A 75-year old male patient had been regularly visiting our hospital for the management of his type 2 diabetes mellitus since he was diagnosed at age 64 years. When he developed hypoglycemic episodes with sulfonylurea, ipragliflozin (50 mg/day) was started to replace the sulfonylurea therapy. However, 49 days after starting ipragliflozin, his AST increased from 13 to 622 U/L, ALT increased from 9 to 266 U/L, ALP increased from 239 to 752 U/L, and γ-GTP increased from 19 to 176 U/L. ZTT was 3.5 U, TTT was 0.4 U, and total bilirubin was 0.7 mg/dL. IgM hepatitis A antibody, hepatitis B antigen, hepatitis C virus antibody, IgM CMV antibody, and IgM EB VCA antibody were negative, whereas a lymphocyte transformation test for ipragliflozin was positive. Abdominal CT scan showed mild fatty liver but no sign of nodular lesions. Following admission to our hospital, he received liver supportive therapy with the discontinuation of ipragliflozin therapy. He was discharged from the hospital 18 days later with AST and ALT levels reduced to 20 U/L and 13 U/L, respectively. Based on the clinical presentation of this patient, it is highly important to monitor liver function along with other possible clinical complications (e.g., dehydration, ketosis, and urinary tract infection) associated with SGLT2 inhibitor therapy.
Type 2 diabetes mellitus (T2DM) is a significant risk factor for nonalcoholic fatty liver disease (NAFLD), nonalcoholic fatty liver (NAFL), and nonalcoholic steatohep-atitis (NASH).1 For example, 33-50% of patients with diabetes mellitus have NAFLD.2 Recently, the usefulness of sodium glucose cotransporter 2 (SGLT2) inhibitors for animal NASH models has been reported.3 However, because of its relatively short history as an antidiabetic drug, the long-term safety of SGLT2 2 inhibitors has not been established.4 Recently, we encountered a case of ipragli-flozin-induced liver injury. As there is a possibility of the future use of SGLT2 inhibitors to treat patients with diabetes mellitus presenting alone or with concomitant NAFLD, NAFL, and NASH, this case report will provide important clinical information about their safety.
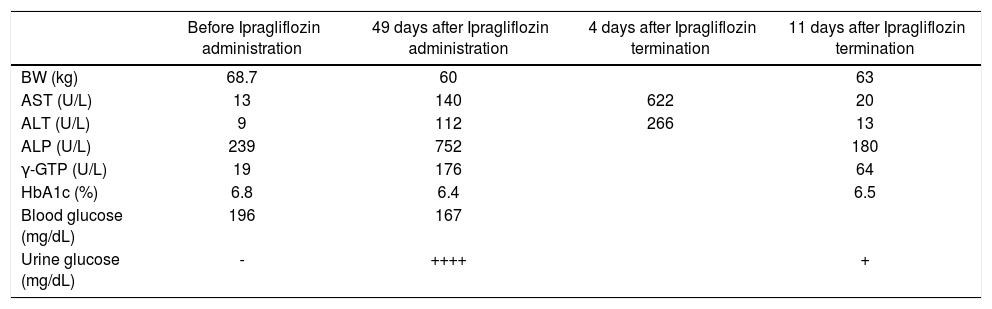
Case ReportA 75-year-old Japanese male patient had been regularly visiting our hospital for control of his type 2 diabetes mel-litus and hypertension since his diagnosis at 64 years of age. He had diabetic peripheral neuropathy but not diabetic retinopathy. This caused slight bilateral peripheral numbness on his legs. A spot urine test showed a urine protein/creatinine ratio of 6.284, and he had an estimated glomerular filtration rate (eGFR) of 63.1 (mL/min/1.73 m2). As he developed hypoglycemic episodes with sulfo-nylurea, it was replaced with ipragliflozin (50 mg/day) in February 2016. However, 49 days after starting ipraglifloz-in, he developed easy fatigability. The following laboratory test results led to a diagnosis of clinical liver injury (Table 1A): aspartate aminotransferase (AST) increased from 13 to 622 U/L (normal range, 10-40 U/L), alanine aminotransferase (ALT) increased from 9 to 266 U/L (normal range, 5-45 U/L), alkaline phosphatase (ALP) increased from 239 to 752 U/L (normal range, 100-325 U/L), and γ-glutamyl transpeptidase (γ-GTP) increased from 19 to 176 U/L (normal value, ≤ 80 U/L). Zinc sulfate turbidity test (ZTT) was 3.5 U (normal range, 2-12 U), thymol turbidity test (TTT) was 0.4 U (normal value, ≤ 4 U), total protein was 6.4 g/dL (normal range, 6.7-8.3 g/dL), albumin was 3.2 g/dL (normal range, 3.8-5.3 g/dL), and total bi-lirubin was 0.7 g/dL (normal range, 0.2-1.2 mg/dL). Hepatitis A virus antibody (Anti-HAV-IgM), hepatitis B virus antigen, antibody, and DNA (HBsAg, anti-HBc-IgM, HBV-DNA), hepatitis C antibody (anti-HCV), IgM cy-tomegalovirus (CMV) antibody and Epstein-Barr virus (EB) viral capsid antigen (VCA)-IgM antibody were negative. Abdominal computed tomography (CT) showed mild fatty liver but there were no nodular lesions. The lymphocyte transformation test (LTT), which is used to determine whether a patient has developed a T-cell response against a certain drug,5 was positive for ipraglifloz-in (Table 1B). Glycosylated hemoglobin (HbA1c) decreased from 6.8% to 6.4% after starting ipragliflozin treatment without any reported hypoglycemic episodes.
