Background and rationale for the study. Previous studies showed that CTLA4Ig and indoleamine 2,3-dioxygenase (IDO) genes played regulatory role in organ transplantation but failed to reach satisfactory effects. In this study, we constructed an adeno-virus-mediated gene expressing CTLA4Ig-IDO and established rat liver transplantation models. Recipients were randomly divided into four groups of 10 rats each. During the operation, CTLA4Ig, IDO, and CTLA4Ig-IDO genes, as well as a blank plasmid, were infused into different rat groups via portal vein to determine their effects on inducing immune tolerance. Survival rate of recipients, histological changes of graft liver, post-transplantation liver function, and cytokine levels were observed at day 14 after operation.
Results. Serum levels of alanine aminotransferase (ALT), aspartate transaminase (AST), and total bilirubin level (TBIL) in the CTLA4Ig-IDO group were lower than those in the other three groups at 14 days post-transplantation (P < 0.05); mRNA and protein expressions of IL-2 and IFN-γ were higher in the control group, but lower in the CTLA4Ig-IDO group (P < 0.05). By contrast, expressions of IL-4, TGF-b, IL-10, and T lymphocyte apoptosis were higher in the CTLA4Ig-IDO group than those in the other three groups (P < 0.05). The CTLA4Ig-IDO group exhibited mild acute rejection and higher survival rate compared with the other groups (P < 0.05).
Conclusion. Compared with using CTLA4Ig or IDO alone, combined transfection of CTLA4Ig-IDO was more effective in inducing immune tolerance after liver transplantation.
Liver transplantation is considered an effective therapy for end-stage liver diseases. Liver is an immune-privileged organ, and the incidence and degree of rejection after liver transplantation are much lower than those of other solid organs.1,2 However, acute and chronic rejection of the liver graft is the major barrier for long-term survival. Furthermore, long-term administration of immunosuppressive drugs results in immunological complications and other severe side effects, such as infections or malignancy development.3 In recent years, an increasing number of studies have focused on alleviating post-transplantation rejection and inducing immune tolerance by inhibiting T lymphocyte activity.4–6
T lymphocytes play a critical role in immune response process and initially require two distinct signals: signal 1 through T-cell receptor major histocompatibility complex plus antigenic peptide complex and signal 2 through costimulatory molecules as the bases of immune response.3,7 The interaction between CD28/CTLA-4 and B7 molecules is a major costimulatory pathway. Binding of CD28/CTLA-4 on T cells to B7-related receptors on antigen-presenting cells (APCs) can produce important antigen-nonspecific costimulatory signals critical for immune responses. Blocking this pathway can lead to a state of antigen-specific unresponsiveness or apoptosis.8,9 Previous studies found that cytotoxic lymphocyte antigen 4-immunoglobulin (CTLA4Ig), a recombinant fusion protein, possesses high avidity with the B molecules of CD28 and can lead to a satisfactory immune result in liver, heart, kidney, and islet transplantation models.3,7,10
Indoleamine 2,3-dioxygenase (IDO) is an IFN-γ-inducible enzyme that catabolizes tryptophan into kynurenine as well as arrests the proliferation of activated T lymphocytes and NK cells.11,12 IDO induces T lymphocyte apoptosis and has emerged as a potent immunosuppressive enzyme in recent years. IDO level is upregulated during rejection, demonstrating that IDO plays a role in the rejection process after transplantation. However, previous studies failed to reach satisfactory effects by transfecting single adenovirus-mediated IDO gene.13,14 This study aims to investigate the effects of adenovirus-mediated combined genes of CTLA4Ig and IDO (CTLA4Ig-IDO) in the immune tolerance after orthotopic liver transplantation in rat model.
