Interferon-free, multi-direct acting antiviral (DAA) therapy for chronic hepatitis C virus (HCV) infection is highly effective and well tolerated, but costly. To gain perspective on the evolving economics of HCV therapy, we compared the cost per cure of a multi-DAA regimen with the prior standard of triple therapy.
Material and methodsPatients infected with HCV genotype 1 who were treated through the University of Colorado Hepatology Clinic between May 2011 and December 2014 comprised the study population. The multi-DAA regimen of simeprevir plus sofosbuvir (SMV/SOF) was compared to the triple therapy regimen consisting of peginterferon and ribavirin, with either boceprevir or telaprevir (TT). Sustained-virologic response (SVR) rates, total costs per treatment and adverse events were recorded. Total cost per SVR were compared for the two treatments, controlling for patient demographics and clinical characteristics.
ResultsOne hundred eighty-three patients received SMV/SOF (n = 70) or TT (n = 113). Patients receiving SMV/SOF were older, more treatment experienced, and had a higher stage of fibrosis. SVRs were 86% and 59%, average total costs per patient were $152,775 and $95,943, and average total costs per SVR were $178,237 vs. $161,813.49 for SMV/SOF and TT groups, respectively. Medication costs accounted for 98% of SMV/SOF and 85% of TT treatment costs.
ConclusionThe high cure rate of multi-DAA treatment of HCV is offset by the high costs of the DAAs, such that the cost per cure from TT to multi-DAA therapy has been relatively constant. In order to cure more patients, either additional financial resources will need to be allocated to the treatment of HCV or drug costs will need to be reduced.
The landscape for treatment of HCV has changed dramatically. Prior to 2011, the standard of care was dual therapy with pegylated interferon (peg-IFN) and ribavirin. That year the first direct-acting antivirals (DAAs), telapre-vir and boceprevir, were approved in combination with peg-IFN and ribavirin. This new triple-therapy (TT) regimen increased rates of SVR for HCV genotype 1 from 40% to over 70%.1-4 The next big advance occurred in 2013 when both simeprevir (SMV) and sofosbuvir (SOF) were FDA-approved. Shortly thereafter, the interferon-free combination of SMV/SOF was shown to achieve rates of SVR in HCV genotype 1 of approximately 90% while significantly improving tolerability and reducing adverse events.5 Subsequently, other multi-DAA regimens have been FDA approved, including ledipasvir (LDV)/ SOF, ritonavir-boosted paritaprevir/ombitasvir with (3D-regi-men) or without (2D-regimen) dasabuvir, and daclatasvir (DAC)/SOF.6-17
Multi-DAA regimens are revolutionizing HCV treatment, expanding the number of patients eligible for treatment, and reducing the resources needed to manage treatment-related side effects. A limitation to treatment is the cost of the DAAs (all values described in US dollars). Average wholesale price (AWP) of telaprevir and boceprevir in were estimated at $87,607 to $106,468 and $64,825 to $95,845 for 24-week treatment regimens with peginterfer-on and ribavirin, respectively.18-20 Combinations of SMV/ SOF, LDV/SOF, 3D-regimen, 2D-regimen, and DAC/ SOF are estimated to cost $66,000 to $100,000 or more for 12 weeks of treatment.21-24
Although Phase III studies illustrated the efficacy of combination DAAs in various patient populations,6-17 identifying real world efficacy and calculating treatment costs is essential for estimating resource utilization for payer sources. In this study we examined the relationship of treatment costs and treatment efficacy (SVR) to define the cost per cure (cost per SVR) using real world observational data in patients who received a SMV/SOF multi-DAA regimen compared to the prior standard of care, protease inhibitor-based TT.
Study sampleThe study included all patients with HCV genotype 1 who underwent either multi-DAA or TT treatment at University of Colorado Health (UCH) Hepatology Clinic between May 1, 2011 and December 31, 2014. Patients were identified using the electronic medical record (EMR) by conducting a manual chart review. The study was approved by the Colorado Multiple Institutional Review Board (COMIRB).
To be included in the analysis, patients had to be infected with HCV genotype 1a or 1b and treated with either simeprevir (150 mg daily) plus sofosbuvir (400 mg daily) for 12 to 24 weeks, telaprevir (750 mg TID) for 12 weeks plus peg-INF and ribavirin for 24 to 48 weeks, or bo-ceprevir (800 mg TID) plus peginterferon and ribavirin for up to 48 weeks. Patients must have initiated treatment on or after May 1, 2011 and completed treatment by September 30, 2014. Patients with HIV/HCV co-infection or HBV/HCV co-infection, and recipients of organ transplantation were excluded.
Data collectionDemographic and clinical informationDemographic information, including gender, race, ethnicity and age at start of treatment were collected. Our clinical variables included baseline body-mass index (BMI), genotype (1a or 1b), prior HCV treatment status, and level of liver fibrosis: minimal (F0-F2) or advanced (F3-F4).
