Many of the 300,000 HCV-infected Canadians live in under-served and remote areas without access to HCV healthcare specialists. Telemedicine (TM) and advances in HCV management can facilitate linkage of these marginalized patients to healthcare.
Materials and MethodsA cohort database analysis was performed on patients followed at The Ottawa Hospital and Regional Viral Hepatitis Program between January 2012 and August 2016. We compared patient characteristics, fibrosis work-up and antiviral treatment outcomes in TM (n = 157) and non-TM (n = 1,130) patients (The Ottawa Hospital Viral Hepatitis Outpatient Clinic) residing in Eastern Ontario.
ResultsTM patients were more often infected with genotype 3 (25.9% vs. 16.4%), were more commonly Indigenous (7.0% vs. 2.2%) had a history of injection drug use (70.1% vs. 54.9%) and incarceration (46.5% vs 35.5%). Groups were comparable in age (48.9 years), gender (63.7% male) and cirrhotic stage (24.0%). 59.2% of TM patients underwent transient elastography during regional outreach blitzes compared to 61.8% of non-TM patients (p = 0.54). Overall, half as many TM patients initiated antiviral therapy as non-TM patients (27.4% vs. 53.8%, p < 0.001). The introduction of DAA regimens is bridging this gap (22.2% of TM patients vs. 34.3% of non-TM patients). SVR rates with interferon-free, DAA regimens were 94.7% and 94.8% in TM and non-TM groups (p = 0.99).
ConclusionOur TM program engages and retains a population that faces many barriers to effective HCV treatment. TM patients initiated HCV therapy and achieved high SVR rates comparable to those obtained using traditional models of care.
Telemedicine (TM) links patients to specialty healthcare in an effort to increase accessibility for rural and otherwise isolated populations.1-8 As many specialist physicians practice in or near urban-based academic centers, TM has the potential to provide much needed specialty care to patients outside these areas. TM has been well described in the management of chronic infections including HCV, HIV and tuberculosis.1-17 TM has been used to link patients directly to specialty healthcare providers, to facilitate consults between primary care providers and specialists, and to facilitate continuing medical education.9,10,14,15 An accumulating body of evidence supports the benefits of TM programs. These include increased access to care, increased uptake of treatment, and potential cost-effectiveness.1-7,9,10,14-16,18,19 Successful provision of HCV care in Canada faces substantial obstacles. There are approximately 300,000 HCV-infected Canadians.20 Many of these individuals live far from a tertiary care center and have no access to a HCV healthcare specialist. TM can provide healthcare to these marginalized patients, who would otherwise have limited access to HCV treatment, by remotely linking patients to healthcare services.
In an effort to engage and retain rural and otherwise isolated HCV patients in care, The Ottawa Hospital and Regional Viral Hepatitis Program began developing a TM program staffed by a multidisciplinary team of HCV-competent healthcare professionals in 2012. The Ottawa TM program provides care for patients residing in Eastern Ontario, predominantly from rural communities. According to the Statistics Canada 2011 National Census, 1,697,989 people reside in Eastern Ontario (area: 35,744.87 square km).21 In an effort to evaluate the effectiveness of this innovative health care delivery program, TM and non-TM (The Ottawa Hospital Viral Hepatitis Outpatient Clinic) patient characteristics were compared. We also assessed key milestones of HCV care including the proportion undergoing fibrosis evaluation, the numbers initiating HCV antiviral treatment as well as HCV treatment outcomes [i.e. Sustained Virologic Response (SVR)] by mode of health care delivery.
Materials and MethodsA cohort database analysis was performed on patients assessed at least once by The Ottawa Hospital and Regional Viral Hepatitis Program between January 2012 and August 2016. Data was collected from patient charts and electronic medical records in The Ottawa Hospital Viral Hepatitis Clinic database (REB 2004-196). Patients 18 years of age and older with chronic HCV infection were included in the analysis. Non-TM patients had all assessments conducted at The Ottawa Hospital outpatient clinic based within a tertiary care centre located in the urban setting of Ottawa, Canada (population: approximately 1,000,000). TM patients were defined as having the majority of clinic visits conducted utilizing the Ontario Tele-Health Network video and audio system. In approximately 80% of cases, all visits were conducted by TM. On occasion, more complex cases defined as those with advanced fibrosis and/or facing multiple obsticles to HCV care were assessed once or more within our outpatient clinic to expedite work-up. The TM system connects 1,583 clinic sites throughout Ontario (162 sites in Eastern Ontario) with a unit located at The Ottawa Hospital-General Campus. The patient and remote site TM nurse are linked by audio and video to The Ottawa Hospital site at which the HCV clinician, nurse and allied health care providers are located.