Material and MethodsAnimals and experiment designInbred male Lewis and Brown Norway rats weighing 180-250 g were used as donors and recipients, respectively. All the rats were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China). Animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health. This research was approved by the regional ethics committee in accordance with the law of China on animal protection. Recipients were randomly divided into four groups, namely, CTLA4Ig-IDO, CTLA4Ig, IDO, and control. Liver grafts of CTLA4Ig-IDO group (n = 10) were infused with 1 mL of adenovirus-mediated combined gene of pWBP-CTLA4Ig-IDO (1 x 1011/mL; Fulen Gene Company, Guang Zhou, China) via the portal vein in the cold ischemia phase with supra- and infra-hepatic vena cava clamping. Liver grafts of CTLA4Ig group (n = 10) were infused with 1 x 1011/mL of pWBP-CTLA4Ig (1 mL) via the portal vein. Liver grafts of IDO group (n = 10) were infused with 1 x 1011/mL of pWBP-IDO (1 mL) via the portal vein. Liver grafts of control group (n = 10) were infused with 1 mL of blank plasmid during transplantation. Five animals were sacrificed at 14 days post-surgery from each group. Blood from vena cava and liver samples were collected for detection. The other five rats in each group were maintained for survival rate assessment. No animal died of operation error, postoperative bleeding, or infection. If the animals could not live to the sample collection because of acute rejection, samples were obtained before their death.
Construction of models of liver transplantationRat orthotopic liver transplantations were performed using the Kamada’s method with slight modifications.15 During the operation, the infra-hepatic vena cava and the portal vein were linked with cuffs and the supra-hepatic vena cava was inosculated with suture. A stent tube was inserted into the common bile duct for bile duct reconstruction. Portal clamping time in all transplantations was < 22 min, and cold ischemic time in compound sodium chloride solution was < 60 min.
Histopathological and immunohistochemical examinationAfter the recipients were sacrificed, graft liver tissues were fixed by immersing in 10% buffered formaldehyde, embedded in paraffin, sectioned, and stained using hematoxylin and eosin. Histological findings, which were used to indicate the degree of acute rejection, were graded according to the Banff schema.16 Rejection activity index (RAI) was calculated from three individual scores: venous endothelial inflammation, bile duct damage, and portal inflammation. Sections were stained using diaminobenzidine (DAB) and analyzed immunohistochemically to detect the transfected gene expression in the graft liver.
Analysis of post-transplantation liver functionBlood of rats was obtained via the infra-hepatic vein. Plasma liver function markers, such as serum levels of alanine aminotransferase (ALT), aspartate transaminase (AST), and total bilirubin level (TBIL), were measured using an automatic biochemical meter (Beckman CX7; Beckman Coulter, CA, USA).
Real-time quantitative PCR (RT-PCR) for cytokine mRNA analysisCytokine RNA was extracted from the liver tissue with a TRIzol reagent kit (Life Technologies, Carlsbad, CA, USA). RT-PCR was performed using an RT-PCR kit (Roche, Los Angeles, CA, USA). The cDNA produced was used for the amplification of the following primers: IFN-γ, IL-2, IL-4, IL-10, TGF-β, and β-actin (Guangzhou Fulen Gene Company, Guangzhou, China). Specific primer sequences of IL-2, IFN-γ, IL-4, IL-10, TGF-β, and β-actin were as follows:
- •
IL-2, forward: 5’-ATG TAC AGG ATG CAA CTC CTG-3’, reverse: 5’-TCA AGT CAG TGT TGA GAT GAT GCT TTG ACA AAA-3’.
- •
IFN-γ, forward: 5’-CCA CGA GGA ATT CTA CGC CCT GGGC-3’, reverse: 5’-AAG CTT GGG GAA CAG GTA GG-3’.
- •
IL-4, forward: 5’-ATG GGT CTC ACC TCC CAA CTG-3, reverse: 5’-TCA GCT CGA ACA CTT TGA ATA TTT CTC TCT CAT-3’.
- •
IL-10, forward: 5’-AGG GCA CCC AGT CTG AGA ACA-3’, reverse: 5’-CGG CCT TGC TCT TGT TTT CAC-3’.
- •
TGF-β, forward: 5’-AAC ATG ATC GTG CGC TCT GCA AGT GCAGC-3’, reverse: 5’-AAG GAA TAG TGC AGA CAG GCA GGA-3’.
- •
β-actin, forward: 5’-GTG GGG CGC CCC AGG CACCA-3’, reverse: 5’-CTC CTT AAT GTC ACG CAC GAT TTC-3’.