HCV related healthcare resource utilizationThe defined HCV treatment duration of therapy was date of initiation of treatment through treatment completion or discontinuation, and post-treatment follow-up. All healthcare utilization related to DAA treatment were captured, including all relevant outpatient and inpatient visits and laboratory tests pre-, during, and post-treatment. Pharmacy utilization included prescribed medications use based on DAA treatment protocols. Recorded utilization included all HCV-related outpatient clinic visits (OP), and all available HCV-related inpatient (IP) and emergency department (ED) encounters. All laboratory monitoring was recorded by monitoring protocols (described in detail below). Finally, the management of hematologic side effects was measured by recording concomitant medications or therapies, including epoetin alfa, filgrastim, and blood transfusions.
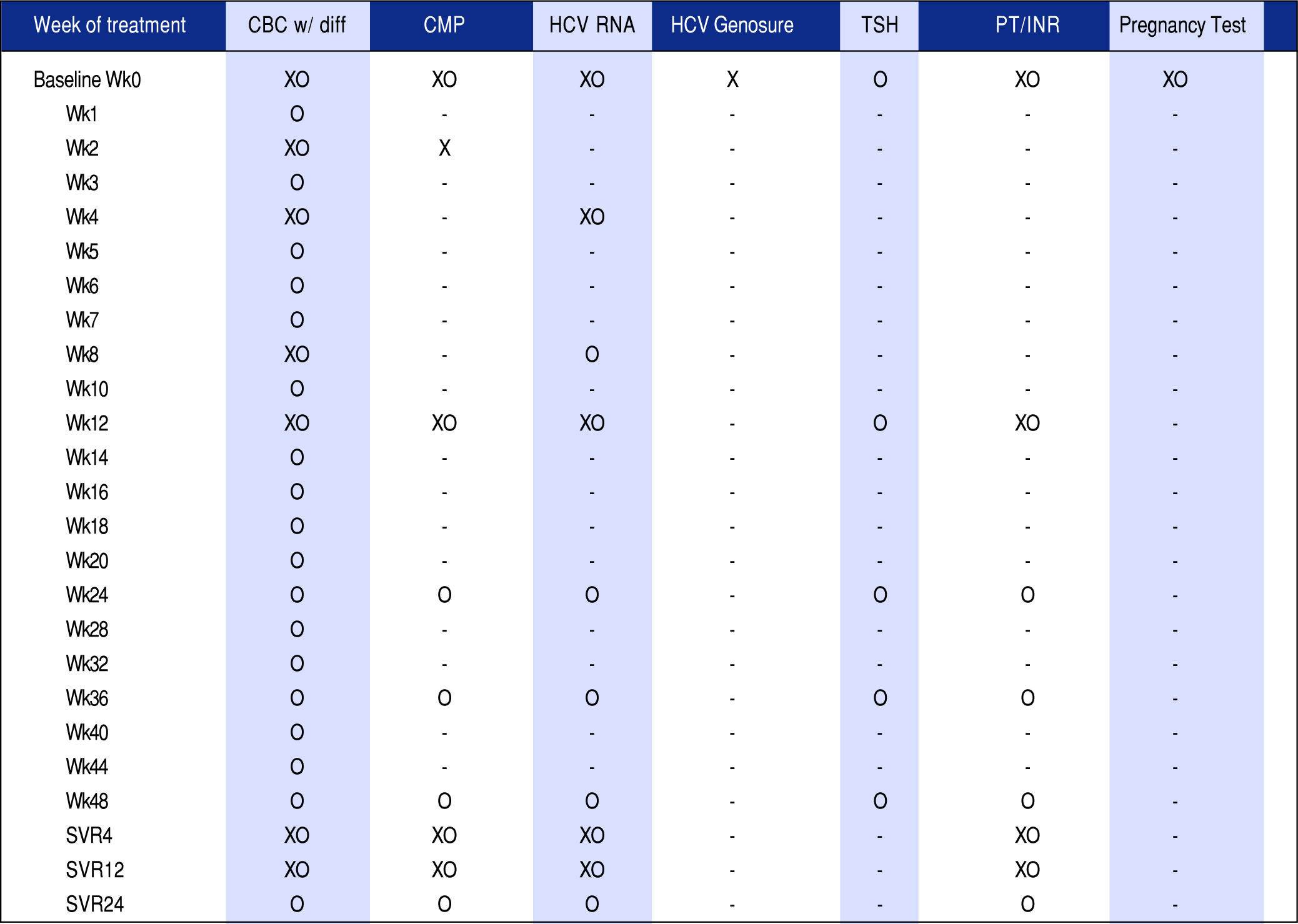
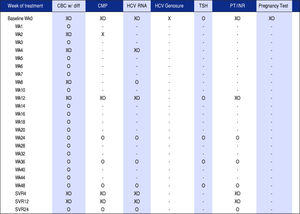
Monitoring protocolsStudy patients receiving drug therapy were divided into unique treatment protocols, defined by the Hepatology Clinic and reflecting national clinical guidelines. Each treatment protocol included monitoring of the following laboratory values: the complete blood count with manual differential (CBC), comprehensive metabolic panel (CMP), HCV RNA, HCV Genosure NS3/4A drug resistance assay (for genotype 1a patients prescribed SMV/SOF only), thyroid-stimulating hormone (TSH), liver function tests and pregnancy test for female patients of childbearing potential prescribed ribavirin. These protocol-based laboratory tests were drawn throughout treatment duration and varied in frequency depending on treatment regimen and length of therapy.
