Hepatitis E virus (HEV) is one of the most common causes of acute hepatitis. In recent years, its role in the development of chronic hepatitis and cirrhosis especially in immunosuppressed patients and its wide range of extrahepatic involvement have increased the amount of research on HEV. In this study we aimed to investigate the presence of HEV infection in individuals with cryptogenic cirrhosis.
Materials and methodsHEV antibodies were analysed using the Anti HEV enzyme-linked immunosorbent assay (ELISA) kit (anti-HEV ELISA; Diapro Prodiagnostic Bioprobes, Milan, Italy). HEV RNA was isolated with using QIAMP Viral RNA mini kit (QIAGEN, Hilden, Germany). The HEV RNA titre was detected with the Rotor Gene 3000 real time polymerase chain reaction (PCR) system using GenoSen's HEV (Rotor Gene) Quantitative Real Time PCR Kit (Genome Diagnostics Private Limited, the Netherlands).
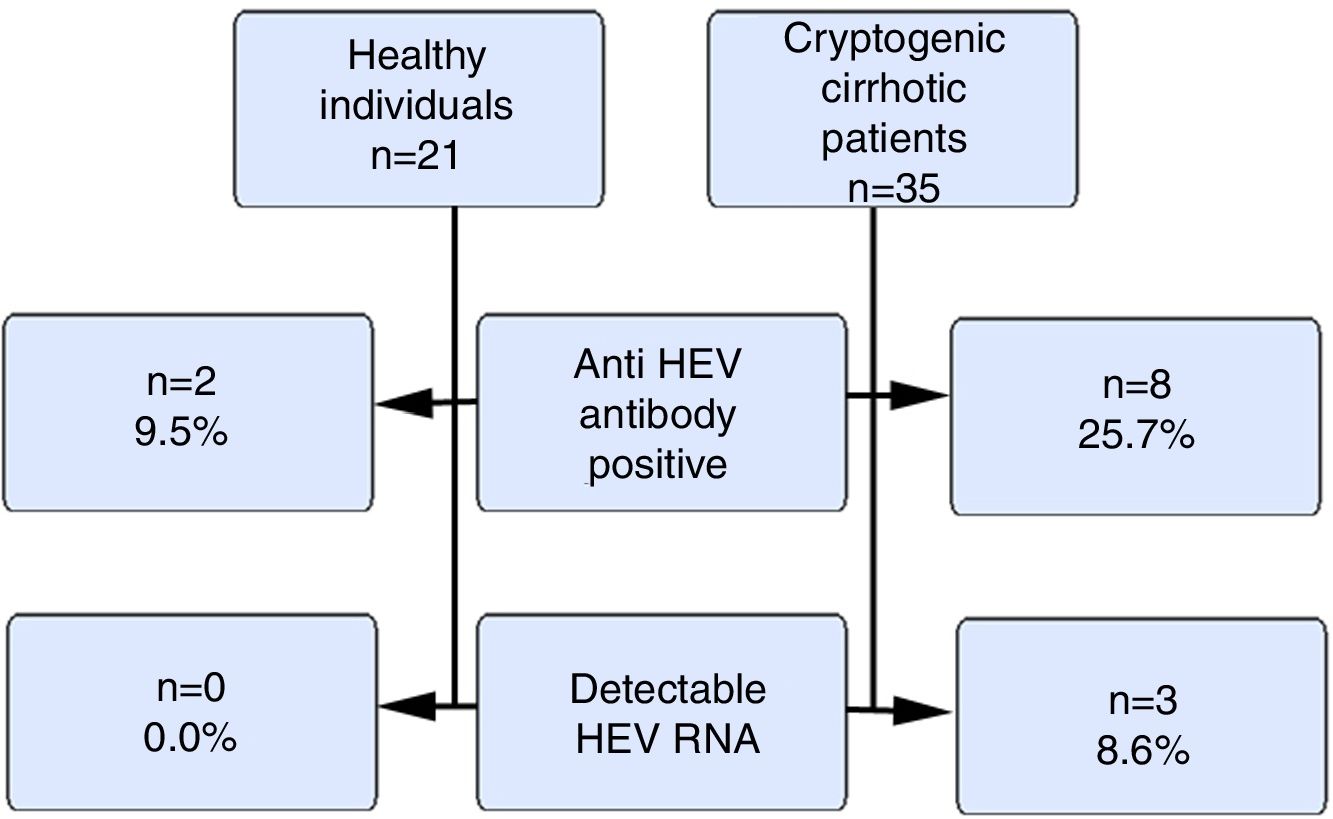
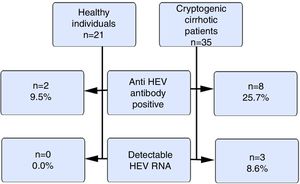
ResultsOur study included 21 healthy volunteers (12 males) and 35 cryptogenic cirrhosis patients (19 males). The ages of the patients and the controls were similar (46±12.1 vs. 37.5±9.7years). The mean Child–Pugh score was 8±2.5. The anti HEV immunoglobulin G(IgG) positivity rate was 9.5% and 25.7% in the control and patient groups respectively (p>0.05). HEV RNA positivity was not detected in the control group, but 3 cases (8.6%) in the patient group were positive (p>0.05). The HEV RNA, aspartate aminotransferase (AST) and alanine aminotransferase(ALT) levels for these 3 cases were 326.461copies/mL, 91IU/L and 67IU/L; 480copies/mL, 68IU/L and 36IU/L and 72copies/mL, 42IU/L and 24IU/L respectively. There were positive correlations between HEV RNA levels and AST and ALT levels (p<0.05).