The PCR conditions were 30 cycles of denaturation at 94 °C for 60 s, annealing at 58 °C for 60 s, extension at 72 °C for 60 s, and a final extension at 72 °C for 7 min. Agarose gel electrophoresis was used to separate PCR products. Ethidium bromide staining and a gelatin imaging and figure analysis system (GelDoc 2000; Bio-Rad, Hercules, CA, USA) were also used to observe and semiquantitatively count relative quantities, expressed as relative optical density with normalization to β-actin.
Western blot analysisTotal protein was extracted using radioimmunoprecipitation assay buffer on ice. The protein sample was then mixed with loading buffer, boiled for 5 min, and subjected to SDS-PAGE. After electrophoresis, the proteins were transferred onto polyvinylidene difluoride (PVDF) membranes at 4 °C and blotted against anti-rat CTLA4Ig and IDO (IgG; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4 °C. PVDF membranes were washed with Tris-buffered saline with Tween 20 buffer before incubating with a 1:2,000 dilution of horseradish peroxidase-conjugated rabbit anti-rat IgG (Santa Cruz Biotechnology, Inc.) for 60 min at room temperature.
Detection of T lymphocyte apoptosisThe Annexin V-FITC apoptosis kit was obtained from Boyou Biotechnology Company (Shanghai, China). The cells were digested with EDTA-free trypsin (Carlsbad, CA, USA), centrifuged, and washed with pre-cold PBS. Annexin V (500 μL) was then added to bind suspension cells in buffer. Annexin V-FITC (5 μL) was also added and then blended before adding 5 μL of propidium iodide. Subsequently, the solution was mixed, protected from light, and reacted for 10 min at room temperature. The apoptotic rate was then detected using flow cytometry.
Statistical analysisGraft survival was calculated according to the Kaplan-Meier method. Data were expressed as mean ± SD. Statistical analyses were carried out using SPSS 18.0 software package. ANOVA was used to compare groups. Pearson’s correlation was employed to analyze the differences between parameters. P < 0.05 was considered statistically significant.
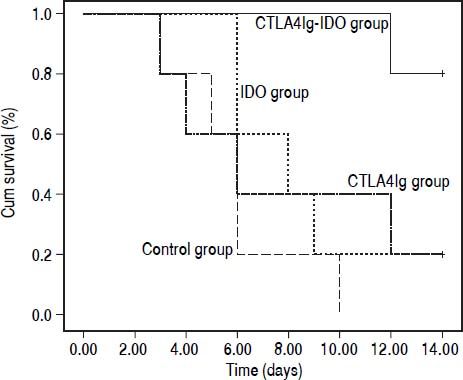
ResultsSurvival rate of recipientsAll the animals were allowed to drink and eat ad libitum after transplantation without any special treatment. Animals in the control group initially showed symptoms of acute rejection, such as weakness, reduced activities, loss of appetite, continuous weight loss, and increasingly elevated serum TBILs, on the fifth post-operative day; all recipients died within 10 days. In the CTLA4Ig and IDO groups, acute rejection occurred later and milder than that in the control group. Meanwhile, fewer rejection symptoms were observed in the CTLA4Ig-IDO group, and the mean survival time of rats was much longer than that of the other three groups (P = 0.000). The survival time of rats in the CTLA4Ig group did not differ from that of the IDO group, but significant differences existed compared with the control group (Figure 1). This finding shows that the adenovirus-mediated genes of CTLA4Ig and IDO, especially the combined gene of CTLA4Ig-IDO, can improve post-liver transplantation survival rate in rats.
