The Liver Unit at the “Dr. José E. González” University Hospital and School of Medicine of the Autonomous University of Nuevo León in Monterrey was founded in 1983. Over the years, it has become a referral center for the northeast of Mexico. The frequency of diagnosis has changed: in 1983, the most common liver disease seen was alcoholic liver disease, today it is chronic hepatitis C. Amebic liver abscess, which used to be common, was hardly seen in 2001. Non-alcoholic fatty liver disease was unidentified 18 years ago, whereas in 2001 it was seen in 10% of patients. The development of five laboratories within the unit has allowed us to implement basic and clinical research trials, and to offer a high quality diagnostic service.
The experimental liver transplant program started in 1987 and a clinical program in humans in 1991: four patients received an orthotopic liver transplantation in its first phase. In the second phase, 20 patients received allografts from September 1999 to March 2002. Technical complications have been encountered in only one patient, with a biliary leak, and there have been three perioperative deaths. Infections occurred in eight patients; all resolved. Acute postoperative rejection occurred in two patients, and in the first seven months in another five; all of them resolved. The twoyear survival rate is 80%.
This unit offers a highly specialized diagnosis, standardized specialized laboratory services and a transplant program that guarantees a higher quality of medical attention to patients with liver diseases.
The Liver Unit at the “Dr: José E, González” University Hospital and School of Medicine of the Autonomous University of Nuevo León in the city of Monterrey, Mexico, was founded in 1983. The aims were to develop a highly specialized center for the diagnosis and treatment of liver diseases, to develop basic and clinical research according to the most frequent liver pathologies seen in the northeast of Mexico, and to develop teaching programs for pre-graduate medical students, as well as postgraduate Master’s and Doctorate programs. This unit was the second one founded in Mexico, and the first one outside Mexico City.
To comply with the original aims of the Unit, a description of the most frequently seen liver diseases was made.1 The major causes of liver diseases have changed over the years. During 1982 and 1983, when the Unit was starting, the major cause of liver disease was alcohol abuse (29%); amebic liver abscess (ALA) was the third most common (19.5%), and chronic active hepatitis (CAH) the fourth (10.2%). Twenty-five percent of CAH patients had chronic hepatitis B, and non-A-non-B chronic liver disease (CLD) represented 54% of all non-alcoholic CLD cases. As the Unit has grown over the years and has become a reference center, the most common liver disease seen in 2001 was chronic hepatitis C. With the spread of anti-amebic treatment, we now very rarely see ALA; no patient was seen with this pathology in 2001.
Five laboratories have been established in the Liver Unit, in which diagnostic tests are performed, as well as basic and clinical research. The Unit has had a steady growth. The mean number of laboratory studies done from 1983 to 1997 was 1,537 per year, whereas during 1998 it went up to 4,039, and was maintained in the years to follow. Diagnostic laboratory studies are performed on patients referred from the unit as well as from different laboratory institutions that require highly specialized laboratory tests.
During 1987, together with the Department of Surgery, we began an experimental program on liver transplantation in dogs. Technical skills were developed by a group of surgeons. Over 100 liver transplants were performed in dogs before the Liver Transplant Program (LTP) was started in humans. In September 1991, the first liver transplant was performed. This procedure, performed in a oneyear- old baby, was successful, but the patient died of pulmonary complications five days after the operation. In the first phase of the program, four patients received an orthotopic liver transplantation (OLT); three were children and one was an adult, who survived for 3.5 years, and died of gastrointestinal bleeding.2 In 1993, the first living-relation donor transplant in Mexico was performed in Monterrey in a 28-month-old child. Because of financial problems, the program was closed for six years, re-opening in September 1999. In the second phase of the LTP, 20 patients have received an OLT: four were aged 18 years or younger and the rest were older than 18 years.3
This study reports the development of a Liver Unit in Latin America. We have chosen to analyze three of the most representative aspects of the unit during the year 2001: the most common diagnoses of the patients seen, the most common abnormalities seen in laboratory diagnostic tests, and the progress of the Liver Transplant Program.
Most common liver diseases seenDuring 2001, 960 consultations or hospitalizations were seen at the Unit. For the purpose of this review, we have analyzed the most common diagnoses made in 159 patients who first attended the Unit during 2001. Patients attending the Unit come from 10 different states of Mexico, although most of them (66%) are from Monterrey. The northeastern states of Coahuila and Tamaulipas account for 25.5% of patients, and the rest come from Mexico City, San Luis Potosí, Durango, Veracruz, Michoacán, and Sonora.