Figure 1 shows an overall comparison of SMV/SOF protocol to the TT protocol, highlighting the difference in frequency of monitoring tests across the two therapies. Following baseline, monitoring for SMV/ SOF and TT protocols is marked on the vertical timeline through 12-48 week treatment regimens and follow-up viral load tests post-treatment. SMV/SOF patients received CBC and CMP less frequently than TT patients and were followed by post treatment HCV viral load tests four and twelve weeks after treatment, while TT patients received an additional HCV RNA test twenty-four weeks post treatment. The monitoring protocol was adjusted for patients who discontinued treatment prematurely.
OutcomesThe primary outcomes were:
- •
Rate of sustained virologic response. The number of patients in a given treatment regimen (SMV/SOF or TT) achieving SVR (through HCV RNA results) following treatment divided by the total number of patients prescribed that drug regimen.
- •
Total HCV treatment cost per patient. Total per patient costs for all utilization during treatment duration in each regimen, including cost of drug treatment regimen, all monitoring defined by treatment protocols, all available IP, OP, and ED encounters, and any treatment prescribed for hematologic side effects.
- •
Total costs per SVR (cost per cure). The total costs of treatment (defined above) divided by the average SVR rate achieved among patients in that particular treatment regimen.
We calculated the rate of SVR as our primary estimate of drug effectiveness. An SVR value at 12 weeks was estimated for patients on SMV/SOF; an SVR value at 24 weeks was estimated for patients on TT. Secondary outcome measure was treatment completion rates (the number of people who completed treatment on each drug regimen divided by total number of people in each drug regimen). TT is known to cause harsh side effects and thus we compared the amount of patients able to complete the recommended regimen.
Data analysisDescriptive (unadjusted) and adjusted regression analysis were conducted between both groups. The unadjusted analysis compared means and proportions for all specified outcome variables. Patient clinical characteristics, including average actual length of treatment, treatment experience and level of liver fibrosis were compared using F-statistics. Depending on the type of outcome variable, different statistical models were employed for the adjusted regression analysis. Logistic regression models were utilized for the binary outcome of achieving SVR. The explanatory variables for all models included age (age 20-49 years was the reference group), gender (male as the reference group), race (white was the reference group), ethnicity (non-Hispanic was the reference group), BMI categories (normal weight BMI 18-24.99 is the reference group), and treatment experience (treatment experienced patients was the reference group).