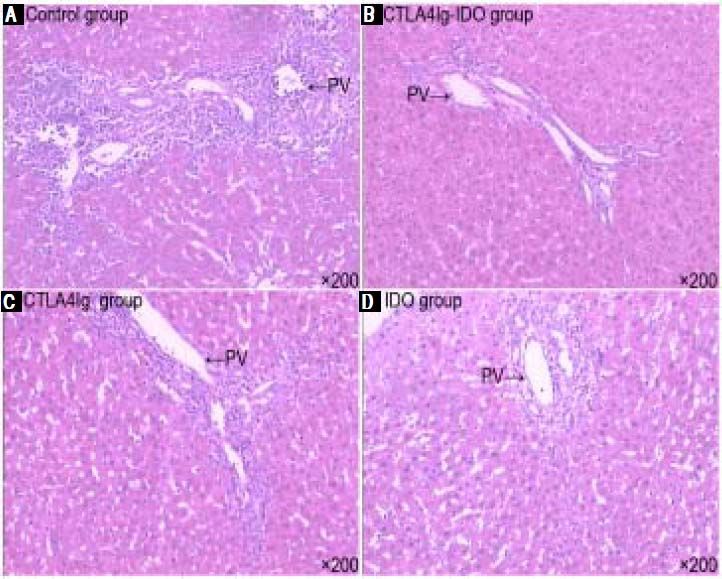
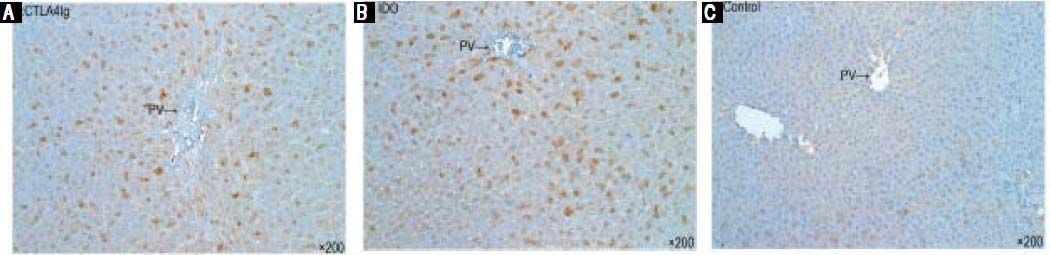
Histopathological examination and immunohistochemical stainingHistological examination of the liver graft was conducted 14 days after surgery. In the control group, signs of severe acute rejection, such as numerous lymphocytes and neutrophils, mononuclear infiltration in the portal tracts, extensive lobular architecture destruction, vacuolated degeneration, spotted and focal necrosis of hepatocyte, and bile duct epithelium damage, were observed. These findings indicate severe acute rejection with progressive liver necrosis (Figure 2A). In the CTLA4Ig-IDO group, the majority of liver grafts showed almost normal liver architecture with only mild portal lym- phocytic infiltration and infiltration of lobular areas (Figure 2B). According to the RAI scoring criteria, the rejection grade of the CTLA4Ig-IDO group was significantly lower than that of the control group on the 14th day after transplantation. Meanwhile, the acute rejection signs of the CTLA4Ig and IDO groups were more severe than those of the CTLA4Ig-IDO group but milder than those of the control group (Figures 2C and 2D). Immunohistochemical staining showed that CTLA4Ig and IDO expressions were positive in the CTLA4Ig-IDO group after portal infusion (Figures 3A and 3B), but these expressions were undetected in the control group (Figure 3C), indicating that adenovirus-mediated gene was transfected and expressed effectively.
Histological changes of the graft liver (HE staining, original magnification x200). In the control group, infammatory cells infiltrated in the portal tracts; lobular architecture destruction, vacuolated degeneration, spotted necrosis of hepatocyte, and damage of the bile duct epithelium were observed. In the CTLA4Ig-IDO group, liver grafts showed almost normal liver architecture and only mild porta lymphocytic infiltration. In the CTLA4Ig and IDO groups, signs of inflammatory cell infiltration and lobular architecture destruction were between control and CTLA4Ig-IDO groups.
Presence of CTLA4Ig-IDO in the liver after adenovirus-mediated gene transfer. Cryostat sections were analyzed immunohistochemically. A and B. CTLA4Ig and IDO expressions were positive in liver tissues in the CTLA4Ig-IDO group on day 14 after gene transfer (DAB staining, original magnification x 200). C. Liver grafts in the control group lacked detectable CTLA4Ig and IDO expressions.