ConclusionsAnti HEVIgG and HEV RNA positivity rates are high in cryptogenic cirrhosis although it is not statistically significant and there is a positive correlation between HEV RNA and aminotransferases.
Hepatitis E virus (HEV), one of the most common causes of acute hepatitis, affects 20 million people annually; 3 million cases are symptomatic, and 60,000 are fatal [1,2]. In the 1980s HEV was first classified as non-hepatitis A, non-hepatitis B virus. Waterborne HEV exposures led to epidemic outbreaks similar to those caused by hepatitis A. The virus was later described by Balayan [3] as HEV based on the evaluation of stool specimens using the principles of immune electron microscopy [3].
HEV is a small, enveloped, single-stranded RNA virus of the Hepeviridae family[3,4]. According to its epidemiological characteristics and survival indicators, 4 different HEV genotypes that affect humans have been described. Genotypes 1 and 2 cause disease in humans only and are responsible for epidemics through faecal-oral and waterborne transmissions. Genotypes 3 and 4 are more likely to infect domestic and wild pigs and cause infections in humans [4–6].
HEV, one of the most common causes of acute hepatitis, is mostly asymptomatic although it may present with symptoms such as jaundice, fatigue, nausea, loss of appetite and muscle and joint aches that are self-limiting in 4–6 weeks in symptomatic individuals [3,7]. Acute HEV is usually asymptomatic, but especially during pregnancy, in individuals with chronic liver disease, and in patients with active alcohol consumption, resulting in a clinical picture of acute hepatic insufficiency [7]. HEV not only presents a clinical picture of acute hepatitis, but also causes chronic hepatitis and cirrhosis in immunocompromised patients especially in those with a history of solid organ transplantation, haematological malignancies, and HIV positivity [8,9]. In addition to acute and chronic hepatitis, and cirrhosis, HEV can manifest as many extrahepatic clinical findings mainly neurological, renal, pancreatic and haematological clinical findings. Neurological findings related to HEV include polyradiculopathy, Guillain–Barré syndrome, Bell's palsy, ataxia, and mental confusion which make the diagnosis difficult because liver function tests in HEV infection show only a mild hepatitis [3,8].
While the existence of HEV has been known for many years, it is not well characterized, especially in its co-existence with cirrhotic diseases. In particular, there are not many studies in the literature evaluating the relationship between hepatitis E, which is known to be chronic in the immunosuppressed group of patients with impaired T-cell response, and its relationship with cirrhosis which is now considered an immunosuppressive table as it named as cirrhosis-associated immune dysfunction. We think that the investigation of the presence of HEV, especially in the cirrhotic group of patients whose aetiology is not clear, can provide different perspectives in the literature and open new doors to the evaluation and management of the virus. In this study we examined the presence of HEV in cryptogenic cirrhotic patients.
2Materials and methods2.1Sample sizeOur study included total 56 subjects, 21 healthy volunteers (12 males) and 35 cryptogenic cirrhosis patients (19 males) without malignancy, HIV positivity, a history of immunosuppressive drugs use and also without a history of organ transplantation. To determine the sample size of our study power was defined as at least 0.80 and the type 1 error was set to 0.05. The diagnosis of cryptogenic cirrhosis was based on clinical, laboratory and radiological findings. Blood samples were taken from patients diagnosed with cryptogenic liver disease after other aetiologies that could have caused chronic liver disease were excluded.
