Background and rationale. The Transplanted Organ Questionnaire (TOQ), developed in France, is a disease-targeted instrument designed to evaluate what the transplanted organ represents to the recipient in patients who have undergone liver transplantation. The present study sought to validate a version of the TOQ for use in the Brazilian population. Translation and cross-cultural adaptation were carried out in accordance with international standard practices. Convergent validity was measured by correlations between TOQ domains and the Beck Depression Inventory (BDI), while reliability was assessed by measurement of internal consistency (Cronbach’s alpha coefficient), reproducibility (intraclass correlation coefficient), sensitivity to change (effect size), and floor and ceiling effects.
Results. The study sample comprised 122 liver transplant recipients, with a mean age of 56.7 ± 9.9 years, treated at the outpatient clinic of a tertiary hospital in Southern Brazil. The sample was largely male (57.4%), and the predominant indication for liver transplant was hepatocellular carcinoma (34.4%). The mean total TOQ score was 32.9 ± 18.0. Cronbach’s alpha for the total score was 0.89 (95%CI 0.86-0.92). Correlations between TOQ and BDI domains were acceptable, with the rejection domain correlating most strongly (r = 0.37; p ≤ 0.001). In conclusion, the Brazilian Portuguese version of the TOQ exhibited good psychometric performance, suggesting that it can be a useful tool in the Brazilian cultural context.
Liver transplantation (LT) has been considered the gold-standard treatment for end-stage liver disease of most etiologies. About 8,500 LTs are performed each year in North America, Latin America, and Europe, and more than 70% of recipients survive for at least 5 years at most centers.1,2 A 2015 publication estimates that 2,270,859 life-years were saved to date during the 25 years of solid-organ transplant practice.3 In the United States, according to data from the United Network for Organ Sharing (UNOS), in 2014, over 50,000 people were living with a hepatic allograft.4 Living with the organ of another person can be challenging for some patients. The transplanted organ may become a burden for the recipient’s everyday life, potentially causing substantial emotional stress due to fear, guilt, and questions about the donor.5,6 Usually, patients do not report these concerns to their health care team, and health professionals do not routinely ask. Thus, the representation of the transplanted organ is practically not considered.
French researchers developed a specific instrument, the Transplanted Organ Questionnaire (TOQ),7 with the objective of evaluating what a transplanted organ represents to its recipient. This instrument proved to be valid and consistent in the population of origin. Considering the importance of this issue in the field of transplant medicine, the aim of the present study was to validate a version of the TOQ translated and adapted for use in the Brazilian context.
Material and MethodsPatientsA convenience sample of adult LT recipients (age > 18 years) and treated at the outpatient clinic of the Transplantation Group at Complexo Hospitalar da Santa Casa de Misericordia de Porto Alegre, Brazil, was included in the study. Patients were excluded if they had undergone combined liver-kidney transplantation.
Transplanted Organ QuestionnaireThe TOQ7 assesses the representation of the transplanted organ in transplanted individuals or, in other words, the recipient’s positive and negative feelings towards the donor (indebtedness, guilt, and gratitude) as well as toward transplantation itself. The questionnaire includes the following three dimensions:
- •
“Donor”, representing concerns about the donor;
- •
“Positive attitude toward the transplant”, representing positive idealization regarding the transplanted organ; and
- •
“Psychological rejection”, representing a negative attitude regarding the transplanted organ. It consists of 24 questions scored on a six-level Likert-type scale, anchored at “never” and “all the time”(scoring 0 and 5 points respectively).7
The Beck Depression Inventory (BDI) is a self-rating scale previously validated for use in Brazil.8,9 It includes 21 multiple-choice items about depressive symptoms in the last 15 days that are rated on a 0-to-3 ordinal scale, yielding a total score that ranges from 0 to 63. The suggested thresholds for levels of severity are as follows: < 10, minimal/no depression; 10-18, mild depression; 19-29, moderate/severe depression; and 30-63, severe depression.10 Both questionnaires, the TOQ (Table 1) and BDI, were administered by an interviewer.