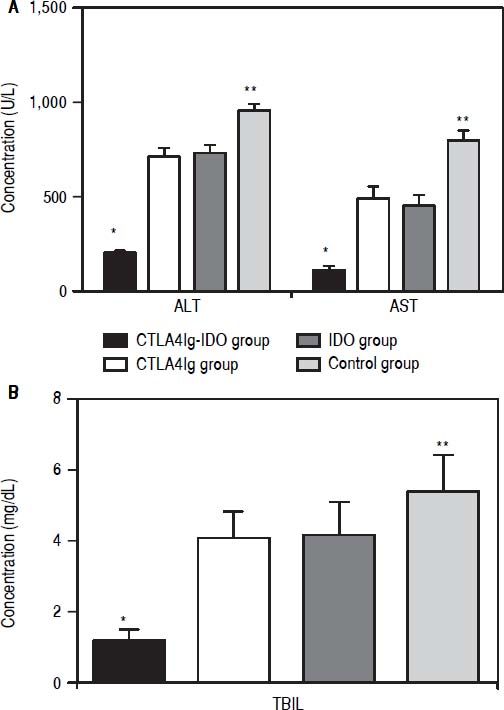
In the CTLA4Ig-IDO group, the ALT, AST, and TBIL levels were clearly lower than those in the other three groups (P < 0.05) on the 14th day after transplantation. Meanwhile, the levels of ALT, AST, and TBIL in the CTLA4Ig group did not differ from those of the IDO group (P > 0.05), but had significant difference when compared with the control group (P < 0.05). Findings showed that transfer adenovirus-mediated single gene of CTLA4Ig or IDO into the liver graft can protect the liver function post-transplantation, which is consistent with previous studies. Transferring the combined gene of CTLA4Ig-IDO via the liver graft portal could effectively improve postoperative functions (Figure 4).
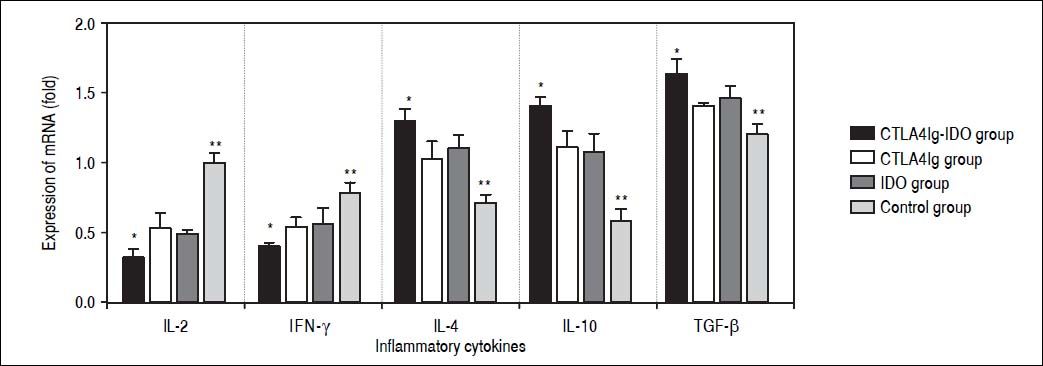
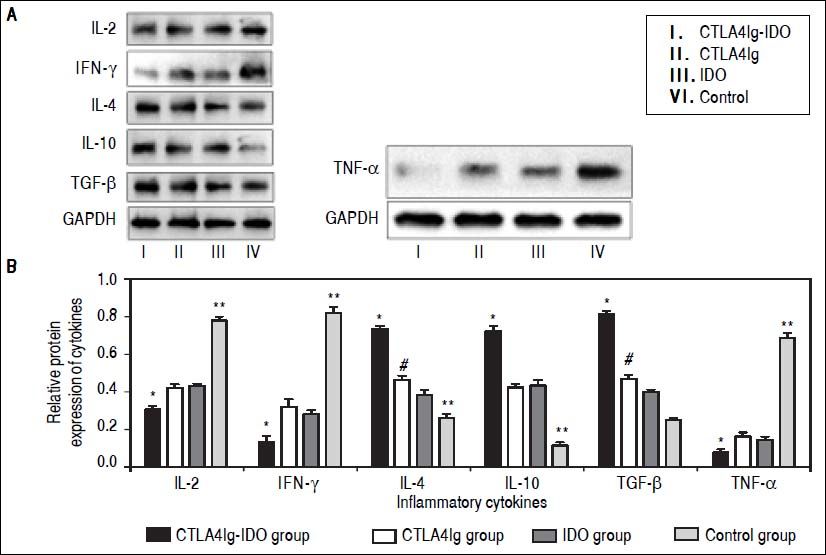
Protein and mRNA expression by Western blot and RT-PCROn the 14th day after operation, the expression levels of IFN-γ mRNA and IL-2 mRNA in the control group were evidently higher than those in the other three groups (P < 0.05), especially that these expression levels were low in the CTLA4Ig-IDO group. The levels of IL-10 mRNA, IL-4 mRNA, and TGF-β mRNA in the CTLA4Ig-IDO group were higher than those of the control group (P < 0.05), but no significant differences appeared between the CTLA4Ig and IDO groups (P > 0.05) (Figure 5). Western blot analysis indicated that protein expression displayed a similar trend to mRNA results, and TNF-α had a high expression in the control group but low expression in the CTLA4Ig-IDO group, revealing that T lymphocyte activity was suppressed by the gene transfer (Figure 6).