2.2PatientsHepatitis B, hepatitis C, autoimmune aetiologies, Wilson's disease, haemochromatosis and alcohol consumption were excluded in patients with clinical, laboratory and radiologically diagnosed cryptogenic cirrhosis. Therefore, hepatitis B virus surface antigen, anti hepatitis C virus, hepatitis B virus DNA, hepatitis C virus RNA levels were evaluated in the patients’ blood samples and were negative in all patients. In addition, in terms of causes of liver enzyme reactivation cytomegalovirus DNA and Epstein–Barr virus DNA values were negative. Antinuclear autoantibodies, anti-liver-kidney microsomal antibodies, anti-muscle cell antibodies and antimitochondrial antibodies were evaluated to exclude autoimmune hepatitis and all were found to be negative in all patients. Haemochromatosis and Wilson's disease were excluded by calculating the transferrin saturations, iron parameters and ceruloplasmin levels in all cryptogenic cirrhotic patients. Alpha 1 antitrypsin deficiency was evaluated using protein electrophoresis and the alpha bands of the all patients were determined to be normal. Histological evaluation was not performed with biopsy, which was accepted as the gold standard for the discrimination of cryptogenic cirrhosis and non alcoholic liver disease related cirrhosis. Because all patients were in the cirrhotic stage, non alcoholic liver disease discrimination was done by non-invasive methods. Accordingly, the metabolic parameters and body mass indexes of the patients were taken into consideration. All biochemical parameters of the patients were also evaluated. None of the patients had a history of diabetes mellitus. The lipid profiles of the patients were checked and no dyslipidemia was observed when these findings were evaluated. Body mass indexes of HEV RNA positive patients were determined to be <30kg/m2.
The patients were informed about the study and their written consents were obtained in accordance with the Declaration of Helsinki. Approval of local ethical committee was also obtained.
2.3Serum samplesSerum samples were obtained upon admission, stored at −85°C and were used for the analyses. Simultaneous biochemical studies were performed on the day that the patients’ serum samples were taken.
2.3.1Detection of anti-HEV antibodiesHEV antibodies were analysed by the Anti HEV ELISA kit (anti HEV ELISA; Diapro Prodiagnostic Bioprobes, Milano, Italy).
2.3.2HEV RNA detection by real-time PCRHEV RNA, was isolated using the QIAMP Viral RNA mini kit (QIAGEN, Hilden, Germany). The HEV RNA titres were detected with a Rotor Gene 3000 real time PCR system using GenoSen's HEV (Rotor Gene) Quantitative Real Time PCR Kit (Genome Diagnostics Private Limited, The Netherlands).
