino-Matus J, Uribe-Esquivel M and Chavez-Tapia NC developed the concept and designed the study; Manzo-Francisco LA and Aquino-Matus J collected data; Chavez-Tapia NC and Vidaña-Pérez D participated in statistical analysis and interpretation of data; Vidaña-Pérez D, Manzo-Francisco LA and Aquino-Matus J wrote the original draft; Uribe-Esquivel M and Chavez-Tapia NC reviewed the final manuscript. All authors approved the final version of the manuscript.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.







![Forest plot for stage of fibrosis F4 sensitivity and specificity (Q=0.263, df=2.00, P=0.438, I2 0.00 [0-100]). Forest plot for stage of fibrosis F4 sensitivity and specificity (Q=0.263, df=2.00, P=0.438, I2 0.00 [0-100]).](https://static.elsevier.es/multimedia/16652681/0000002800000004/v1_202306271139/S1665268123002119/v1_202306271139/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Forest plot for stage of fibrosis F3 sensitivity and specificity (Q=5.780, df=2.00, P=0.028, I2 65.00 [22.01-98.99]). Forest plot for stage of fibrosis F3 sensitivity and specificity (Q=5.780, df=2.00, P=0.028, I2 65.00 [22.01-98.99]).](https://static.elsevier.es/multimedia/16652681/0000002800000004/v1_202306271139/S1665268123002119/v1_202306271139/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)