Protein expression of inflammatory medium in liver tissues. A. IL-2, IFN-γ and TNFα expressions were higher in the control group but lower in the other three groups. IL-10, IL-4 and TGF-β in the CTLA4Ig-IDO group were higher than those of the control group. B. Relative protein expression of cytokines. * P < 0.01 vs. CTLA4Ig, IDO, and control groups; ** P < 0.05 vs. CTLA4Ig and IDO groups; # P < 0.05 vs. IDO group.
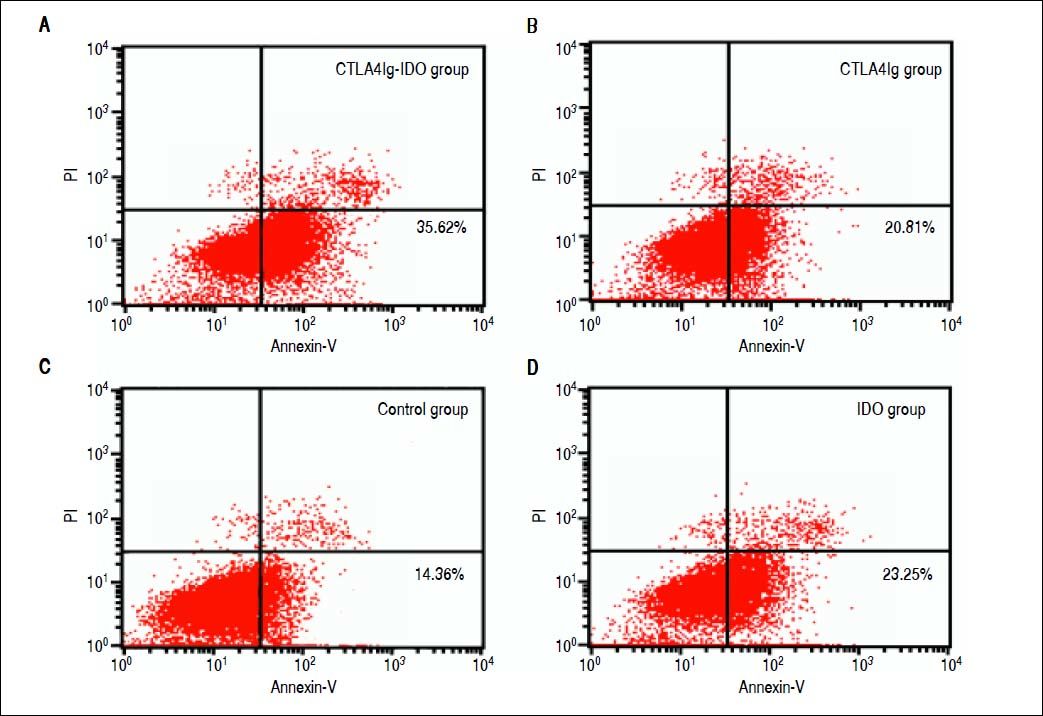
The peripheral T lymphocyte apoptosis detected by Annexin V-FITC/PI method showed that apoptosis index was higher in the CTLA4Ig-IDO group (36.63%) than those of the other three groups (P < 0.05). The apoptosis index in the CTLA4Ig group (21.32%) did not differ from the IDO group (23.87%), but showed significant difference compared with the control group (15.10%) (Figure 7). Results indicate that overexpression of CTLA4Ig or IDO in the liver grafts could enhance peripheral T lymphocyte apoptosis post-transplantation, and significant differences were found between their efficacies. Furthermore, overexpression of combined gene of CTLA4Ig-IDO in the liver graft enhanced the efficacy of CTLA4Ig and IDO, which could boost the T lymphocyte apoptosis and induce immune tolerance after liver transplantation.