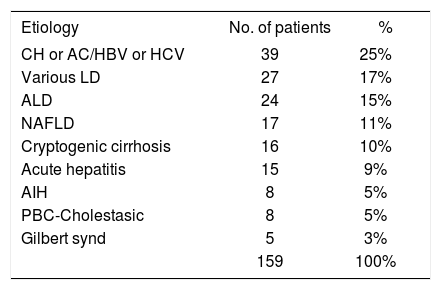
Nineteen years after the Unit’s foundation, the frequency of diseases has changed. The major original etiology was chronic viral hepatitis (25%, see Table), accounting for 39/88 (44%) of patients with non-alcoholic chronic liver disease. In fact chronic hepatitis (CH), regardless of the etiology, constituted the major cause of liver disease (n = 47, 30%). Alcoholic liver disease (ALD) was the third most common liver disease seen (n = 24, 15%), and non-alcoholic fatty liver disease (NAFLD) was seen in 17 (11%) patients (Table). Sixteen patients remain with an unknown etiology for their cirrhosis.
Diagnosis of patients first attending to the Liver Unit during 2001.
| Etiology | No. of patients | % |
|---|---|---|
| CH or AC/HBV or HCV | 39 | 25% |
| Various LD | 27 | 17% |
| ALD | 24 | 15% |
| NAFLD | 17 | 11% |
| Cryptogenic cirrhosis | 16 | 10% |
| Acute hepatitis | 15 | 9% |
| AIH | 8 | 5% |
| PBC-Cholestasic | 8 | 5% |
| Gilbert synd | 5 | 3% |
| 159 | 100% |
CH: chronic hepatitis; AC: asymptomatic carrier; LD: liver diseases; ALD: alcoholic liver disease; NAFLD: non-alcoholic fatly liver disease; AIH: autoimmune hepatitis; PBC: primary biliary cirrhosis.
Fifty-nine of the 159 patients (37%) had cirrhosis, and their etiologies are shown in figure 1. In the group of patients with chronic hepatitis (CH) due to hepatitis C virus (HCV) and/or hepatitis B virus (HBV), 38% were shown to have cirrhosis, whereas 83% of ALD patients were cirrhotic when first seen. On the other hand, when patients with viral hepatitis were separated by etiology, 37% of those with HCV infection were cirrhotic, whereas more of the patients with HBV (67%) had cirrhosis (Figure 2). It is important to note that CH due to HCV was far more common than CH caused by HBV (30/39, 77%, vs 6/39, 15%). Only three patients (8%) had a simultaneous HCV/ HBV infection (Figure 2).
Ten percent of patients (n = 16) presented with autoimmune liver disease, either primary biliary cirrhosis (PBC) or autoimmune hepatitis (AIH). A high proportion of patients with AIH already had cirrhosis (63%) when they were first seen. Most of the patients with AIH had type 1 disease; in a series of 50 patients with this diagnosis only two had type 2 AIH with anti-liver and anti-kidney microsomal antibody type 1 (unpublished observations).
Laboratory studiesDuring 2001, 4,226 laboratory studies were performed at the Liver Unit. Of these, 3,210 (76%) were done for diagnostic purposes, whereas 1,016 (24%) were performed in clinical or research projects.
The Liver Unit is a reference center in the northeast of Mexico, which means that diagnostic laboratory studies were carried out for patients from the Unit and for patients referred from 11 different institutions in the state of Nuevo León. Figure 3 shows the biochemical tests performed and the percentage of abnormal results obtained during 2001.
Biochemical characteristics in patients seen at the Liver Unit during 2001.
Total protein (TP), Albumin (Alb), Total bilirubin (TB), Direct bilirubin (DB), Aspartate transaminase (AST), Alanine transaminase (ALT), Alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γGT), α-fetoprotein (αFP), Ferritin (FER).