Clinical outcomes including pre-treatment access to biopsy, transient elastography (i.e. Fibroscan), initiation and type of HCV antiviral treatment, and SVR (defined as free of virus 12 or more weeks after treatment completion) were assessed dichotomously. Only the most recent treatment was considered in those who received multiple courses of HCV antiviral therapy. Of note, interferon-rib-avirin-protease inhibitor regimens became widely available for prescription in May 2012 and interferon-free, DAA regimens in January 2015. HCV risk factors (including history of excessive alcohol use, injection drug use (IDU), tattooing, and incarceration), demographic and socioeco-nomic characteristics were self-reported by patients at baseline. Laboratory results including HCV genotype, viral load, and transaminase (AST and ALT) levels were gathered at baseline from electronic medical records. Area-level income and material/social deprivation were used as indicators of socioeconomic status (SES) and were based on patients’ residential postal codes. Postal codes were mapped to census geography using postal code conversion files (PCCF) provided by Statistics Canada. Quin-tiles of neighborhood level income per person based on the 2006 Census were then merged to the study dataset. For these analyses, we defined low income as the patients residing in the lowest (poorest) quintile on this measure. Material and social deprivation was defined at the area level using data on six indicators (the proportion of population with no high school diploma; the employment/ population ratio; mean income; the proportion of individuals living alone; the proportion of individuals who are separated, divorced or widowed; and the proportion of single-parent families) from the 2006 Census. These indicators were combined through a principle component analysis with the first component representing material deprivation and second representing social deprivation.22 Quintiles for each of the deprivation indices were used with the lowest quintile representing greatest material/social deprivation.