Cost per SVR (Cost/SVR) was calculated by dividing the total per patient cost by the SVR rate for each regimen. We modeled Cost/SVR across treatment groups using a generalized linear model (GLM) with log transformation controlling for age, fibrosis level, treatment experience, BMI, ethnicity, race and gender. Likelihood of treatment completion was modeled using multivariate logistic regression.
HCV drug treatment costs were obtained from AWP in the RED Book. Unit costs for all medical services, including all IP/OP/ED visits, laboratory tests, and non-HCV related prescription drug fills were obtained from a 10% random sample (8 million covered lives) of the IMS Life-Link PharMetrics Health Plan Claims Database (Phar-Metrics),25 a national database of administrative claims and managed care enrollment. Unit costs estimates for medical utilization were assessed from utilization for all claims from the HCV-diagnosed population January 2007-De-cember 2013. Cost estimates from different years were inflated to common 2013 prices, using medical care component of consumer price index. Statistical analyses were performed using STATA 13.1.
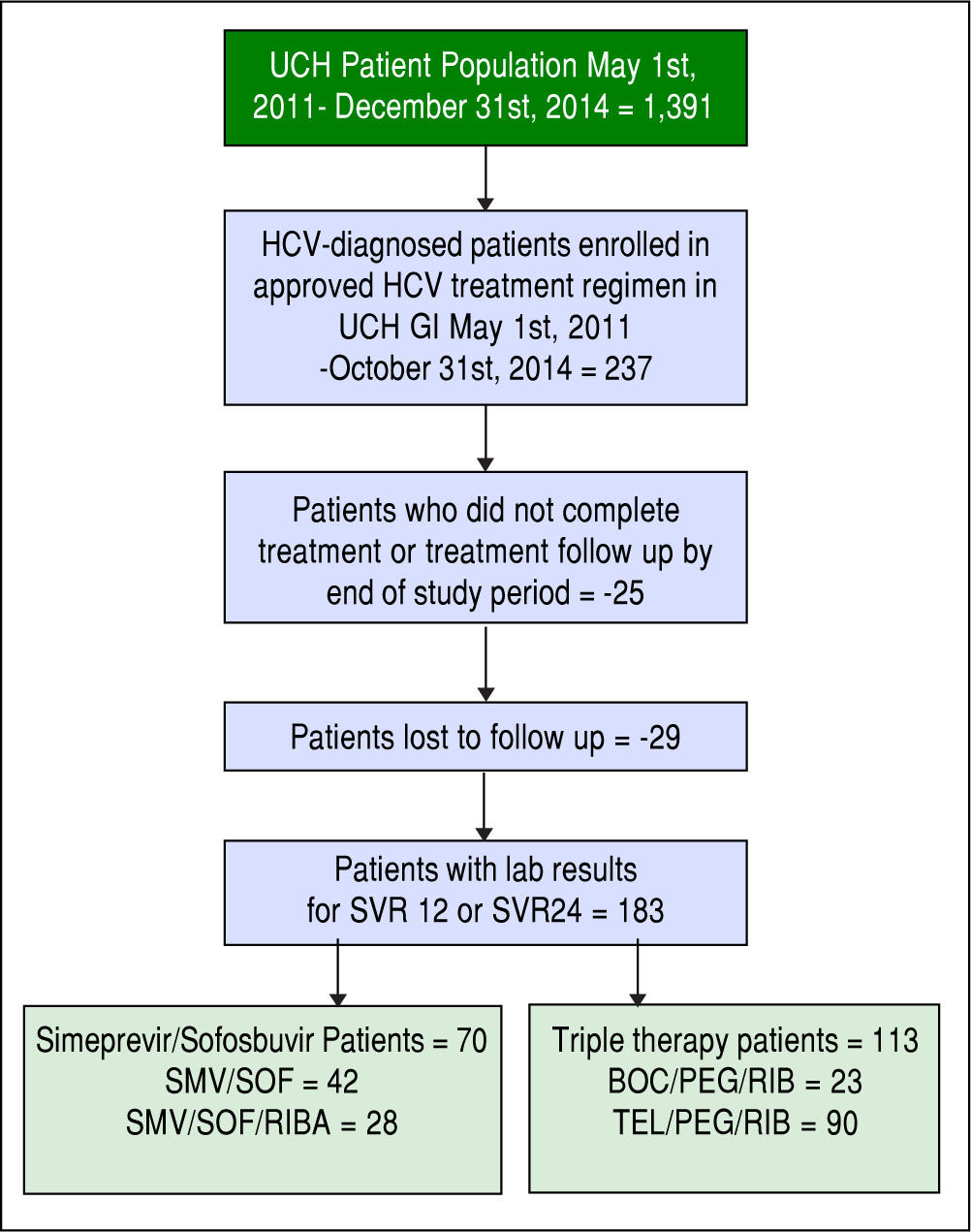
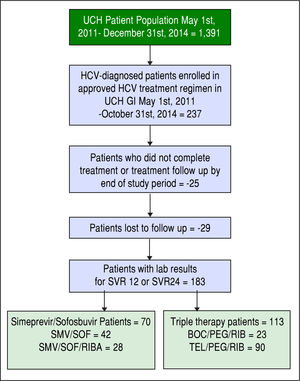
ResultsOne hundred eighty-three patients meeting the inclusion and exclusion criteria were identified. Our final HCV cohort contained 70 SMV/SOF patients (Figure 2) (42 patients receiving SMV/SOF; 28 patients receiving SMV/ SOF with ribavirin) and 113 TT patients (90 patients receiving telaprevir/peginteferon/ribavirin treatment; 23 receiving boceprevir/peginterferon/ribavirin treatments).
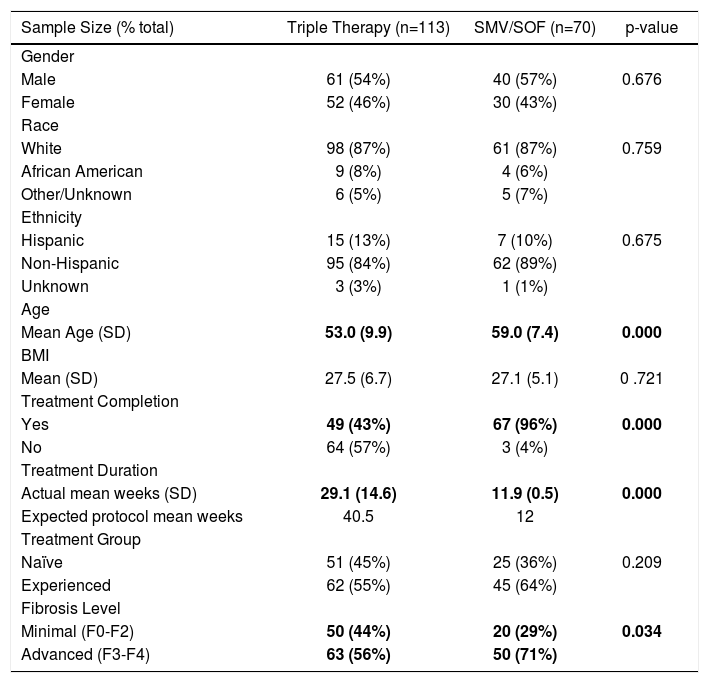
Characteristics of the treatments cohortsResults from our descriptive analysis are shown in table 1. The majority of patients in the SMV/SOF and TT treatment groups were male (57% and 54%, respectively), white (87% in both groups) and non-Hispanic (89% and 84%, respectively). The average age of SMV/SOF patients was greater than TT patients (59 years vs. 53 years, respectively) and the mean BMI for each group was similar (27.1 kg/m2 and 27.5 kg/m2, respectively). SMV/SOF patients were much more likely to complete treatment (96%) vs. TT patients (43%). Majority of patients were of advanced fibrosis level in both treatment groups (64% SMV/SOF; 55% TT).