DiscussionStrategies to reduce post-transplantation graft rejection by transferring adenovirus-mediated gene into allografts have gained increasing attention. CTLA4Ig is a fusion protein formed in an extracellular domain of CTLA4 and a constant region of human IgG1, which has high avidity with B7-1 molecules; CTLA4Ig blocks the costimulatory signal pathway from APC to antigen-specific T cells and induces immune tolerance.3,17,18 Previous studies found that CTLA4Ig gene is constantly expressed in the graft liver and peripheral serum via single penile dorsal vein or portal vein injection preoperatively by using adenovirus as vector and remains positive after transplantation. Results show that CTLA4Ig gene can inhibit infiltration of immune activator cells and induce T lymphocyte apoptosis in grafts, resulting in reduced rejection and prolonged survival of recipients.3,7,19 Therefore, CTLA4Ig gene not only plays an important role in autoimmune diseases, but also induces immune tolerance after transplantation.20,21
IDO mediates tryptophan metabolism and is preferentially found among the APC population. Recent studies proposed a profound immune regulatory role of IDO in many responses, including transplant immunity, tumor immunology, autoimmunity, and HIV infection.22–24 The considered mechanisms by which IDO regulates the immune response are as follows:
- •
Local tryptophan depletion, because tryptophan is essential for many cellular organisms to survive and divide; and
- •
Tryptophan metabolites arrest cycling responsive T cells and render them susceptible to apoptosis.12,25
The effects of IDO in reducing rejection response and inducing immune tolerance in the field of transplantation were investigated in a few studies with different results. Luan, et al.10 found that IDO level is closely associated with acute rejection and is important in immune tolerance induction. Hill, et al.26,27 found that in rat recipients of heart allografts, CTLA4Ig-induced tolerance in vivo depends on IDO metabolism. However, other studies demonstrated that IDO has no critical role in the processes of acute rejection and tolerance induction in in vivo models, even when IDO expression was upregulated in rejecting grafts;13–16,28 this finding may be caused by the rejection process being too strong to be overcome by IDO or that insufficient IDO activity was induced in these models. Increased IDO activity in a variety of cell lines, whether upregulated endogenous IDO or via transfection with IDO cDNA, can suppress T-cell activity in vitro.29
In this study, we constructed recombinant adenovirus-mediated vectors pWBP-CTLA4Ig-IDO, pWBP-CTLA4Ig, and pWBP-IDO expressing CTLA4Ig-IDO, CTLA4Ig, and IDO genes, respectively. These genes were positive in liver allografts in each group 14 days after transplantation, indicating that pWBP-CTLA4Ig-IDO, pWBP-CTLA4Ig, and pWBP-IDO were effectively expressed in liver allografts via portal vein infusion. We observed the cytokine expression and histology changes of acute rejection in different groups to determine the effects of transfection of combined and single genes. The histological examination of transplanted liver on the 14th day indicated that majority of the liver grafts in the CTLA4Ig-IDO group showed almost normal liver architecture with only mild portal lymphocytic infiltration and infiltration of lobular areas. By contrast, signs of severe acute rejection, such as numerous lymphocytes and neutrophils, mononuclear infiltration in the portal tracts, extensive lobular architecture destruction, vacuolated degeneration, necrosis of hepatocyte, and bile duct epithelium damage, were observed in the control group. The changes in the CTLA4Ig and IDO group were between the two mentioned above. These findings indicate that CTLA4Ig-IDO induced the greatest reduction of acute rejection in all groups.