Comparisons between TM and non-TM populations weremadebystandardstatisticalmethodsincludingχ2 tests for dichotomous/categorical variables and t-tests for continuous variables. Logistic regression was utilized to evaluate differences in dichotomous clinical outcomes and SVR between TM and non-TM populations with adjustment for age and sex. Estimates are presented as odds ratios (OR) and 95% confidence intervals (CI).
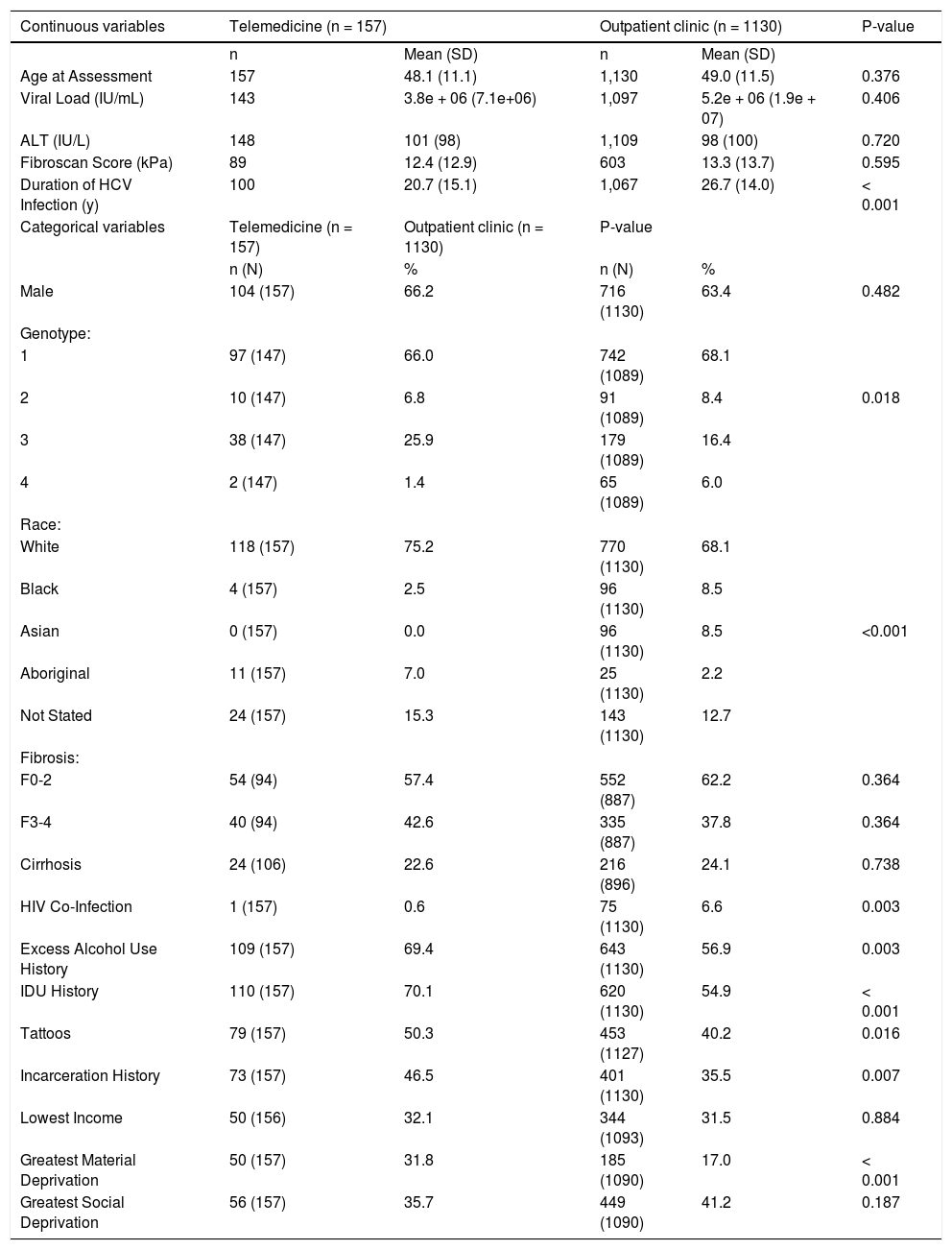
ResultsA total of 157 TM and 1130 non-TM patients were evaluated. TM patients were more frequently genotype 3-infected (25.9% vs. 16.4%), Indigenous (7.0% vs. 2.2%), and had a history of injection drug use (70.1% vs. 54.9%), excess alcohol use (69.4% vs. 56.9%), and incarceration (46.5% vs. 35.5%) (Table 1). TM patients were more likely to be characterized as materially deprived (31.8% vs. 17.0%). Groups were comparable in age (mean 48.9), gender (63.7% male) and fibrosis stage (24.0% cirrhotic). The estimated length of HCV infection was greater in non-TM patients (26.7 years vs. 20.7 years). Non-TM patients were more likely to be HIV co-infected (6.6% vs. 0.6%).
Summary Statistics for the Overall Telemedicine/Clinic Populations (January 2012 to August 2016).
| Continuous variables | Telemedicine (n = 157) | Outpatient clinic (n = 1130) | P-value | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Age at Assessment | 157 | 48.1 (11.1) | 1,130 | 49.0 (11.5) | 0.376 |
| Viral Load (IU/mL) | 143 | 3.8e + 06 (7.1e+06) | 1,097 | 5.2e + 06 (1.9e + 07) | 0.406 |
| ALT (IU/L) | 148 | 101 (98) | 1,109 | 98 (100) | 0.720 |
| Fibroscan Score (kPa) | 89 | 12.4 (12.9) | 603 | 13.3 (13.7) | 0.595 |
| Duration of HCV Infection (y) | 100 | 20.7 (15.1) | 1,067 | 26.7 (14.0) | < 0.001 |
| Categorical variables | Telemedicine (n = 157) | Outpatient clinic (n = 1130) | P-value | ||
| n (N) | % | n (N) | % | ||