A prior study has evaluated the sensitivity and specificity of the kits we used to measure anti-HEV immunoglobulin G (IgG) (anti HEV ELISA; Diapro Prodiagnostic Bioprobes, Milano, Italy). This study reported that the sensitivity was 77.5%–98% and the specificity was 96% [10].
3Statistical analysisDescriptive statistics for the continuous (numeric) variables are presented as medians, means, standard deviations, minimums and maximums; whereas the categorical variables are presented as numbers and percentages. Non-parametric tests were used because the continuous variables did not have a normal distribution. The Mann–Whitney U test was used for comparisons between the two groups. Chi-square tests were used to determine the relationship between the groups and the categorical variables. The level of statistical significance was set to (α) 5% and the SPSS (IBM SPSS for Windows, version 24) was used for analyses.
4ResultsThe mean ages of the patient and control groups in this study were similar. The mean age of the patients was 46±12.1 years while the mean age of healthy volunteers was 37.5±9.7 years. The mean values for AST, ALT, alkaline phosphatase (ALP), gamma glutamyltransferase (GGT), and total bilirubin levels of the patients were 47.4±23IU/L, 34.2±18IU/L, 200.8±93IU/L, 69.6±86IU/L, and 2.14±1.4mg/dL, respectively. Thirty percent of the patients were diagnosed with compensated cirrhosis and, 70% were diagnosed with decompensated cirrhosis. The mean Child–Pugh score was 8±2.5(range 5–12).
Fig. 1 summarizes the anti HEVIgG and HEV RNA findings of the control and the patient groups.
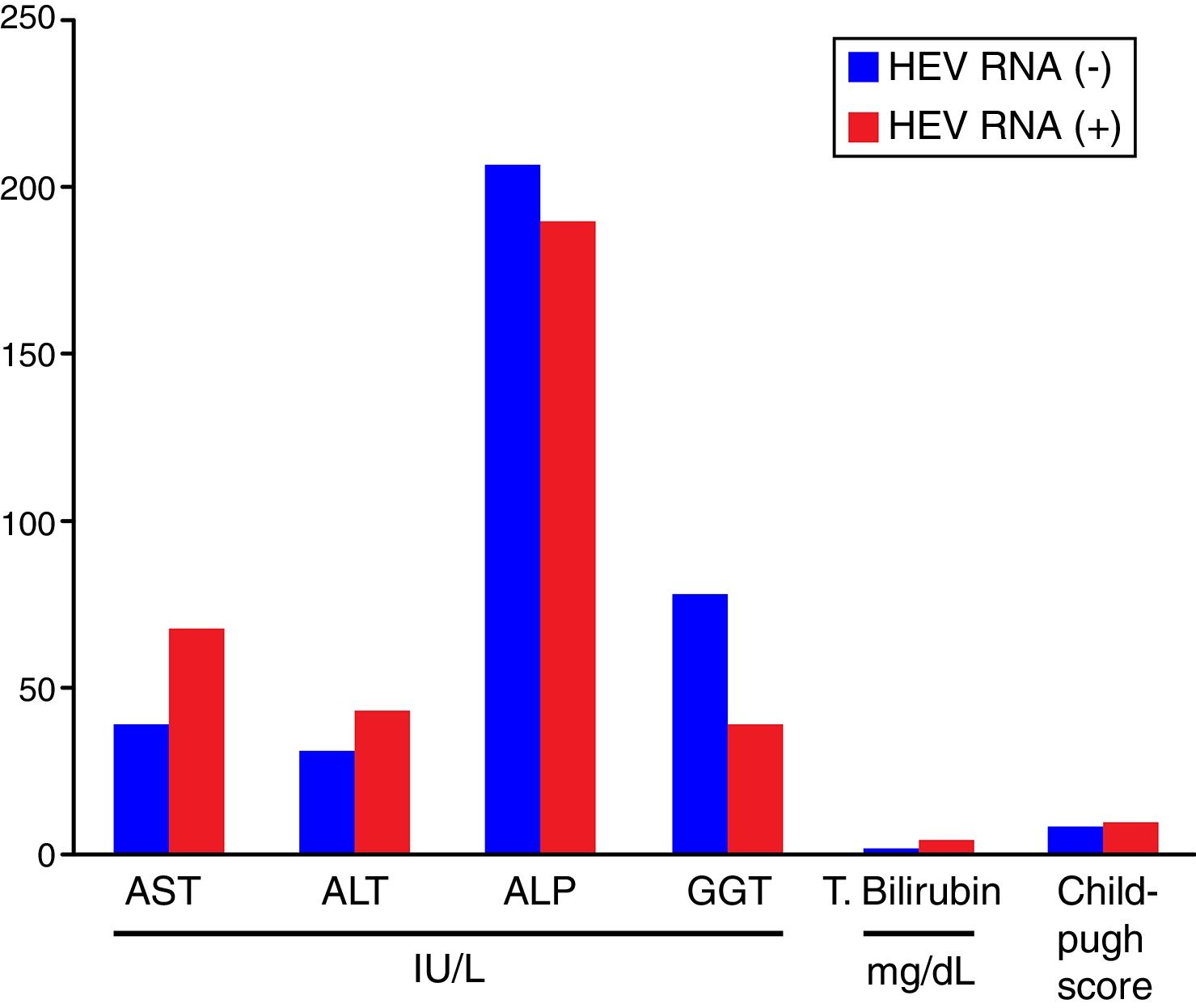
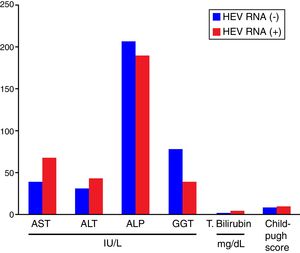
Comparisons of the biochemical parameters and the Child–Pugh scores between the HEV RNA positive and negative patient groups showed that AST, ALT, total bilirubin, and the Child–Pugh scores were higher in the HEV RNA positive patients than in the negative group, but this difference was statistically significant only for total bilirubin levels (p=0.03). The mean values for AST, ALT, ALP, GGT, total bilirubin, and the Child–Pugh scores in the HEV RNA positive and negative patient groups were 67±24.5 vs. 39±19.5IU/L (p=0.087), 42±22.19 vs. 30±16.9IU/L (p=0.360), 188±110.9IU/L vs. 206±94.9IU/L (p=0.569), 39±27.7 vs. 77±102.9IU/L (p=0.648), 3.72±1.4 vs. 1.47±0.75mg/dL (p=0.03) and 8.6±2.08 vs. 8.6±2.8 (p=0.565), respectively. Fig. 2 shows the comparison of the biochemical parameters and Child–Pugh scores of the HEV RNA positive and the negative groups.