Brazilian Portuguese of the Transplanted Organ Questionnaire (TOQ).
| 1. Fico pensando sobre o transplante. Nunca/ Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 2. Fico pensando sobre a pessoa que me deu o órgão dela. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 3. Fico pensando sobre como o transplante salvou a minha vida. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 4. Fico pensando que a minha vida tem sido mais difícil desde o transplante. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 5. Fico pensando que não deveria ter concordado em fazer o transplante. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 6. Fico pensando que tenho mais liberdade agora do que antes do transplante. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 7. Fico pensando que o meu transplante na verdade não me pertence. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 8. Fico pensando que o meu transplante é alheio a mim. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 9. Fico pensando que o meu transplante é uma restrição ou obstáculo. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 10. Fico pensando que poderia viver muito bem sem o transplante. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 11. Tenho sentimentos em relação à pessoa que doou o órgão. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 12. Fico pensando que devo minha vida à pessoa que doou o órgão. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 13. Fico pensando que tenho uma divida para com a pessoa que doou o órgão. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 14. Fico pensando sobre o que o meu doador passou Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 15. Fico me sentindo responsável pelo que o meu doador passou. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 16. Fico pensando que me tornei parecido(a) com a pessoa que doou o órgão. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 17. Fico desejando poder fazer o mesmo pela pessoa que doou o órgão. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 18. Fico pensando que o meu transplante é um de meus aliados na vida Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 19. Fico pensando que a minha vida tem sido melhor desde o transplante. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 20. Sinto ressentimento em relação à pessoa que doou o órgão. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 21. Sinto uma espécie de proximidade com o meu transplante. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 22. Tenho sentimentos em relação ao meu transplante. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 23. Sinto ressentimento em relação ao transplante. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
| 24. Fico me sentindo responsável pelo meu transplante. Nunca /Raramente /Às Vezes /Frequentemente /Com Muita Frequência /O Tempo Inteiro. |
The study consisted of two stages: Translation of the TOQ and assessment of its psychometric properties.
ValidationThe validation process was conducted in accordance with international recommendations, with the following steps:11,12
- •
Initial translation to the target language. In this step, the items of the original English-language version of the TOQ were conceptually translated to Brazilian Portuguese by two independent, bilingual health professionals. Potential differences between both versions were discussed, resulting in a unified version.
- •
Back-translation of the Brazilian-Portuguese version to English by two different and independent bilingual translators. Again, potential differences were discussed, leading to a unified version which should be similar to the original English instrument. Any discrepancies were discussed among all translators, and a final Brazilian-Portuguese version resulted. This resulting version was discussed in a pilot sample of target subjects to check for clarity.
The psychometric properties of interest were validity and reliability. Validity was evaluated by measuring convergent validity, assessing whether the TOQ domains correlated well with the equivalent BDI domains and between total score and domain scores. Reliability was assessed by internal consistency, by means of Cronbach’s alpha (α) coefficient.13
Clinical variables of interestData on the following variables were collected: Sociodemographic characteristics, etiology and severity of liver disease on the day of LT, and presence of comorbidities. The Brazil-validated version of the Model for End-Stage Liver Disease (MELD) severity score14 was used to assess the severity of liver disease at the time of transplantation. The MELD score was calculated using the UNOS formula.15 For those patients who underwent LT with supplementary MELD points, such as those with hepatocellular carcinoma within the Milan criteria, the MELD score was calculated based on immediate pre-transplant laboratory results. The time elapsed between transplantation and administration of the questionnaire was stratified as follows: 0 to 6 months; 6 to 12 months; or > 12 months. The presence or absence of comorbidities was recorded, with particular emphasis on diagnoses of diabetes mellitus, hypertension, osteoporosis, and obesity.
Statistical analysisThe sample size was estimated at 120 participants, considering the inclusion of five participants per item of the instrument. Categorical variables were expressed as absolute and relative frequencies, and continuous variables, as mean and standard deviation or median and interquartile range as appropriate. Convergent validity was measured by assessing whether the TOQ domains correlated with the equivalent BDI domains by means of Spearman correlation coefficients (r); values > 0.3 were deemed acceptable.15 Cronbach’s α values ≥ 0.7 were considered adequate.
Statistical analyses were performed in SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The significance level was set at 5%.