| Male | 104 (157) | 66.2 | 716 (1130) | 63.4 | 0.482 |
| Genotype: | |||||
| 1 | 97 (147) | 66.0 | 742 (1089) | 68.1 | |
| 2 | 10 (147) | 6.8 | 91 (1089) | 8.4 | 0.018 |
| 3 | 38 (147) | 25.9 | 179 (1089) | 16.4 | |
| 4 | 2 (147) | 1.4 | 65 (1089) | 6.0 | |
| Race: | |||||
| White | 118 (157) | 75.2 | 770 (1130) | 68.1 | |
| Black | 4 (157) | 2.5 | 96 (1130) | 8.5 | |
| Asian | 0 (157) | 0.0 | 96 (1130) | 8.5 | <0.001 |
| Aboriginal | 11 (157) | 7.0 | 25 (1130) | 2.2 | |
| Not Stated | 24 (157) | 15.3 | 143 (1130) | 12.7 | |
| Fibrosis: | |||||
| F0-2 | 54 (94) | 57.4 | 552 (887) | 62.2 | 0.364 |
| F3-4 | 40 (94) | 42.6 | 335 (887) | 37.8 | 0.364 |
| Cirrhosis | 24 (106) | 22.6 | 216 (896) | 24.1 | 0.738 |
| HIV Co-Infection | 1 (157) | 0.6 | 75 (1130) | 6.6 | 0.003 |
| Excess Alcohol Use History | 109 (157) | 69.4 | 643 (1130) | 56.9 | 0.003 |
| IDU History | 110 (157) | 70.1 | 620 (1130) | 54.9 | < 0.001 |
| Tattoos | 79 (157) | 50.3 | 453 (1127) | 40.2 | 0.016 |
| Incarceration History | 73 (157) | 46.5 | 401 (1130) | 35.5 | 0.007 |
| Lowest Income | 50 (156) | 32.1 | 344 (1093) | 31.5 | 0.884 |
| Greatest Material Deprivation | 50 (157) | 31.8 | 185 (1090) | 17.0 | < 0.001 |
| Greatest Social Deprivation | 56 (157) | 35.7 | 449 (1090) | 41.2 | 0.187 |
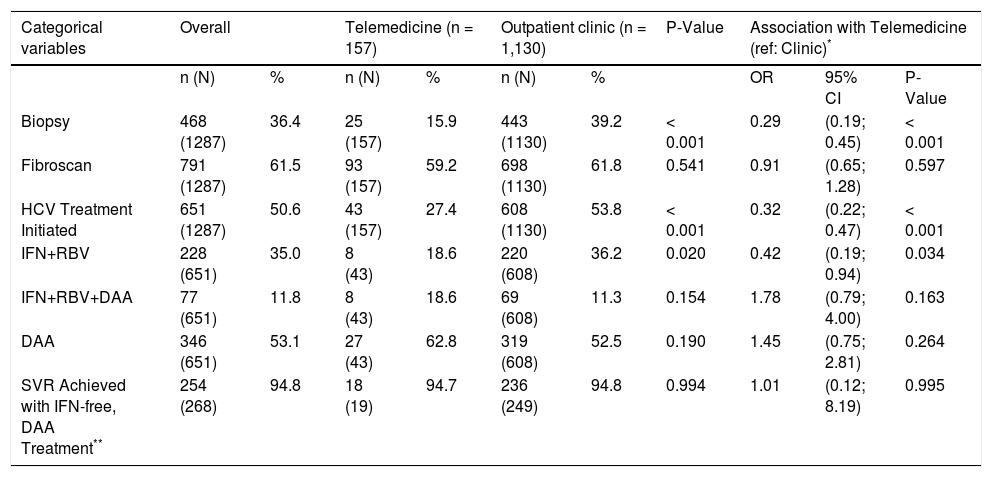
TM patients were less likely to have ever undergone a liver biopsy (15.9% vs. 39.2%) and less likely to have initiated interferon-ribavirin HCV antiviral treatment [5.1% (8/157) vs. 19.5% (220/1130)] (Table 2). In contrast, TM patients were equally likely to have undergone fibrosis assessment by Fibroscan (59.2% vs. 61.8%). Treatment uptake increased in both groups compared to when interferon-ribavirin-only regimens were available. Overall, half as many TM patients initiated antiviral therapy as non-TM patients (27.4% vs. 53.8%, p < 0.001). The introduction of DAA regimens is bridging this gap [22.2% of TM patients (35/157) vs. 34.3% of non-TM patients (388/ 1130)].