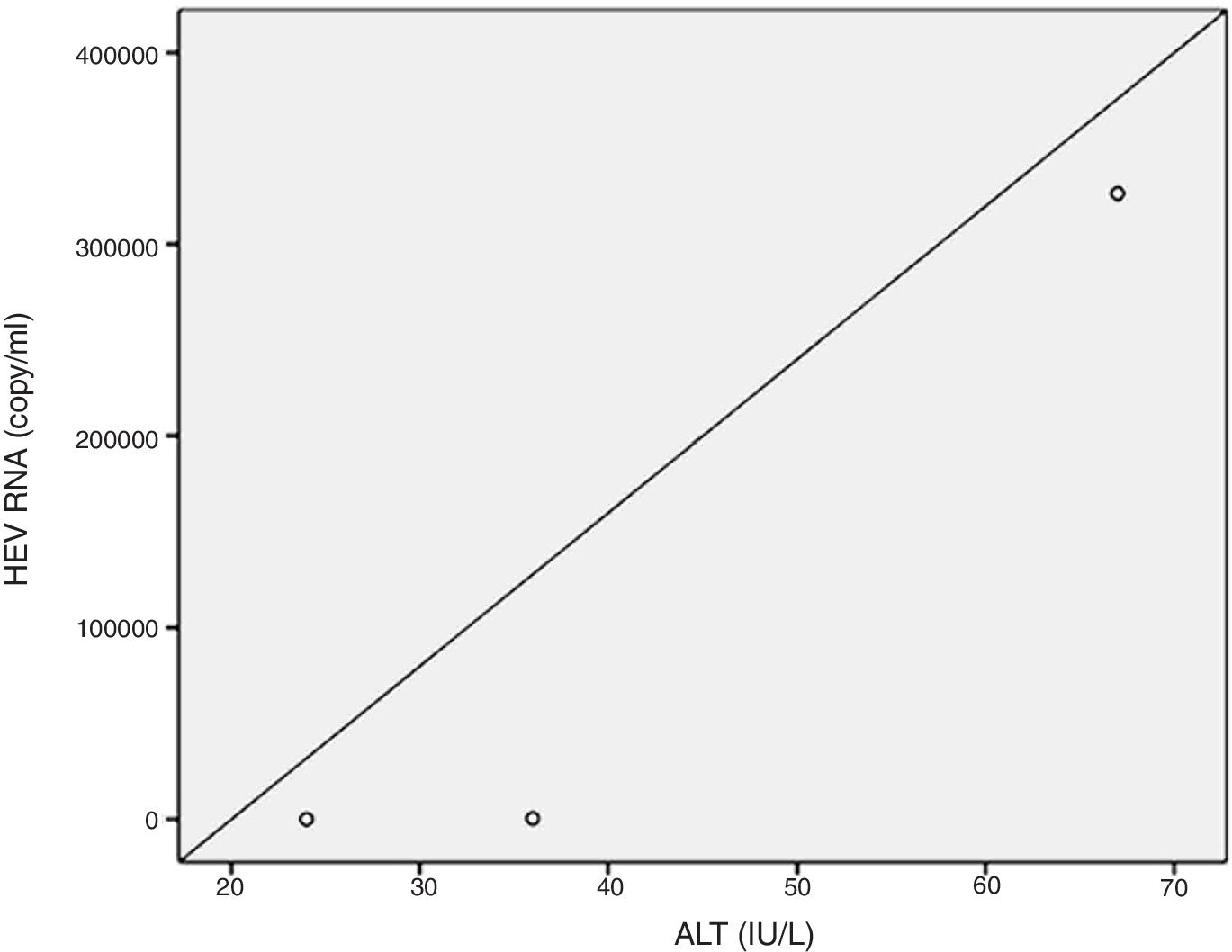
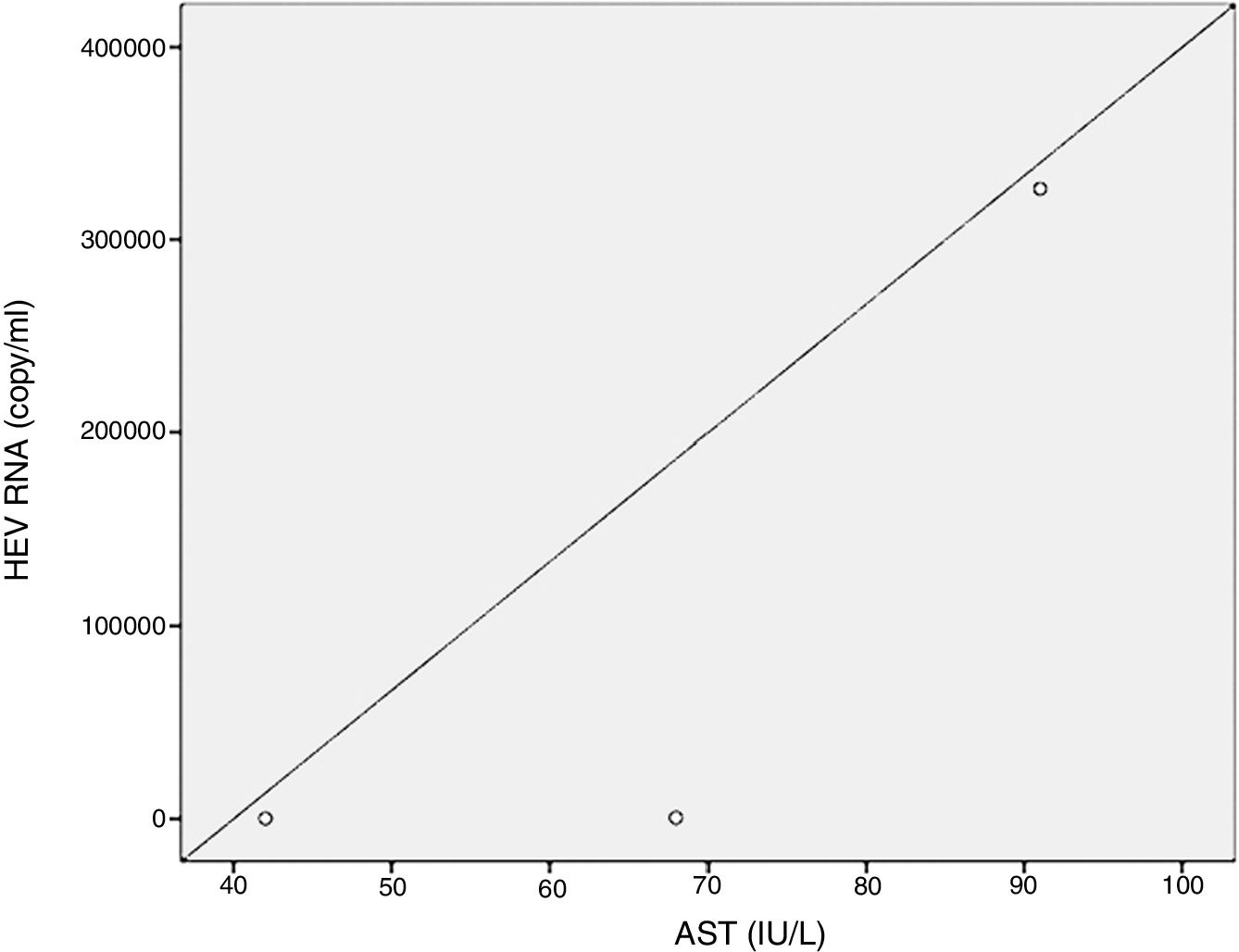
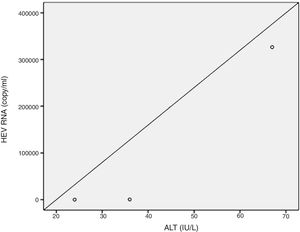
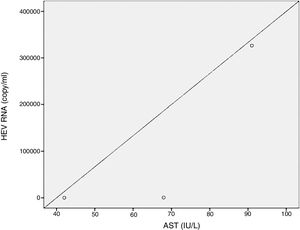
Positive correlations were found between HEV RNA levels and AST (p=0.046, r=0.642), and HEV RNA levels and ALT (p=0.049, r=0.632) levels. Figs. 3 and 4 summarize the relationship between liver aminotransferases and HEV RNA levels. HEV RNA positivity and serum levels were not correlated with Child–Pugh score (p>0.05). Table 1 lists the clinical, biochemical and demographic characteristics of the HEV RNA positive patients. In Table 2 Odd's ratio and confidence interval for the liver enzymes in HEV RNA positivity is listed.
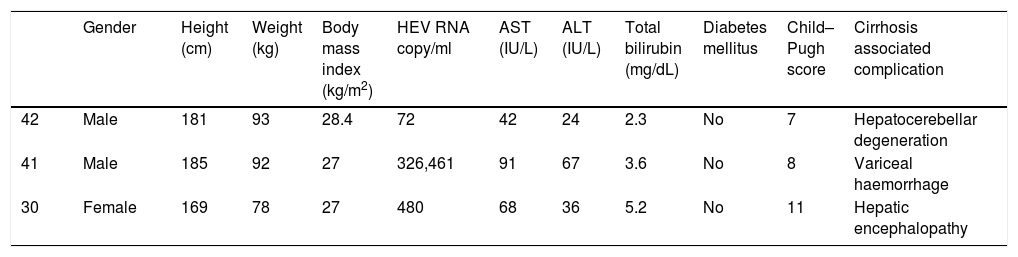
Demographic features of the HEV RNA positive patients.
