Optimal treatment of hepatocellular carcinoma (HCC) involving portal vein tumor thrombus (PVTT) remains controversial.
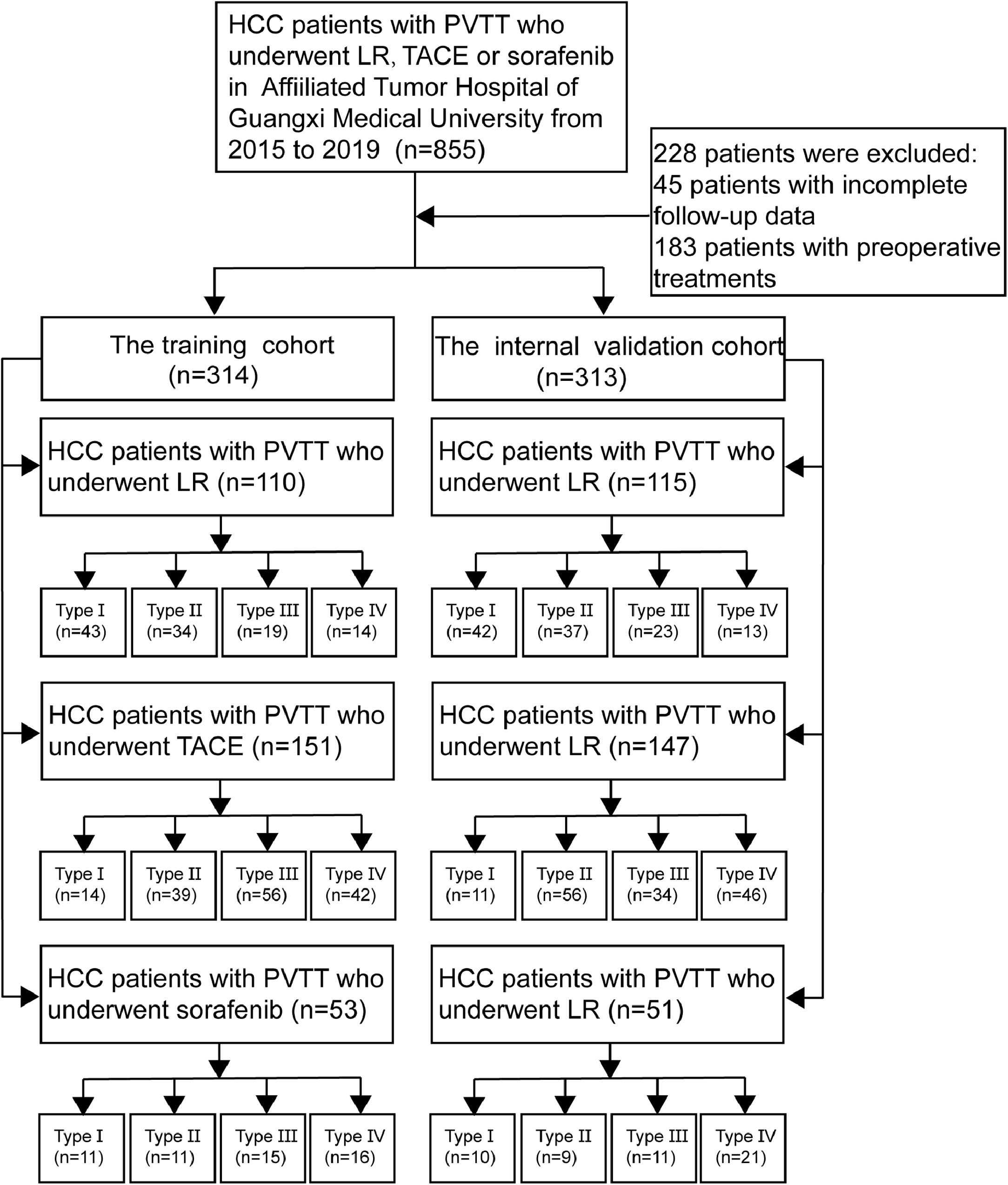
Materials and methodsA total of 627 HCC patients with PVTT after initial treatment with one of the following at Affiliated Tumor Hospital of Guangxi Medical University: liver resection (LR, n = 225), transarterial chemoembolization (TACE, n = 298) or sorafenib (n = 104) were recruited and randomly divided into the training cohort (n = 314) and internal validation cohort (n = 313). Survival analysis were repeated after stratifying patients by Cheng PVTT type.