Characteristics of the treatment cohorts.
| Sample Size (% total) | Triple Therapy (n=113) | SMV/SOF (n=70) | p-value |
|---|---|---|---|
| Gender | |||
| Male | 61 (54%) | 40 (57%) | 0.676 |
| Female | 52 (46%) | 30 (43%) | |
| Race | |||
| White | 98 (87%) | 61 (87%) | 0.759 |
| African American | 9 (8%) | 4 (6%) | |
| Other/Unknown | 6 (5%) | 5 (7%) | |
| Ethnicity | |||
| Hispanic | 15 (13%) | 7 (10%) | 0.675 |
| Non-Hispanic | 95 (84%) | 62 (89%) | |
| Unknown | 3 (3%) | 1 (1%) | |
| Age | |||
| Mean Age (SD) | 53.0 (9.9) | 59.0 (7.4) | 0.000 |
| BMI | |||
| Mean (SD) | 27.5 (6.7) | 27.1 (5.1) | 0 .721 |
| Treatment Completion | |||
| Yes | 49 (43%) | 67 (96%) | 0.000 |
| No | 64 (57%) | 3 (4%) | |
| Treatment Duration | |||
| Actual mean weeks (SD) | 29.1 (14.6) | 11.9 (0.5) | 0.000 |
| Expected protocol mean weeks | 40.5 | 12 | |
| Treatment Group | |||
| Naïve | 51 (45%) | 25 (36%) | 0.209 |
| Experienced | 62 (55%) | 45 (64%) | |
| Fibrosis Level | |||
| Minimal (F0-F2) | 50 (44%) | 20 (29%) | 0.034 |
| Advanced (F3-F4) | 63 (56%) | 50 (71%) |
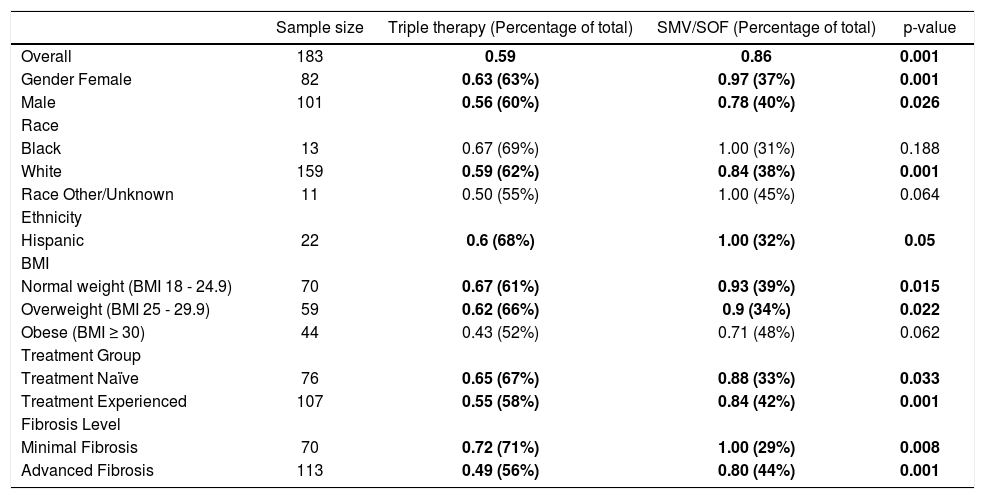
The primary outcome, unadjusted SVR rates by treatment group, stratified by subgroups of patient demographics and clinical characteristics, is represented in table 2. The SVR rates between the two treatment groups are significantly different from one another at the 5% level of significance. Males and females, as well as whites and Hispanics, have significantly higher SVR rates in the SMV/ SOF treatment group compared to the TT control group. Normal weight (BMI 18 - 24.9) and overweight patients (BMI 25 - 29.9) also had significantly higher SVR rates compared to obese patients (BMI ≥ 30) in both treatment groups. SMV/SOF patients had significantly higher SVR rates compared to the TT group, regardless of whether they were treatment naïve or treatment experienced or had minimal vs. advanced fibrosis.
SVR rates by subgroups (descriptive).
