Objective. Thyroid hormones profile in patients with hepatic cirrhosis due to chronic HBV and HCV infections was evaluated in order to find any relationship between thyroid hormones and severity of liver damage.
Material and methods. Patients with the diagnosis of hepatic cirrhosis due to hepatitis B or C were screened for thyroid function status. Child-Pugh and model for end-stage liver disease (MELD) scores were calculated. Considering each thyroid function test, patients were divided into two groups with lower than normal and normal range of thyroid hormones, separately for each (for TSH, normal and upper than normal). The correlation between thyroid function tests and severity of liver disease was taken into account.
Results. Number of patients with a T3 level lower than normal range (70-110 ng/dL) significantly increased along with Child-Pugh scores A, B and C. A negative correlation was found between Child-Pugh scores and total serum T3 level (r = -0.453, P < 0.001). Also a reverse correlation was observed between MELD score and T3 levels (r = -0.305, P = 0.14).
Conclusion. In conclusion serum T3 concentration is a good index of hepatic function, decreasing by the severity of liver damage.
Liver plays an important role in the metabolism of thyroid hormones, as it is the most important organ in the peripheral conversion of tetraiodothyronine (T4) to triiodothyronine (T3) by type I iodinase resulting to 5, deiodination of T4.1 Moreover, it is involved in conjugation and circulation of thyroid hormones by synthesis of thyroid binding proteins.2,3
There are evidences showing an association between chronic liver diseases and changes in thyroid gland.2-6 A 17% increase in thyroid glandular volume in cirrhotic patients is reported when compared with controls.4 Furthermore, it is demonstrated that levels of thyroid hormones and their binding proteins are altered in patients with hepatic disorders, especially cirrhosis;5 however, almost all are clinically euthyroid.3 The most frequent change in plasma levels of thyroid hormones is decreased total T3 and free T3 concentration which is reported to be associated with the severity of hepatic dysfunction.2,3,6
The relative high prevalence of hepatitis B and C viruses (HBV, HCV) infection in Iran7,8 and their major role in development of hepatic cirrhosis led us to evaluate thyroid hormones profile in patients with hepatic cirrhosis due to chronic HBV and HCV infections to find any relationship between thyroid hormones and severity of liver damage.
Material and MethodsIn a cross-sectional study, all patients attending the outpatient liver clinic of Razi Hospital, Rasht, Iran with the diagnosis of hepatic cirrhosis due to hepatitis B or C during March 2008 to March 2009 were consecutively screened for thyroid function status. The diagnosis of cirrhosis was confirmed by either liver biopsy, laboratory findings of liver failure or ultrasound proven portal hypertension and small size liver. Chronic hepatitis was defined as more than 6 months of abnormal serum aminoteransferase; if associated with positive serum HBS antigen, was considered due to HBV, and if accompanied by positive serum anti-HCV antibody and positive HCV RNA PCR, was considered due to HCV. Patients suffering from previously known thyroid dysfunction or with clinical findings of thyroid disease and those positive for both HBV and HCV infections, were not considered in the study. Patients on medications that could affect the study outcome, including Carbamazepine, Phenytoin, Phenobarbitone, Salicylates and Nonsteroidal anti-inflamatory druges (NSAIDs) were also excluded; none of the patients had been under treatment by thyroid stimulating/inhibiting agents. As we wanted to study the changes in thyroid hormones in patients suffering from HBV or HCV related cirrhosis, subjects with a history of alcohol consumption in the last 6 months were omitted.
Gastrointestinal and Liver Diseases Research Center of Guilan University of Medical Sciences ethics committee approved the study protocol. Patients fulfilling the inclusion criteria were recruited in the study after taking informed written consent. Thyroid function, liver function and clinical complications including ascites, encephalopathy, bleeding varices, and spontaneous bacterial peritonitis (SBP) were evaluated. Child-Pugh score was recorded9 and MELD risk score (RS) was calculated according to the following formula:10
RS = 0.957 x loge (creatinine mg/dL) + 0.378 x loge (bilirubin mg/dL) + 1.120 x loge (INR) + 0.643 x (cause of cirrhosis).
For which the value for cause of cirrhosis, as it was viral in all the patients, was considered 1.
Circulating free T3 (reference, 1.5 to 4.1 ng/dL), free T4 (reference, 0.9 to 1.7 mcg/dL), total T3 (reference, 70 to 190 ng/dL), total T4 (reference, 4.8 to 12 mcg/dL) and TSH (reference, 0.3 to 5.5 mcIU/ mL), total albumin and thyroxine-binding globulin (TBG) levels were measured with radioimmunological assay kits (Monobind, USA). Liver tests were examined using Pars Azmoon-co kits (Tehran, Iran).