Ethical aspectsWe were authorized by the creators of the original TOQ to validate the instrument for use in the Brazilian population. The study was approved by the Institutional Review Board of Irmandade Santa Casa de Misericórdia de Porto Alegre, and all subjects provided written informed consent for participation.
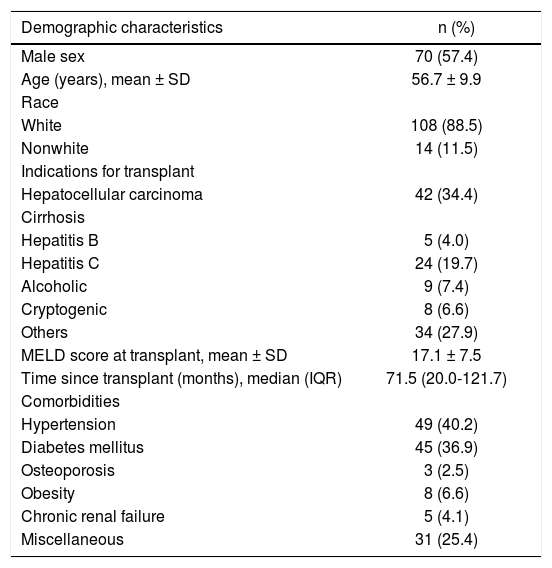
ResultsOverall, 132 patients were eligible for inclusion; of these, two were excluded because they had undergone combined liver-kidney transplantation, two refused to participate, five were on interferon therapy, and one was mentally handicapped. The characteristics of the 122 patients included in the analysis are listed in table 2. Most patients were male (57.4%) and white (88.5%); the mean age was 56.7 (± 10.4) years. Hepatocellular carcinoma associated with hepatitis C virus infection was the main indication for LT (34.4% of cases). The overall mean pre-transplant MELD score was 17.1 ± 7. The median time since transplantation was 71 months.
Demographic data of liver transplant recipients (n = 122).
| Demographic characteristics | n (%) |
|---|---|
| Male sex | 70 (57.4) |
| Age (years), mean ± SD | 56.7 ± 9.9 |
| Race | |
| White | 108 (88.5) |
| Nonwhite | 14 (11.5) |
| Indications for transplant | |
| Hepatocellular carcinoma | 42 (34.4) |
| Cirrhosis | |
| Hepatitis B | 5 (4.0) |
| Hepatitis C | 24 (19.7) |
| Alcoholic | 9 (7.4) |
| Cryptogenic | 8 (6.6) |
| Others | 34 (27.9) |
| MELD score at transplant, mean ± SD | 17.1 ± 7.5 |
| Time since transplant (months), median (IQR) | 71.5 (20.0-121.7) |
| Comorbidities | |
| Hypertension | 49 (40.2) |
| Diabetes mellitus | 45 (36.9) |
| Osteoporosis | 3 (2.5) |
| Obesity | 8 (6.6) |
| Chronic renal failure | 5 (4.1) |
| Miscellaneous | 31 (25.4) |
SD: Standard deviation. MELD: Model for End-Stage Liver Disease. IQR: Interquartile range.
The mean time required for the TOQ interview was 6 min. The mean total score was 32.9 ± 18.0, while the median domain scores were as follows: Donor, 10.9 (5-14); Idealization, 18.8 (5-14); Psychological rejection, 3.0 (0-4).
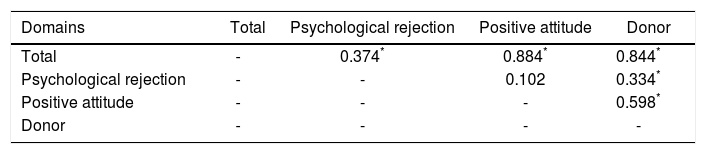
Correlations are presented in tables 3 and 4. Significant correlations were observed between domains, with the exception of the positive rejection domain. Correlation between the Donor and Psychological rejection domains was r = 0.33 (p < 0.001). Among the domains evaluated, the positive attitude domain showed the strongest correlation with the total score (r = 0.88, p < 0.001). We also found a correlation between the donor domain and the positive attitude domain (r = 0.60, p < 0.001). This suggests that the recipient’s concern toward the donor leads to positive feelings about the transplanted organ.