| Sample size | Triple therapy (Percentage of total) | SMV/SOF (Percentage of total) | p-value | |
|---|---|---|---|---|
| Overall | 183 | 0.59 | 0.86 | 0.001 |
| Gender Female | 82 | 0.63 (63%) | 0.97 (37%) | 0.001 |
| Male | 101 | 0.56 (60%) | 0.78 (40%) | 0.026 |
| Race | ||||
| Black | 13 | 0.67 (69%) | 1.00 (31%) | 0.188 |
| White | 159 | 0.59 (62%) | 0.84 (38%) | 0.001 |
| Race Other/Unknown | 11 | 0.50 (55%) | 1.00 (45%) | 0.064 |
| Ethnicity | ||||
| Hispanic | 22 | 0.6 (68%) | 1.00 (32%) | 0.05 |
| BMI | ||||
| Normal weight (BMI 18 - 24.9) | 70 | 0.67 (61%) | 0.93 (39%) | 0.015 |
| Overweight (BMI 25 - 29.9) | 59 | 0.62 (66%) | 0.9 (34%) | 0.022 |
| Obese (BMI ≥ 30) | 44 | 0.43 (52%) | 0.71 (48%) | 0.062 |
| Treatment Group | ||||
| Treatment Naïve | 76 | 0.65 (67%) | 0.88 (33%) | 0.033 |
| Treatment Experienced | 107 | 0.55 (58%) | 0.84 (42%) | 0.001 |
| Fibrosis Level | ||||
| Minimal Fibrosis | 70 | 0.72 (71%) | 1.00 (29%) | 0.008 |
| Advanced Fibrosis | 113 | 0.49 (56%) | 0.80 (44%) | 0.001 |
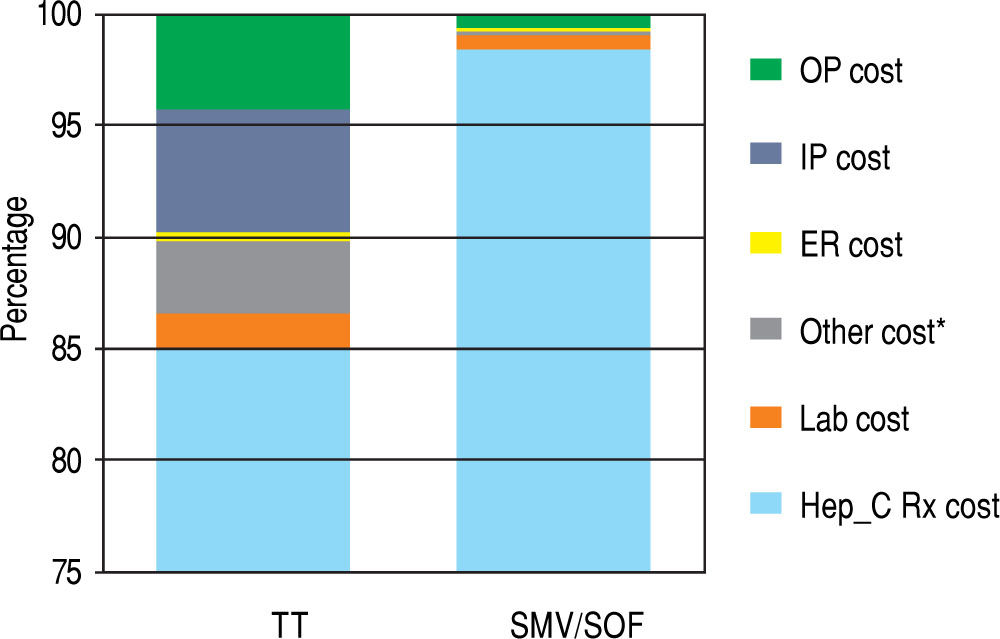
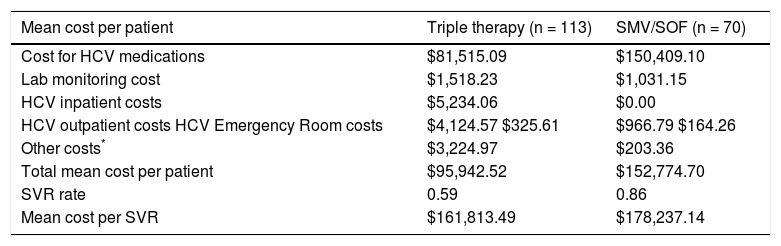
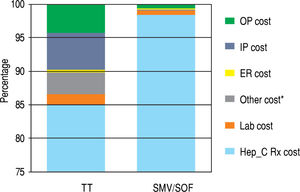
Table 3 represents the unadjusted components of costs that constitute the cost component of the model and the unadjusted costs per SVR for treatment and control groups. HCV medication-related costs make up the majority of the total costs (98% of total costs for SMV/SOF; 85% for TT). The data are presented graphically in Figure 3. Lab monitoring costs for TT are higher than SMV/SOF due to longer duration of treatment and need for closer monitoring. 9.8% of the TT cost components result from OP, IP, and ED visits compared to less than 1% of costs for SMV/SOF treatment. No SMV/SOF patients had an IP visit during treatment. Over 3% of costs for TT patients were due to other treatment-related expenses and management of hematologic side effects, such as blood transfusions and epoetin alfa for treatment-related anemia and fil-grastim for leukopenia. SMV/SOF patients achieved a higher mean SVR rate compared to TT (0.86 vs. 0.59). As a result, the cost per SVR becomes comparable ($178,237.14 vs. $161,813.49) between the 2 groups.
Mean total costs (unadjusted).
| Mean cost per patient | Triple therapy (n = 113) | SMV/SOF (n = 70) |
|---|---|---|
| Cost for HCV medications | $81,515.09 | $150,409.10 |
| Lab monitoring cost | $1,518.23 | $1,031.15 |
| HCV inpatient costs | $5,234.06 | $0.00 |
| HCV outpatient costs HCV Emergency Room costs | $4,124.57 $325.61 | $966.79 $164.26 |
| Other costs* | $3,224.97 | $203.36 |
| Total mean cost per patient | $95,942.52 | $152,774.70 |
| SVR rate | 0.59 | 0.86 |
| Mean cost per SVR | $161,813.49 | $178,237.14 |
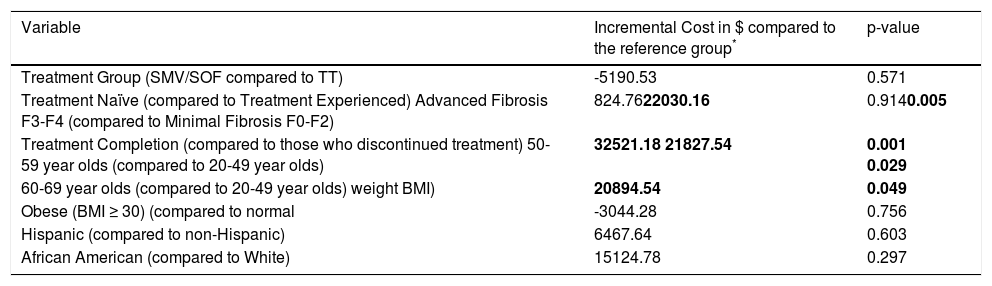
The incremental cost per SVR across treatment groups is summarized in Table 4. In contrast to the unadjusted costs per SVR, adjusted costs per SVR are not significantly different between treatment groups (p = 0.571). Covari-ates that were statistically significant include advanced versus minimal fibrosis (advanced fibrosis patients had higher costs per SVR than minimal fibrosis patients); treatment completion vs. discontinuation (completing treatment resulted in higher costs per SVR); and older vs. younger patients (older patients had higher costs per SVR than younger patients).