| Gender | Height (cm) | Weight (kg) | Body mass index (kg/m2) | HEV RNA copy/ml | AST (IU/L) | ALT (IU/L) | Total bilirubin (mg/dL) | Diabetes mellitus | Child–Pugh score | Cirrhosis associated complication | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 42 | Male | 181 | 93 | 28.4 | 72 | 42 | 24 | 2.3 | No | 7 | Hepatocerebellar degeneration |
| 41 | Male | 185 | 92 | 27 | 326,461 | 91 | 67 | 3.6 | No | 8 | Variceal haemorrhage |
| 30 | Female | 169 | 78 | 27 | 480 | 68 | 36 | 5.2 | No | 11 | Hepatic encephalopathy |
HEV RNA: hepatitis E virus ribonucleic acid, AST: aspartate aminotransferase, ALT: alanine aminotransferase.
Odds ratio and confidence interval for the liver enzymes in HEV RNA positivity.
| Independent variables | Dependent variables HEV RNA (copy/ml) |
|---|---|
| Odds ratio (CI) | |
| AST (IU/L) | 1068 (0.981–1163) |
| ALT (IU/L) | 1039 (0.959–1126) |
HEV RNA: hepatitis E virus ribonucleic acid, AST: aspartate aminotransferase, ALT: alanin aminotransferase.