Access to fibrosis assessment, treatment starts, HCV regimen type and Sustained Virological Response (SVR) in Telemedi-cine and Outpatient Clinic HCV patients (reference: Outpatient Clinic).
| Categorical variables | Overall | Telemedicine (n = 157) | Outpatient clinic (n = 1,130) | P-Value | Association with Telemedicine (ref: Clinic)* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (N) | % | n (N) | % | n (N) | % | OR | 95% CI | P-Value | ||
| Biopsy | 468 (1287) | 36.4 | 25 (157) | 15.9 | 443 (1130) | 39.2 | < 0.001 | 0.29 | (0.19; 0.45) | < 0.001 |
| Fibroscan | 791 (1287) | 61.5 | 93 (157) | 59.2 | 698 (1130) | 61.8 | 0.541 | 0.91 | (0.65; 1.28) | 0.597 |
| HCV Treatment Initiated | 651 (1287) | 50.6 | 43 (157) | 27.4 | 608 (1130) | 53.8 | < 0.001 | 0.32 | (0.22; 0.47) | < 0.001 |
| IFN+RBV | 228 (651) | 35.0 | 8 (43) | 18.6 | 220 (608) | 36.2 | 0.020 | 0.42 | (0.19; 0.94) | 0.034 |
| IFN+RBV+DAA | 77 (651) | 11.8 | 8 (43) | 18.6 | 69 (608) | 11.3 | 0.154 | 1.78 | (0.79; 4.00) | 0.163 |
| DAA | 346 (651) | 53.1 | 27 (43) | 62.8 | 319 (608) | 52.5 | 0.190 | 1.45 | (0.75; 2.81) | 0.264 |
| SVR Achieved with IFN-free, DAA Treatment** | 254 (268) | 94.8 | 18 (19) | 94.7 | 236 (249) | 94.8 | 0.994 | 1.01 | (0.12; 8.19) | 0.995 |
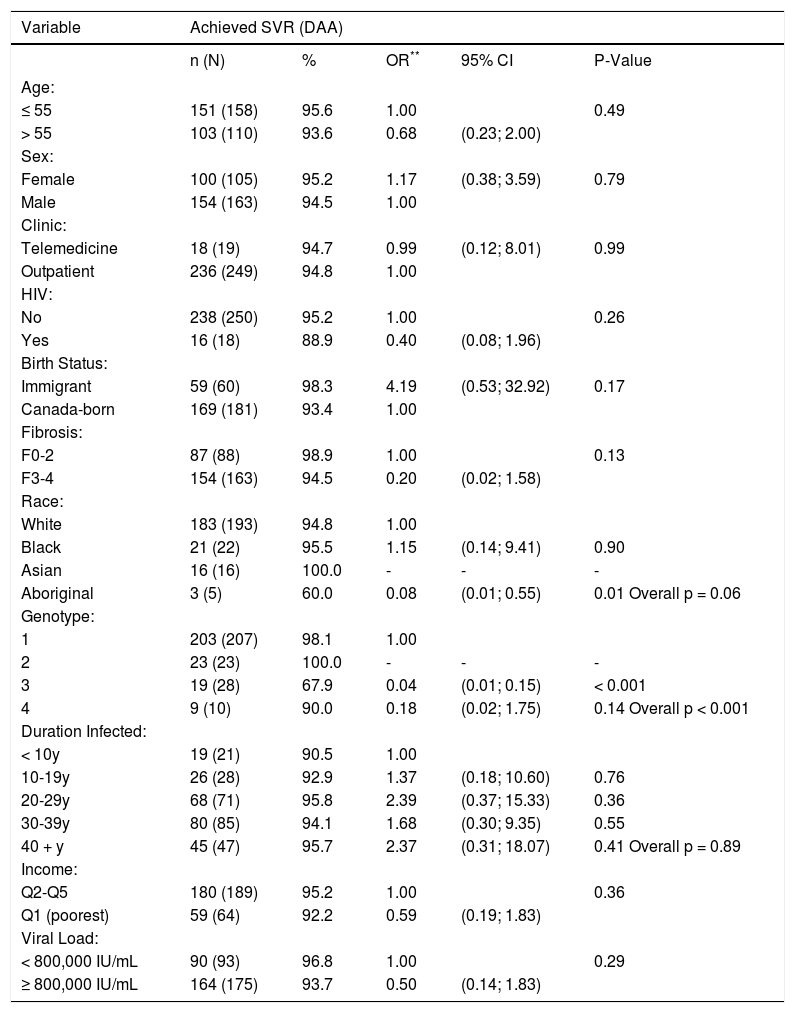
The SVR rate with interferon-free, DAA regimens was similar by group: 94.7% (n = 18/19) in the TM group and 94.8% (n = 236/249) in the non-TM group (Table 2). Multiple key factors were evaluated as predictors of SVR in DAA recipients (Table 3). Mode of HCV DAA treatment delivery (TM vs. standard outpatient clinic model) was not identified as a predictor of SVR. Indigenous race and genotype 3-infection were associated with reduced likelihood of achieving therapeutic success.