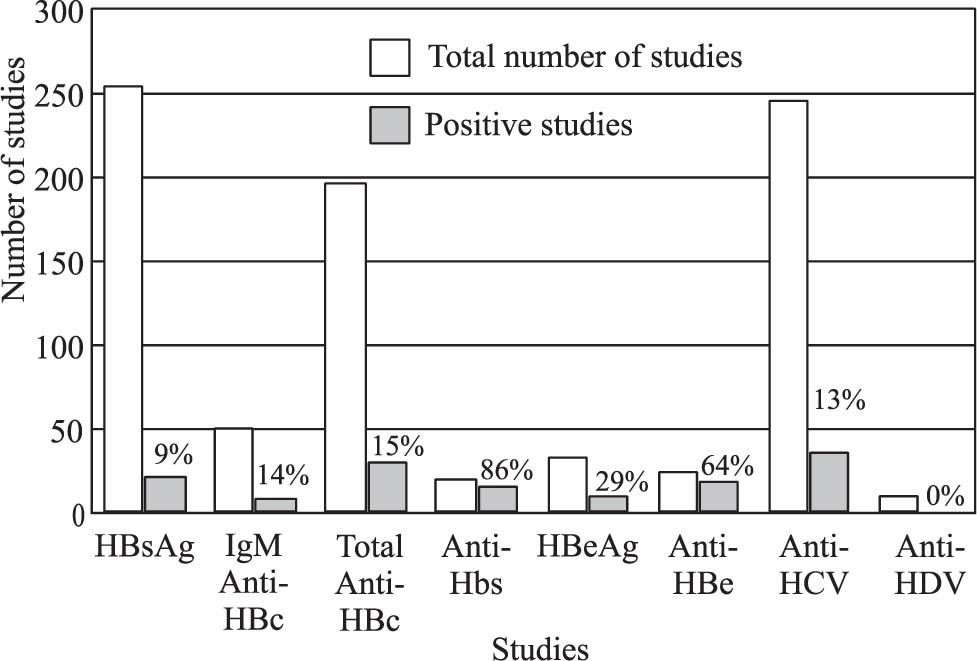
Figures 4and5 show the most common viral markers measured and the percentage of positive results. The viral markers with the most demand for measurement were HBsAg (n = 257), total anticore (n = 197) and Anti-HCV (n = 245); however, their rates of positive results were not high (9%, 15% and 13%, respectively). The tests with the greatest proportions of positive results were IgM-HAV (41%) and IgG-HAV (88%), which confirms that HAV is an endemic disease in Mexico (Figure 5). HCV-RNA was confirmed positive in 53 of cases (43%) and HBV-DNA in 35 (23%).
Viral characteristics seen in patients of the Liver Unit in 2001.1
Virological characteristics seen in patients of the Liver Unit in 2001.2
The liver transplant program was started with the orchestration of a multidisciplinary group including surgeons, intensive care specialists, hepatologists, nurses and psychologists, as well as various highly trained internal medicine specialists who contributed to the correct selection, preparation and care of patients before and after their OLT.
Twenty patients have received a liver transplant from September 1999 to March 2002. One transplant was from a living relative and 19 were from cadavers. All the patients had cirrhosis. Etiologies were hepatitis C (3 patients), PBC (3 patients), alcohol (3 patients), cryptogenic (3 patients), AIH (2 patients), biliary atresia (2 patients), hepatitis B (1 patient), autoimmune cholangitis (1 patient), methotrexate treatment (1 patient) and biliary hypoplasia (1 patient).
During 2001, 22 patients were evaluated in the OLT protocol to see whether they could fill our criteria to benefit from the procedure. Three patients had very advanced liver disease, and died before completing their evaluation. Two were rejected because they had advanced hepatocellular carcinoma (HCC). One patient was rejected because he tested positive for HIV antibodies, confirmed by western blotting, in addition to being positive for HCV-RNA. One patient died while on the waiting list (Child-Pugh class C with a score of 11). Twelve patients received an OLT: 11 from cadavers and one from a living relation. The remaining three patients are presently in the 2002 waiting list, out of a total of eight.
Figure 6 shows the cumulative survival of 18 patients who received an OLT and who had a follow-up for at least five months. The survival at two years was 80%. Three patients (15%) died shortly after the operation. One child died of cardiac failure at six days. Two adults died at two and five days of primary non-functioning allografts. One patient died of acute rejection four months after the OLT in her referral institution, and one patient died of disseminated HCC at five months. There has been only one patient with a significant cytomegalovirus infection, presenting as a severe pancreatitis; that patient died seven months after the OLT. Another two patients have received treatment for CMV with ganciclovir: one because the donor was positive for IgG antibodies while the recipient was negative; the other because he received multiple transfusions and a bolus of metilprednisolone to treat an acute rejection episode. The outcomes were successful for both, without any clinical evidence of CMV disease. Eight patients (44%) presented with infections in the postoperative period: these pathogens were CMV, herpes virus (HV), Candida, Mycobacterium tuberculosis bacteria, and anaerobes. All patients had adequate responses to treatment, except for the patient with CMV infection, which was resistant to both ganciclovir and foscarnet. No patient developed de novo infections with HBV or HCV.