CI: confidence interval. Bold denotes statistical significance at p<0.05
Biochemical values and Child–Pugh scores between anti HEVIgG positive and negative patients were compared. The mean values for AST, ALT, ALP, GGT, total bilirubin and Child–Pugh scores in the anti-HEVIgG positive and negative patient groups were 51.7±15.7 vs. 44.5±29.22IU/L (p=0.522), 26.75±7.63 vs. 39.17±22.14IU/L (p=0.336), 190.25±103.59 vs. 207.83±96.39IU/L (p=0.831), 33±18.38 vs.87.33±109.12IU/L (p=0.109), 2.64±1.76 vs. 1.82±1.20mg/dL (p=0.593), 9±2.31 vs. 7.5±2.74 (p=0.389), respectively.
The AST, ALT, ALP, GGT, total bilirubin values and Child–Pugh scores did not show statistically significant difference in the anti HEVIgG positive and negative patient groups (p>0.05).
5DiscussionAlthough we have been familiar with HEV for more than 40 years, it is obvious that little is known about the virus. Many new and different effects have been demonstrated, such as the development of chronicity in immunosuppressed patients, its possible role in cirrhosis, and its role in extrahepatic involvement that may affect the neurological, haematological and gastrointestinal systems.
HEV causes epidemic outbreaks especially in developing countries. Its prevalence in Turkey has been found to vary between 3% and 29% in several studies [11,12]. A meta-analysis assessed HEV seroprevalence in Europe and included 73 studies from 11 countries that evaluated 129,254 individuals, of whom 116,043 were healthy individuals from the general population. In that meta-analysis, the HEV seroprevalence in healthy individuals from the general population varied between 7% and 17% [13]. We also found our total anti HEVIgG positivity to be 17.85%. Similar to studies from both Turkey and Europe, the healthy group positivity rate was approximately, 9.5%. On the other hand, among patients with cryptogenic cirrhosis, we found that the rate of anti-HEVIgG positivity was as high as 25.7%.
Mellgren et al. [14] evaluated the HEV prevalence among 204 patients in Sweeden with hepatitis C infections and various stages of fibrosis and found a HEV prevalence of 30%. They compared this rate with the 17% anti HEVIgG positivity rate which is found in blood donors in Sweden and detected a statistically significant increase. One potential reason for this was considered to be the higher mean age of patients with chronic hepatitis C than the mean age of the control group. In our study, we found that the rate of anti HEVIgG positivity (25.7%) was higher in the cirrhotic group, although the mean age of the healthy and patients group was similar. Therefore, we believe that the high anti HEVIgG positivity rate is associated with parameters other than age. In the same study, Mellgren et al. [14] found a rate of anti HEVIgG positivity of 38% in 98 patients with a Meta-analysis of Histological Data in Viral Hepatitis (METAVIR) fibrosis scores of F2 and above, which was statistically significant [14]. The detection of high anti HEVIgG positivity in the patient group with a high fibrosis score in that study supports the similarly high rate of anti HEVIgG positivity (25.7%) found in the cirrhotic patient group in our study. In a study by Atiq et al. [15] that evaluated the prevalence of anti-HEV IgG in patients with chronic liver disease, anti-HEVIgG positivity was found in29.3% of the patients with chronic hepatitis, regardless of its aetiology [15]. A study by Schulz et al. [16] in Germany found anti HEVIgG positivity in 21% of 295 patients with chronic liver disease, which was similar to the proportion found in those without a known liver disease (24.4%). In addition, 15 (23.8%) of 62 chronic liver disease patients with anti HEVIgG positivity had a cirrhotic stage due to hepatitis B, hepatitis C, alcohol consumption, autoimmune hepatitis, secondary biliary cirrhosis and primary sclerosing cholangitis. HEV RNA was not assessed in that study [16]. A study that reported findings consistent with our data was conducted in Spain by Riveiro-Barciela et al. [17]. In that study, HEV seroprevalence was 17.5% in cirrhotic patients and was32.1% in patients who developed hepatic cirrhosis after liver transplantation and 7.4% in patients with hepatic transplantation that did not develop cirrhosis. An important finding of this study was the higher prevalence of HEV in the cirrhotic group when both immunosuppressed groups were compared [17], which is a more interesting finding than the association of HEV with chronicity in immunosuppressed patients.
Although the higher anti HEVIgG positivity in patients with cryptogenic cirrhosis does not indicate that HEV alone can cause cryptogenic cirrhosis, it suggests that encountering HEV contributes to the fibrotic process.