Associations between patient characteristics, Telemedicine utilization, and achieving SVR in DAA-based treatment recipients (n = 268).*
| Variable | Achieved SVR (DAA) | ||||
|---|---|---|---|---|---|
| n (N) | % | OR** | 95% CI | P-Value | |
| Age: | |||||
| ≤ 55 | 151 (158) | 95.6 | 1.00 | 0.49 | |
| > 55 | 103 (110) | 93.6 | 0.68 | (0.23; 2.00) | |
| Sex: | |||||
| Female | 100 (105) | 95.2 | 1.17 | (0.38; 3.59) | 0.79 |
| Male | 154 (163) | 94.5 | 1.00 | ||
| Clinic: | |||||
| Telemedicine | 18 (19) | 94.7 | 0.99 | (0.12; 8.01) | 0.99 |
| Outpatient | 236 (249) | 94.8 | 1.00 | ||
| HIV: | |||||
| No | 238 (250) | 95.2 | 1.00 | 0.26 | |
| Yes | 16 (18) | 88.9 | 0.40 | (0.08; 1.96) | |
| Birth Status: | |||||
| Immigrant | 59 (60) | 98.3 | 4.19 | (0.53; 32.92) | 0.17 |
| Canada-born | 169 (181) | 93.4 | 1.00 | ||
| Fibrosis: | |||||
| F0-2 | 87 (88) | 98.9 | 1.00 | 0.13 | |
| F3-4 | 154 (163) | 94.5 | 0.20 | (0.02; 1.58) | |
| Race: | |||||
| White | 183 (193) | 94.8 | 1.00 | ||
| Black | 21 (22) | 95.5 | 1.15 | (0.14; 9.41) | 0.90 |
| Asian | 16 (16) | 100.0 | - | - | - |
| Aboriginal | 3 (5) | 60.0 | 0.08 | (0.01; 0.55) | 0.01 Overall p = 0.06 |
| Genotype: | |||||
| 1 | 203 (207) | 98.1 | 1.00 | ||
| 2 | 23 (23) | 100.0 | - | - | - |
| 3 | 19 (28) | 67.9 | 0.04 | (0.01; 0.15) | < 0.001 |
| 4 | 9 (10) | 90.0 | 0.18 | (0.02; 1.75) | 0.14 Overall p < 0.001 |
| Duration Infected: | |||||
| < 10y | 19 (21) | 90.5 | 1.00 | ||
| 10-19y | 26 (28) | 92.9 | 1.37 | (0.18; 10.60) | 0.76 |
| 20-29y | 68 (71) | 95.8 | 2.39 | (0.37; 15.33) | 0.36 |
| 30-39y | 80 (85) | 94.1 | 1.68 | (0.30; 9.35) | 0.55 |
| 40 + y | 45 (47) | 95.7 | 2.37 | (0.31; 18.07) | 0.41 Overall p = 0.89 |
| Income: | |||||
| Q2-Q5 | 180 (189) | 95.2 | 1.00 | 0.36 | |
| Q1 (poorest) | 59 (64) | 92.2 | 0.59 | (0.19; 1.83) | |
| Viral Load: | |||||
| < 800,000 IU/mL | 90 (93) | 96.8 | 1.00 | 0.29 | |
| ≥ 800,000 IU/mL | 164 (175) | 93.7 | 0.50 | (0.14; 1.83) | |
The use of TM to manage patients with chronic infectious diseases including HCV is increasingly com-mon.1,2,4,6,7,16,23 Although manageable as a chronic medical condition by non-specialized health care providers, HCV often requires the involvement of a specialist to provide guidance on advanced liver disease management, antiviral regimen selection, antiviral resistance profile interpretation, drug-drug interactions, timing of treatment initiation and on-treatment side effect management.1,6,16 HCV specialists can also provide long-term follow-up for clinically stable patients not on antiviral therapy utilizing TM infrastructure.8 A primary advantage of TM is increased patient access to specialty care. This is a particular benefit for isolated populations including nursing home residents, the incarcerated and people living in rural communities.1-7 A lack of specialty care for HCV has been identified as a major obstacle to receiving optimal care.16 Highlighting this point, rurally located HCV patients without access to specialty care were historically less likely to initiate interferon-based antiviral treatment.4 Our data corroborates this observation.