Technical complications were seen in only one patient (5.5%), who developed a biliary leak that resolved with conservative treatment. Two patients (11%) developed an episode of acute rejection in the postoperative period at days four and 10; these were both resolved with methylprednisolone boluses. Another five patients (28%) developed episodes of acute rejection at four months (two patients), and at two, five and seven months. Among these, four episodes were resolved by adjusting the immunosuppressive regime. Two patients (11%) showed cholestasis due to preservation damage to the transplant, which resolved satisfactorily
CommentsFounding the Liver Unit was a great challenge, particularly as facilities were not there initially, but had to be built up according to needs. The first key ingredient was the presence of highly trained professionals with enthusiasm and high tolerance to frustration. There is still a long way to go. A multidisciplinary group is the backbone of a highly specialized center, with experienced Radiologists, Pathologists, Surgeons, Intensive Care specialists, nurses, Infectologists, Gastroenterologists and Hepatologists, who need to work together to offer a precise diagnosis to patients with liver diseases, for their monitoring and in preparation for a liver transplant.4,5 Over the years, as the Liver Unit evolved to become a referral center, the types of liver diseases changed in frequency, with hepatitis C now being the most common liver disease seen. Most of the patients have come from the northeast of Mexico.
The development of five laboratories in the Liver Unit has facilitated clinical and basic research to expand, as well as offering the patients laboratory studies with high standards. Most of the tests were developed for research projects before they were used for diagnostic purposes, for example our molecular biological studies to detect hepatitis B or C viral genomes, as well as hepatitis C genotypes. During 2001, 35 clinical or basic research projects, either multicentric or multinational, were performed at the Liver Unit for the treatment of chronic hepatitis B or C. Collaborative studies have been established with other institutions within Mexico, with different Schools within the Autonomous University of Nuevo León, and with different Departments within the School of Medicine and University Hospital.
When the Liver Unit was started, the diagnosis of NAFLD was unknown. Today 10% of patients are seen with this diagnosis, most of them with well-recognized risk factors such as diabetes mellitus, hyperlipidemia and obesity. Ten percent of patients present with cryptogenic cirrhosis, and we are still in the process of analyzing how many of these patients belong to the NAFLD or to other groups. A high proportion of patients had cirrhosis due to alcohol abuse (20/59, 34%); however, this is no longer the most frequent cause of liver disease seen.
The Liver Transplant Program became successful when it reopened in 1999, with a two-year survival rate of 80%. There have been only minor technical problems. The first living-relation donor transplant done in Mexico was performed in Monterrey. One third of transplanted patients had autoimmune liver diseases, and 22% had viral hepatitis B or C.
We also reported the first successfully transplanted patients with cirrhosis due to hepatitis B in Mexico. They were treated with lamivudine to get a low viral replication pattern and hyperimmune gamma globulin from the anhepatic phase.6 However, the high cost of this treatment limits its wider application.
Infections occur in the postoperative period in 30% to 90% of cases.7 Of the patients reported here, 44% had an episode of acute infection, which was resolved satisfactorily. Immunosuppressive treatment reduces cell-mediated immunity, which increases the susceptibility to infections. Other factors, such as malnutrition uremia and hyperglycemia, are also risk factors for infections, as are invasion with catheters, intravenous lines, chest and biliary tubes.
The immunosuppressive regime used in our program was either a combination of prednisone and cyclosporine, or prednisone, cyclosporine and mycophenolate mofetil.8 Three patients were switched from cyclosporine to tacrolimus, two of them during an acute episode or rejection and the other one because of suboptimal immunosuppression with cyclosporine; all of them had good results. The rescue of failing grafts using FK 506, one of the initial observations in Pittsburgh in the early clinical trials, has been confirmed in a number of centers.9
Although the cost of a liver transplant is much lower in Mexico than in USA (equivalent to US $ 43,000), it is still a costly procedure taking into consideration the economic limitations in Mexico and Latin America.
The development of this Liver Unit has allowed us to offer a highly specialized medical diagnosis, standardized specialized laboratory services. Combined with a Liver Transplant Program, it guarantees a higher quality of medical attention to patients with liver diseases. This in turn has allowed us to improve basic and clinical research lines, to offer possible solutions to local problems, and allows the advancement of knowledge that can be applied to the specific problems seen in Latin America.