Statistical analysisDuring analysis, in order to figure out significant differences, patients were divided into different groups according to type of hepatitis, comorbidities, Child-Pugh classes and MELD score more vs. less than 20. Statistical analysis was performed using SPSS 14.0 (SPSS Inc., Chicago, IL). Chi-square test, Fisher exact test and univariate logistic regression model were used as appropriate. As child-Pugh and MELD scores were not normally distributed, Spearman's rho was used to report the correlations. A P-value < 0.05 was considered statistically significant.
ResultsSeventy two cirrhotic patients attended our liver clinic during the study period; 3 were excluded due to chronic alcohol consumption and 5 due to history of hypothyroidism. A total of 64 patients (42 male and 22 female) with a mean age of 55.03 ± 12.05 were enrolled in the study.
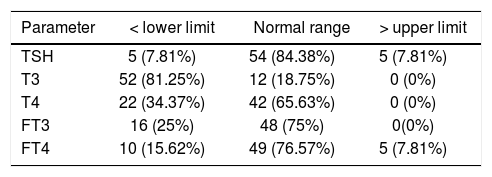
The mean duration of cirrhosis before enrollment was 25 months (range = 1 month to 6 years). HBV was specified in 34 patients as the etiology of cirrhosis while in 30 patients HCV was responsible. Associated comorbidities included ascites (n = 59), encephalopathy (n = 31), bleeding varices (n = 21), and SBP (n = 10). The results of thyroid function tests are expressed in table 1. The total albumin and the TBG levels were measured as 3.04 ± 0.6 (NL: 4-5.3 gr/dL) and 1.02 ± 0.9 (NL: 1.3-3.0 mgr/dL), respectively.
Thyroid function tests in the study population cate-gorized according to normal ranges (n; %).
| Parameter | < lower limit | Normal range | > upper limit |
|---|---|---|---|
| TSH | 5 (7.81%) | 54 (84.38%) | 5 (7.81%) |
| T3 | 52 (81.25%) | 12 (18.75%) | 0 (0%) |
| T4 | 22 (34.37%) | 42 (65.63%) | 0 (0%) |
| FT3 | 16 (25%) | 48 (75%) | 0(0%) |
| FT4 | 10 (15.62%) | 49 (76.57%) | 5 (7.81%) |
When patients were divided into two groups with lower than normal and normal range of thyroid hormones, separately for each thyroid hormone test (for TSH, normal and upper than normal), only for total T3, significant differences were found in number of patients suffering from ascites, bleeding varices, and Child-Pugh scores. Among the patients presented with ascites, 16 had severe (all with T3 < 70 ng/dL), 31 moderate (26 with T3 < 70 ng/dL), and 12 mild (2 with T3 < 70 ng/dL) ascites. In a logistic regression model adjusted only for ascites, patients with a higher grade ascites were more probable to have a serum T3 level below the normal range (OR = 3.56, 95% CI = 1.54 to 8.23); hence, the severity of ascites was correlated with the number of patients with a serum T3 level of less than the normal range (P = 0.011). For all 20 patients with bleeding varices, serum T3 level was < 70; compared with the rest (32 with serum T3 lower than normal range out of 44), the difference was significant (P = 0.012). However, TSH, T4, FT3 and FT4 were not different in patients with and without comorbidities.
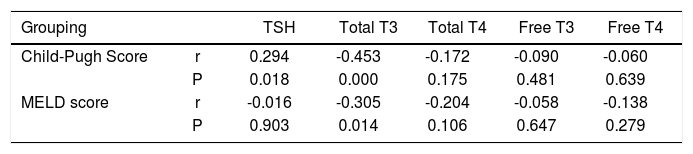
Patients were categorized into Child-Pugh scores A, B and C. Dividing patients into subgroups with thyroid function test variables lower than normal and normal (for TSH, normal and upper than normal), the difference was again significant only for T3; number of patients with T3 < 70 ng/dL was 2 (out of 5), 16 (out of 22) and 34 (out of 35) for Child-Pugh scores A, B and C, respectively (P < 0.001). Also a negative correlation was found between Child-Pugh scores and total serum T3 level (r = -0.453, P < 0.001). The same analysis for MELD score over 20 versus less than 20 showed no significant difference for any of the thyroid function test variables. However, a reverse correlation was observed between MELD score and T3 levels (r = -0.305, P = 0.014). Also there was a positive correlation between TSH and Child-Pugh score (r = 0.294, P = 0.018) (Table 2). Patients with HBV/HCV related cirrhosis were classified by thyroid test variables lower than normal/within normal range, separately for each thyroid hormone test (for TSH, normal and upper than normal). No significant difference was recorded for any thyroid test variables (for TSH, P = 0.540; T3, P = 0.810; T4, P = 0.081; free T3, P = 0.583; free T4, P = 0.810).