Cost per SVR (adjusted using GLM).
| Variable | Incremental Cost in $ compared to the reference group* | p-value |
|---|---|---|
| Treatment Group (SMV/SOF compared to TT) | -5190.53 | 0.571 |
| Treatment Naïve (compared to Treatment Experienced) Advanced Fibrosis F3-F4 (compared to Minimal Fibrosis F0-F2) | 824.7622030.16 | 0.9140.005 |
| Treatment Completion (compared to those who discontinued treatment) 50-59 year olds (compared to 20-49 year olds) | 32521.18 21827.54 | 0.001 0.029 |
| 60-69 year olds (compared to 20-49 year olds) weight BMI) | 20894.54 | 0.049 |
| Obese (BMI ≥ 30) (compared to normal | -3044.28 | 0.756 |
| Hispanic (compared to non-Hispanic) | 6467.64 | 0.603 |
| African American (compared to White) | 15124.78 | 0.297 |
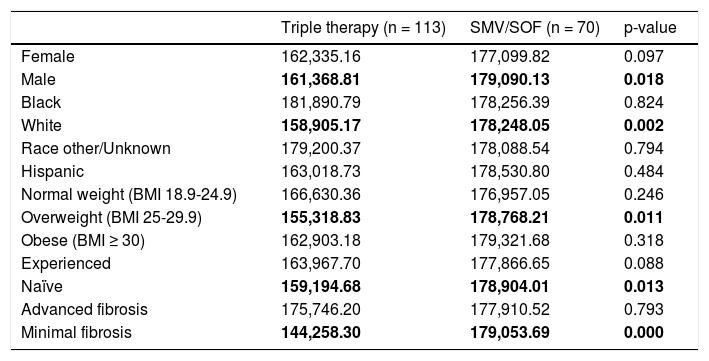
A sub analysis of unadjusted cost per SVR of the aforementioned covariates was performed (Table 5). There is considerably less variation in costs among SMV/SOF group compared to the TT group. This is due to the cost of HCV medication driving the total costs in SMV/SOF, whereas costs related to longer treatment and adverse events during treatment increase the variability of costs in the TT group. Finally, the odds ratio for completing treatment and achieving SVR in the two treatment groups was calculated. The odds of completing the treatment were 39 times higher for SMV/SOF group compared to TT patients. Additionally, SMV/SOF patients had a six times higher odds of achieving SVR vs. TT patients.
Cost per SVR by subgroups (descriptive).
| Triple therapy (n = 113) | SMV/SOF (n = 70) | p-value | |
|---|---|---|---|
| Female | 162,335.16 | 177,099.82 | 0.097 |
| Male | 161,368.81 | 179,090.13 | 0.018 |
| Black | 181,890.79 | 178,256.39 | 0.824 |
| White | 158,905.17 | 178,248.05 | 0.002 |
| Race other/Unknown | 179,200.37 | 178,088.54 | 0.794 |
| Hispanic | 163,018.73 | 178,530.80 | 0.484 |
| Normal weight (BMI 18.9-24.9) | 166,630.36 | 176,957.05 | 0.246 |
| Overweight (BMI 25-29.9) | 155,318.83 | 178,768.21 | 0.011 |
| Obese (BMI ≥ 30) | 162,903.18 | 179,321.68 | 0.318 |
| Experienced | 163,967.70 | 177,866.65 | 0.088 |
| Naïve | 159,194.68 | 178,904.01 | 0.013 |
| Advanced fibrosis | 175,746.20 | 177,910.52 | 0.793 |
| Minimal fibrosis | 144,258.30 | 179,053.69 | 0.000 |
Tolerability and efficacy were two main barriers to HCV treatment before the first combination DAA treatment, SMV/SOF. Many patients were not eligible for treatment with peg-IFN due to mental health concerns or other comorbidities. Of the patients who were healthy enough to start treatment, only 43% were able to finish the full course (mainly due to recommended 48 week treatment course). Only 59% achieved an SVR (Table 2). Medication costs of TT resulted in 85% of the total costs, but patients also had inpatient and outpatient visits, as well as frequent laboratory monitoring that increased the total healthcare utilization costs. This led to an average of $95,952.52 in total cost per patient. But when taking into account the efficacy, it was $161,813.49 per SVR. In comparison, the costs per patient was significantly higher ($152,774.70) for the SMV/SOF regimen, although this translated to a cost per cure that was similar at $178,237.14.