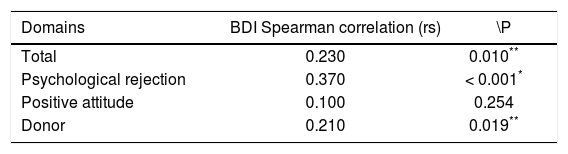
Significant correlations were found between the TOQ domains and the BDI, with the exception of the positive attitude domain (r = 0.100; p = 0.254). The strongest correlation with the BDI was in the Psychological rejection domain (r = 0.37, p < 0.001). In other words, greater severity of depression in the transplant recipient was associated with greater odds of psychological rejection of the organ.
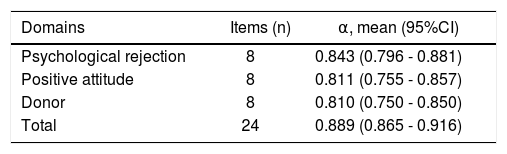
ReliabilityCronbach’s alpha values consistently exceeded 0.80, with α = 0.889 (0.859-0.916) for the overall instrument and ranging from 0.81-0.8915 for individual domains (Table 5).
Characteristics of the Transplanted Organ Questionnaire in a Brazilian population.
| Domains | Items (n) | α, mean (95%CI) |
|---|---|---|
| Psychological rejection | 8 | 0.843 (0.796 - 0.881) |
| Positive attitude | 8 | 0.811 (0.755 - 0.857) |
| Donor | 8 | 0.810 (0.750 - 0.850) |
| Total | 24 | 0.889 (0.865 - 0.916) |
α: Cronbach’s alpha coefficient. CI: Confidence interval.
The present study led to the cross-cultural validation of the Brazilian version of the Transplanted Organ Questionnaire. The validated questionnaire showed good performance in the psychometric properties assessed, proving to be a valid and reliable version for the proposed population. As expected, it was also easily administered to and understood by transplant recipients.
Coping with a transplanted organ is complex and, for some, it may become a burden, potentially jeopardizing the procedure’s long-term success. It is equally important to prepare the recipient before and after transplantation. Grief from the loss of one’s own organ and for the donor’s life or organ are known to occur and have an impact on the recipient’s well-being.16–18
As part of coping, transplant recipients must learn to deal with a complex but promising new reality, interacting with their feelings towards the acquired organ, the multi-disciplinary healthcare team, and new possibilities in their own life. A recent study explored self-management tasks in view of medical role-related and emotional tasks in liver transplant candidates and recipients and their respective close caregivers. Researchers found that patients and families prioritized self-management tasks in a particular order: first medical, then role-related, and, finally, emotional management. The authors conclude it is important to ask and keep in mind how patients and their families prioritize these three components in order to better tailor health care to this population.19
The number of liver transplants in Brazil has been rising over the years,20 and the advent of quantitative as well as qualitative instruments that help appraise the overall condition of patients is more than welcome, as long as the proper measures to use them are taken; cultural validation of the TOQ is part of this process. When comparing our results with those of the original TOQ study, we found very similar mean scores on all three domains. Similarly, there was also a significant and positive correlation between depressive symptoms and Psychological Rejection domain scores in our participants, supporting the questionnaire’s ability to signal patients that may need special support; after all, depressive symptoms have been associated with lower quality of life and detrimental outcomes in transplantation medicine.
Considering the sociocultural diversity of Brazil, the main limitation of our study could be its single-center design. However, the clarity of the questions facilitated the use of standard expressions common to all regions of the country. Ideally, new studies should be conducted in other states to confirm this.
In conclusion, the Brazilian Portuguese version of the Transplanted Organ Questionnaire was found to be valid and reliable for use in Brazilian liver transplantation recipients.
Abbreviations- •
LT: Liver transplantation
- •
TOQ: Transplanted Organ Questionnaire
- •
MELD: Model for End-Stage Liver Disease
The authors have no financial relationships relevant to this article to disclose.
Conflict of InterestThe authors have no conflicts of interest to disclose.
AcknowledgmentsThe authors thank the Liver Transplantation Group at Complexo Hospitalar da Irmandade Santa Casa de Misericórdia de Porto Alegre, Porto Alegre, RS, Brazil.