The correlation between thyroid profile and Child-Pugh score and MELD score.
| Grouping | TSH | Total T3 | Total T4 | Free T3 | Free T4 | |
|---|---|---|---|---|---|---|
| Child-Pugh Score | r | 0.294 | -0.453 | -0.172 | -0.090 | -0.060 |
| P | 0.018 | 0.000 | 0.175 | 0.481 | 0.639 | |
| MELD score | r | -0.016 | -0.305 | -0.204 | -0.058 | -0.138 |
| P | 0.903 | 0.014 | 0.106 | 0.647 | 0.279 |
r: Spearman’s rho. P: P-value.
In the present study, it was confirmed that a more severe liver status was inversely associated with serum total T3 levels; using Child-Pugh score and MELD risk score such a negative correlation was observed. Categorizing patients according to Child-Pugh score to A, B and C, we found that the number of patients with a serum T3 below the normal range significantly increased with Child-Pugh score; such relationship was not present when setting a cutoff of 20 as the indicator of severity of hepatic status in MELD risk score. Severity of ascites and presence of bleeding varices, as indicators of severity of cirrhosis, also showed negative correlation with serum T3 levels. However, no significant difference was found according to the viral etiology of cirrhosis (HBV/HCV).
It is proved that hypothyroidism is associated with conditions mimicking liver diseases, including elevated aspartate amino transferase and myxoedema ascites.11,12 As a result, patients with a history of treated or under treatment hypothyroidism were excluded in this study. Alcohol is stated to be toxic for thyroid gland parenchyma;13 hence, patients with a history of alcohol consumption were also omitted. In this study, in addition to Child-Pugh score, the correlation between MELD score and thyroid function tests was also examined. We also tried to investigate the decrease in serum T3 level in cirrhotic patients according to severity of liver damage by classifying the patients into under normal/normal range T3 groups along with finding the correlation between T3 and severity of the liver disease.
Thyroxine and tri-iodothyronine modulate hepatic function by regulating hepatocyte basal metabolic rate, besides all other body cells. Liver also regulates circulating T3 and T4 by hormone metabolizing, resulting in a supervised endocrine effect.6 Two main enzymes acting in the liver as part of the iodothyronine seleno-deiodinase enzyme system are type 1 and type 3 deiodinases, responsible for extrathyroidal production of T3 and inactivation of thyroid hormones, respectively.14-16 The decrease in total T3 probably reflects a decrease in deiodinase 1 activity in the liver of cirrhotic patients.2,6,17 However, a contemporary increase in rT3 values18,19 associated with sick euthyroid syndrome is attributed to factors inhibiting conversion of rT3 to rT2,6,20 which was not observed in our study.
Lipophilic thyroid hormones in plasma are more than 99% bound to thyroxine-binding globulin, thyroxine-binding prealbumin and albumin. The liver role in synthesis of these proteins justifies a lower serum total T3 after hepatocyte damage; negative correlation between thyroxine-binding globulin and Child-Pugh score is reported. Decreased serum T3 is, as a result, associated with severity of Child-Pugh score.21,22 However, serum TSH and T4 are reported to be at steady levels2,3,6,17,22 despite alteration in serum T3, indicating adaptive mechanisms by which the body reduces basal metabolic rate to decrease caloric requirements, however, preserving the patient euthyroid. Moreover, lower total serum T3 dedicates to a lower basal metabolic rate within hepatocytes and the resultant preservation of liver function and total body protein storage.6
Experimental studies on rats showed subclinical hypothyroidism to be beneficial both in protecting the liver from further damage and in regression of established fibrosis in induced liver fibrosis.23 It is also suggested that liver function in hypothyroid patients tend to be better compared with euthyroid controls.24 Besides, Oren et al also showed that a controlled hypothyroidism might be beneficial for euthyroid cirrhotic patients.25 These studies, however, could be suggestive of a protective mechanism in the body in which lower circulating T3 in the body contributes to protection of liver from further fibrosis and helps the liver reverse the damages.
Hepatitis C is reported to be more associated with hypothyroidism rather than hepatitis B,22,25 however, we did not find any significant relation between types of hepatitis and serum level of thyroid hormones which was in accordance with the results of Zietz, et al.21
ConclusionOur results confirm other studies in which serum T3 concentration is reported to be a good indicator of hepatic function. No difference was observed in thyroid hormones regarding etiology of viral hepatitis.
Abbreviations- •
MELD: model for end-stage liver disease.
- •
SBP: spontaneous bacterial peritonitis.
- •
RS: risk score.
- •
HBV, HCV: hepatitis B and C viruses.
The authors would like to thank members of Gastroenterology ward, Laboratory personnel's of Razi Hospital for their priceless assistance in performing this study. We also thank Farzan Institute of Science Research and Technology for technical assistance.