In our study HEV RNA levels were not detectable in the healthy group. Among worldwide studies in which HEV RNA prevalence was investigated in healthy groups, the prevalence rate was between 0.08 and 0.14% in Germany, 0.04% in England and between 1.5 and 3.7% in India. It was thought that the detection of measurable HEV RNA without clinical evidence of disease may have been associated with spontaneous clearance of the virus after exposure in healthy individuals [18,19]. We detected a significantly higher HEV RNA positivity rate in patients with cryptogenic hepatic cirrhosis compared to the healthy control group. Mellgren et al. [14] while evaluating the seroprevalence of hepatitis E in 204 patients that have chronic hepatitis C infection at various fibrotic stages, detected a low titre HEV RNA positivity only in one patient. This patient had a cirrhotic stage and mildly elevated hepatic transaminase values were similar to those that we observed in our study [14]. The pathogenesis of HEV in liver injury still remains unclear [20]. Because HEV is not cytopathic, it is thought that the immune response associated with cytotoxic T and natural killer cells likely plays a role in the liver injury. Because the presence of HEV RNA is also an indicator of HEV replication, measurable HEV RNA levels could stimulate a cytotoxic response, which is a possible mechanism leading to the destruction of hepatocytes. Although HEV is mostly asymptomatic throughout the world, the infection it can cause a severe acute hepatitis in pregnant women and people with previously known liver diseases [21]. Barrague et al. [20] detected a high HEV RNA level in a patient with non-alcoholic steatohepatitis background and achieved HEV RNA clearance with ribavirin treatment. That study, which described a patient with chronic hepatitis E and a cirrhotic background, also evaluated the immune response of the patient, whose interferon γ response was similar to that of solid organ transplant patients who subsequently developed chronic hepatitis E [20]. In the current guidelines ribavirin therapy is recommended for immunosuppressive patients with solid organ transplantation after the immunosuppressive medications used for chronic HEV infection are reduced, if possible, and if HEV RNA positivity continues for three months or longer. Ribavirin and/or pegylated-interferon alpha may be used for chronic hepatitis E treatment in cases of haematological disorders or HIV-related immunosuppression in addition to solid organ transplantation. Ribavirin treatment should be considered in cases of severe acute hepatitis E and acute or chronic liver failure [22]. It is still unclear how we should approach this issue in non-immunosuppressed patients and cirrhotic patients, though there have been variations in our approaches to hepatitis E in recent years. One important point that our study suggests relative to this issue, is that in patients with cryptogenic cirrhosis with enzyme activity and HEV RNA levels can be examined to assess whether treatment has positive effects on cirrhosis progression.
The lack of HEV RNA clearance in the cirrhotic patient group in our study may be related to the fact that cirrhosis is a disease that leads to immune dysfunction which is recently named as cirrhosis-associated immune dysfunction. It may also be appropriate to assess hormonal changes, such as increased oestrogen and progesterone, which have been shown to be responsible for pregnancy-associated fulminant hepatitis E infection, in cirrhotic patients as well [23,24]. Hoan et al. [25] evaluated hepatitis E virus superinfection and clinical progression in 1318 Vietnamese patients with hepatitis B related diseases such as acute hepatitis B, chronic hepatitis B, hepatic cirrhosis, hepatocellular carcinoma or cirrhosis with hepatocellular carcinoma. In this study, only one patient with cirrhosis and hepatocellular carcinoma had a measurable level of HEV RNA. Again, the same study found that when participants were classified as Child–Pugh A, B and C, the anti-HEV IgG positivity rate was higher in the Child–Pugh B and C groups than inthe Child–Pugh A group in patients with cirrhosis due to hepatitis B [25]. Similarly in our study, the mean Child–Pugh score was higher in the anti HEVIgG positive patient group than the anti HEVIgG negative patient group.
Although our study evaluating HEV RNA and anti HEVIgG levels in a specific patient group consisted of a small sample size, it is logical to research HEV in a group that has not been previously evaluated, such as patients with cryptogenic cirrhosis. Additionally we could have made better interpretations regarding the chronicity of HEV if we had measured HEV RNA levels again after 3 months, as is generally recommended in the literature. Another limitation of our study is the absence of HEV genotyping, although genotypes 1 and 2 are most commonly seen among patients in Turkey. If we had detected genotype 3, which is responsible for chronicity in immunosuppressed patients, we could have made better interpretations of our data. It is obvious that additional studies are needed to properly evaluate the co-occurrence of HEV with clinical presentations in diseases such as acute hepatitis, chronic hepatitis, with or without extrahepatic involvement.
In conclusion, to the best of our knowledge, this study is the first to evaluate the presence of HEV in patients with cryptogenic cirrhosis in the literature. This study demonstrated that anti HEVIgG and HEV RNA positivity rates were high in patients with cryptogenic cirrhosis although the results were not statistically significant and there was a positive correlation between HEV RNA and aminotransferases.
AbbreviationsALP alkaline phosphatase alanine aminotransferase aspartate aminotransferase Celcius deoxi-nucleic-acid enzyme-linked immunosorbent assay gamma glutamyltransferase hepatitis E virus human immunodeficiency virus immunoglobulin G meta-analysis of histolgical data in viral hepatitis polymerase chain reaction ribonucleic acid Statistical Package for the Social Sciences
There is no financial support for this study to declare.
Conflicts of interestThe authors of this research have no conflicts of interest to declare.