Changes in liver function and HbA1c.
| Before Ipragliflozin administration | 49 days after Ipragliflozin administration | 4 days after Ipragliflozin termination | 11 days after Ipragliflozin termination | |
|---|---|---|---|---|
| BW (kg) | 68.7 | 60 | 63 | |
| AST (U/L) | 13 | 140 | 622 | 20 |
| ALT (U/L) | 9 | 112 | 266 | 13 |
| ALP (U/L) | 239 | 752 | 180 | |
| γ-GTP (U/L) | 19 | 176 | 64 | |
| HbA1c (%) | 6.8 | 6.4 | 6.5 | |
| Blood glucose (mg/dL) | 196 | 167 | ||
| Urine glucose (mg/dL) | - | ++++ | + |
Evaluation of the updated Roussel Uclaf Causality Assessment Method (RUCAM) for the cholestatic or mixed liver injury of DILI and HILI for ipragliflozin.
| Updated RUCAM for the cholestatic or mixed liver injury of DILI and HILI. |
|---|
Items for Cholesta3c or Mixed Liver Injury Score Result
|
| No information, persistence, increase, or continued drug/herb use 0 |
|
He was admitted to our hospital and iplagliflozin was discontinued. He was discharged from the hospital 18 days later with his AST and ALT levels reduced to 20 U/L and 13 U/L, respectively. Thereafter, his liver function remained within the normal range until the time of this writing.
DiscussionIpragliflozin reportedly has therapeutic effects on nonalcoholic steatohepatitis in mice.6 However, we have identified an individual who developed liver injury 49 days after starting ipragliflozin therapy, and recovered 11 days after its termination (Table 1A). We confirmed that hepatitis A virus, hepatitis B virus, hepatitis C virus, cytomegalovirus, and Epstein-Barr virus did not cause the elevated levels of AST, ALT, ALP, or γ-GTP. We also ruled out the potential of a tumor mass by abdominal CT examination. Since the patient had mild fatty liver, we used the updated Roussel Uclaf Causality Assessment Method (RUCAM) scale to determine if patient had cholestatic or mixed liver injury of DILI (drug induced liver injury) or HILI (herb induced liver injury). This patient had cholestatic liver injury as his R (= ALT/ALP) value was ≤ 2. His updated RUCAM score was 7, according to recent literature,7,8 (Table 1B) and indicated that our case was probably of a DILI. We also confirmed that drug-induced lymphocyte transformation test was positive for ipragliflozin (Table 1C). Therefore, we concluded that liver injury was caused by ipragliflozin. At the time of writing this report, we are unable to identify any similar case studies in our PubMed searches. Since 33-50% of patients with diabetes mellitus have NAFLD,2 and NAFLD can be considered as a risk factor for DILI,8,9 it is prudent to occasionally monitor liver function and assess patients for indications, such as dehydration, ketosis, and urinary tract infections, before prescribing SGLT2 inhibitors.
Result of lymphocyte transformation test.
| Measurement (cpm) | Transformation rate (%) | Normal range (%) | Result | |
|---|---|---|---|---|
| Ipragliflozin | 913 | 275 | ≤ 179 | Positive |
We would like to thank Dr. Jeffrey E. Pessin (Albert Einstein College of Medicine, Bronx, NY, U.S.A.) for critical suggestions about our manuscript.
DisclosureNone of the authors have any potential conflicts of interest associated with this case study.
Statement of Human And Animal RightsAll procedures performed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed ConsentInformed consent was obtained from the patient prior to inclusion in the study.