Cytokines play an important role in the induction of immune tolerance and are extensively studied in transplantation. The unique array of cytokines produced by two subsets of CD4+ helper T cells, namely, Th1 and Th2, controls the induction and regulation of cellular and humoral immunity. Th1 subset secretes IL-2, IFN-γ, and TNF-α, which induce inflammatory response and acute rejection. By contrast, Th2 cells produce IL-4, IL-6, IL-10, IL-13, and TGF-ß, which are associated with immune tolerance. In this study, we examined the levels of Th1 (IL-2, IFN-γ, and TNF-α) and Th2 cytokines (IL-4, IL-10, and TGF-β) to investigate the role of CTLA4Ig-IDO in inducing immune tolerance. Results show that the levels of Th1 cytokines in the control group were evidently higher than those in the other three groups, with particularly low expressions in the CTLA4Ig-IDO group. Th2 cytokine expressions were high in the CTLA4Ig-IDO group but low in the CTLA4Ig or IDO group. The differences between Th1 and Th2 cytokine levels were primarily caused by CTLA4Ig-IDO. Elevated Th2 cytokines may alleviate rejection by:
- •
Suppressing Th1 cytokine secretion.
- •
Inhibiting the function of antigen-presenting cells.
- •
Promoting the development of CD4+ CD25 + T lymphocytes, and
- •
Downregulating the expression of the major histocompability complex.30
This observation is in accordance with previous findings, which indicate that cytokine mRNA expression in rejecting allografts plays significant roles in the rejection progress.19,31 Furthermore, the differences between peripheral T lymphocyte apoptosis index and recipient survival 14 days post-transplantation in the groups were also observed. The trends were similar to the changes of histological examination and cytokine expression in the four groups.
Major challenges in clinical liver transplantation include alleviation of rejection and improvement of longterm survival in patients. These challenges are mainly overcome by using immunosuppressants. However, longterm administration of immunosuppressive drugs results in immunological complications and numerous side effects. These problems need to be addressed, and new strategies should be developed to induce immune tolerance. In this study, the combined genes of CTLA4Ig and IDO were transfected via portal vein, and their expressions were positive in the liver after transplantation. This process helped reduce rejection and improve survival. The findings of this study may help in designing novel constructions containing CTLA4Ig-IDO gene (e.g., nanometer materials), which can remain and express positive in the graft liver, to help reduce rejection and induce immune tolerance through infusion during patient operation. The findings can also provide new insights for developing new anti-rejection drugs. This method has potential to become a feasible and effective method of immunological tolerance in liver transplantation and is likely to become a clinical tool for the treatment of liver immune disease.
In conclusion, this study confirms that CTLA4Ig and IDO genes both played regulatory role in acute rejection in rat liver transplantation model. When transferring combined CTLA4Ig-IDO gene into the graft liver, the synergistic effects of CTLA4Ig and IDO were more effective than when using CTLA4Ig or IDO separately. The combined gene transfusion may become a novel strategy in inducing immune tolerance and improving patient survival after liver transplantation. However, further studies on synergistic mechanisms and time-dependent changes in the rejection process are necessary.
Abbreviations- •
ALT: alanine aminotransferase.
- •
AST: aspartate transaminase.
- •
CTLA4Ig: cytotoxic T lymphocyte-associated antigen-4 immunoglobulin.
- •
IDO: indoleamine 2, 3-dioxygenase.
- •
IL: interleukin.
- •
PV: portal vein.
- •
TBIL: total bilirubin.
- •
TGF: transforming growth factor.
This study was supported by the National Natural Science Foundation of China (Nos. 81200329, 81300364, and 81370580). Foundation of Health and family planning comission in ChongQing (2011-1-083).
AcknowledgmentsWe thank the Fulen Gene Company (Guang Zhou, China) for the generous gift of pWBP-CTLA4Ig-IDO. We thank Doctor Jinzheng Li for the technical assistance.