Our analysis suggests that a multidisciplinary TM program can successfully engage and retain a remote population having characteristics associated with barriers to successful HCV treatment. These include poverty, high rates of substance use, and history of incarceration. Other programs, notably Project Echo, have demonstrated similar success in engaging a rural population living with HCV.9,10 The opportunity to connect primary care providers to hepatitis specialists has increased the numbers of HCV patients evaluated and treated.9,10,14
Advances in HCV work-up and antiviral treatment have facilitated access to care for marginalized populations. In the past, fewer of our rural patients underwent liver biopsy given the need to travel to an urban-based tertiary care centre for this procedure. The recent introduction of transient elastography has eliminated this barrier to care. Our TM program team travels to smaller centers located throughout Eastern Ontario to conduct ‘Fibroscan-blitzes’ during which 15 to 30 individuals at a time undergo fibro-sis assessment. This is a critical step in the evaluation of those living with HCV and is essential to qualify for HCV antiviral funding through provincial government reimbursement programs in Canada.24
Acknowledging the potential influence of patient selection and publication biases, previous studies suggested that TM and non-TM HCV patients can achieve similar SVR rates when treated with interferon-ribavirin regimens with comparable rates of antiviral-related side effects.2,4,10 In our cohort, the likelihood of initiating in-terferon-ribavirin treatment was lower in our TM population. The sample in our TM cohort was too small to comment on interferon-ribavirin SVR outcomes relative to our non-TM patients. Prior to our report, there were no published studies evaluating HCV treatment outcomes in interferon-free, DAA regimen recipients receiving care via TM programs. In our experience, DAA therapy has allowed for increased treatment uptake and equivalent SVR rates in both TM and non-TM patients. This is due to reduced duration of treatment and the excellent safety profile of DAAs, which are easier to administer and require less need for intensive on-treatment monitoring. We anticipate that recently announced expanded reimbursement for DAA therapy in Canada with a streamlined approval process will positively impact our ability to provide HCV antiviral therapy through our TM program. Specifically, less time spend on administrative issues will be redirected to HCV patient assessment and treatment initiation.
We do not believe that the success observed with our TM program is entirely due to new fibrosis assessment modalities and better HCV antiviral treatments. Patients have reported that attending remote appointments through TM saves them time, diminishes the distance traveled and reduces missed work days.2,4,6,7,16,19 Satisfaction is generally high. In fact, a majority of TM patients indicated that they would once again choose to have their appointments via this modality if given the opportunity.16 The risk for late appointment arrival and clinic visit cancelations is reduced. Beyond these advantages, the hazards involved with travel, particularly during periods of inclement weather conditions, are eliminated. TM can also be used to provide HCV-specific continuing medical education (CME) to primary care providers.9,10,14,15
TM patients were able to engage in HCV care, achieving high SVR rates comparable to those obtained by traditional models of care. However, not all marginalized populations benefited equally. Although the proportion of persons of Indigenous ethnicity assessed by our TM program was higher, SVR rates with DAA treatment were lower suggesting that this group requires additional attention to maximize outcomes. Moving forward, methods to expand our TM program to improve Indigenous Peoples engagement are being explored. Strategies include utilization of Indigenous peer navigators to assist with HCV education and adherence as well as small group HCV education and delivery of care think tank sessions with Indigenous community members.
Abbreviations- •
HCV: hepatitis C virus.
- •
TM: telemedicine.
- •
DAA: direct acting antiviral.
- •
SVR: sustained virologic response.
- •
HIV: human immunodeficiency virus.
- •
REB: research ethics board.
- •
IDU: injection drug use.
- •
AST: aspartate aminotransferase.
- •
ALT: alanine aminotransferase.
- •
SES: socioeconomic status.
- •
PCCF: postal code conversion files.
- •
OR: odds ratio.
- •
CI: confidence interval.
- •
CME: continuing medical education.
- •
CIHR: Canadian Institutes of Health Research.
- •
NCRTP: National CIHR Research Training Program.
This work was supported by the Ontario HIV Treatment Network Research Chair support to C.C. and the National CIHR Research Training Program in Hepatitis C (NCRTP-HepC) to P.P.