Significant barriers to care still remain despite the development of safe, tolerable, and effective regimens. As more patients are tested and diagnosed with HCV infection and there are less contraindications for patient groups, there is an increased demand for treatment. The number of eligible patients to be treated has significantly increased. The barrier to care is no longer tolerability and comorbidities, but financially driven.
Our analysis shows that the cost per cure of HCV treatment has remained constant during the transition from TT to multi-DAA treatment. If the costs of DAAs are fixed, then health insurance companies will need to allocate additional financial resources in order to cure more patients. If resource allocation for HCV treatment is fixed, then the cost of DAAs must be reduced to cure more patients. The volume of eligible patients for multi-DAA treatment for HCV has led to financial strain on the healthcare system. It is our opinion that reduction in costs of DAAs through competition and market forces and additional allocation of resources by payers are essential to cure more patients with HCV infection.
Additional studies needed to compare this data to newer combination treatments, such as sofosbuvir/ledipasvir, paritaprevir/ritonavir/ombitasvir/dasabuvir, grazoprevir/ elbasvir, and sofosbuvir/velpatasvir.
There are several limitations to the methodology we used in our analyses. The cost of regimen sums up to 98% of total cost in the SMV/SOF group. By using AWP estimates for DAA regimen prices, we did not take into account discounted drug costs or rebates that provider networks and health plans may negotiate in the current marketplace since the time of this analysis. Thus by utilizing the AWP we may have overestimated the cost of both SMV/SOF and TT regimens.
Similarly, our use of PharMetrics for costs of treatment-related inpatient, outpatient and emergency department visits, laboratory tests, drug fills and other patient utilization draws from costs of commercial insurance claims from managed care organizations throughout the US. Commercial insurance claims may not accurately reflect costs incurred by tertiary academic care facilities such as UCH or similar safety net hospitals and clinics that serve a higher percentage of indigent patients and utilize pricing schemes specific to these providers, such as 340 B pricing.
Another limitation of the data is the relatively small sample size and homogeneity within the UCH system. Over 80% of the HCV population for this analysis self-identifies as white, non-Hispanic. Our patient population thus does not fully reflect national disease prevalence among minority populations and therefore cannot necessarily be used for estimations of efficacy and cost at other more heterogeneous treatment centers.
ConclusionThis study defined the cost per cure of HCV in a well-defined patient population with HCV genotype 1 infection. By controlling for a variety of patient characteristics, we showed that the cost per cure has not changed appreciably during the transition from TT to multi-DAA treatment. Lowering drug prices via competition in the marketplace, coupled with an increase in resource allocation by payers, could dramatically increase the number of patients cured of HCV.
Abbreviations- •
2D-regimen: Ritonavir-boosted paritaprevir/ombitasvir.
- •
3D-regimen: Ritonavir-boosted paritaprevir/ombitas-vir with dasabuvir.
- •
AWP: Average wholesale price.
- •
BMI: Body-mass index.
- •
CBC: Complete blood count with manual differential.
- •
CMP: Comprehensive metabolic panel.
- •
Cost/SVR: Cost per sustained virologic response.
- •
DAA: Direct acting antiviral.
- •
DAC: Daclatasvir.
- •
ED: Emergency department.
- •
EMR: Electronic medical record.
- •
GLM: Generalized linear model.
- •
HBV: Hepatitis B virus.
- •
HCV: Hepatitis C virus.
- •
HIV: Human immunodeficiency virus.
- •
IP: Inpatient.
- •
LDV: Ledipasvir.
- •
OP: Outpatient.
- •
Peg-IFN: Pegylated interferon.
- •
SMV: Simeprevir.
- •
SOF: Sofosbuvir.
- •
SVR: Sustained-virologic response.
- •
TID: Three times a day.
- •
TSH: Thyroid-stimulating hormone.
- •
TT: Triple therapy.
- •
UCH: University of Colorado Health.
- •
Langness: Nothing to Disclose.
- •
Tabano: Nothing to Disclose.
- •
Wieland: Grant Funding: Janssen.
- •
Tise: Nothing to Disclose.
- •
Pratt: Nothing to Disclose.
- •
Ayers: Nothing to Disclose.
- •
Lin: Nothing to Disclose.
- •
Ghuschcyan: Nothing to Disclose.
- •
Nair: Grant Funding: Novartis, Biogen Idec. Consultation for Astellas, Genentech.
- •
Everson: Advisory Boards: Roche/Genentech, Vertex, Globeimune, BMS, Abbvie, Eisai, HGS/Novartis, Pfizer, Gilead, Janssen/Tibotec, Abbvie/Abbott. Consulting: Roach-Genentech, HGS/Novartis, BMS, Three Rivers/Kadmon, Vertex, Abbvie, Biotest, Boe-hringer-Ingelheim. DSMB: Merck, Centocor, Galec-tin. Stock/Ownership: HepQuant LLC. Management: HepQuant LLC. Research Grants: Roche/Genentech, Schering/Merck, Vertex, GlobeImmune, Gilead, HGS/Novartis, BMS, Pfizer, Source, Eisai, GSK, Pharmassett, Ortho Biotech, Janssen/Tibotec, Abbie.