ResultsResection led to significantly higher OS than the other two treatments among patients with type I or II PVTT. TACE worked significantly better than the other two treatments for patients with type III. All three treatments were associated with similar OS among patients with type IV. These findings were supported by the internal validation cohort.
ConclusionsOur results suggest that the optimal treatment for HCC involving PVTT depends on the type of PVTT. LR may be more appropriate for type I or II PVTT; TACE, for type III Sorafenib may be more appropriate than invasive treatments for patients with type IV PVTT.
Portal vein tumor thrombus (PVTT) occurs in 40-60% of patients with hepatocellular carcinoma (HCC), which accounts for 80-90% of cases of primary liver cancer [1]. PVTT is a significant predictor of poor prognosis for HCC patients [2,3]. Indeed, HCC patients with PVTT survive a median of 3-4 months without intervention [4].
The optimal treatment for HCC patients with PVTT remains controversial. Currently the only officially recommended treatment is sorafenib, based on the Barcelona Clinic for Liver Cancer (BCLC) staging system [5]. However, the drug shows limited efficacy: one trial reported median survival of only 6.5 months in HCC patients with PVTT on the drug [6]. This, coupled with the drug's high price, leads many patients to refuse the treatment and opt instead for liver resection or transarterial chemoembolization (TACE). Liver resection has been associated with 1-year overall survival (OS) rates of 22-70% for HCC patients with PVTT, which may be higher than with non-surgical treatments [7,8]. TACE has also proven safe and effective for such patients [3,9]; In fact, the Hong Kong Liver Cancer system recommends TACE for patients with intrahepatic vascular invasion [10].
Combined targeted therapy had received great attention from clinicians in recent years. The combination targeted therapy may cover more molecular targets, effectively kill tumors, and weaken tumor proliferation and metastasis [11]. However, there are also some issues. First, the high price makes it unbearable for many HCC patients. Then, combined targeted therapy may be accompanied by more and more complicated complications. At last, the tumor may develop more complicated drug resistance to the combination targeted therapy [12].
In this retrospective study, we compared OS of HCC patients with PVTT following initial liver resection, TACE or sorafenib in order to identify more effective treatments. We repeated the comparison after stratifying patients by extent of portal invasion according to Cheng's PVTT classification [13].
2Material and methods2.1Study populationFrom January 2015 to December 2019, a total of 855 HCC patients with PVTT were conducted at the Affiliated Tumor Hospital of Guangxi Medical University after initial treatment with one of the following at our hospital: liver resection (LR), transarterial chemoembolization (TACE) or sorafenib.
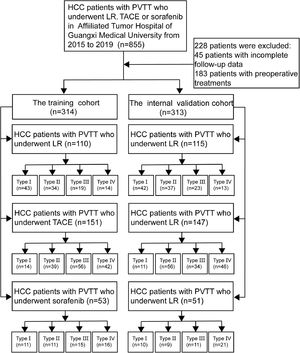
A total of 627 HCC patients with PVTT met the inclusion criteria and were randomly divided into the training cohort (n = 314) and internal validation cohort (n = 313) (Fig. 1). The method of random grouping is through“sample ()”function of R studio. The details are as follows, “Patients_data <- read.table (∼ dirict) Grouping <- function (Patients_data, m, n) {k =1 while (k<=(n-1)) {Grouping <- sample (Patients_data, m, replace = FALSE, prob = NULL) for (i in 1:m) {Patients_data <- Patients_data [-which (Patients_data == Grouping [i])]} k=k+1 Print (Grouping)} Print (Patients_data)} m: the number of patients in each group; n: the number of groups” The study was approved by the Ethics Committees of the Affiliated Tumor Hospital of Guangxi Medical University. Written informed consent was obtained from all patients for their data to be used for scientific purposes.
2.2PVTT DiagnosticsHCC patients were diagnosed with PVTT based on typical preoperative radiological feature: ultrasonography, Doppler ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and intra- or postoperative histopathology examinations. PVTT type was assigned using Cheng's classification [13]: in type I, a tumor thrombus lies in the segmental branches of the main portal vein or above; in type II, a tumor thrombus extends into the right or left portal vein; in type III, a tumor thrombus extends into the main portal vein; and in type IV, a tumor thrombus extends into the main portal vein and superior mesenteric vein. Thus, type I and II PVTT were limited to a first-order branch of the main portal vein or above (Table S1).
2.3The inclusion and exclusion criteriaThe inclusion criteria: (1) they had been diagnosed with PVTT as described above; (2) they did not show macroscopic hepatic vein tumor thrombus, macroscopic bile duct tumor thrombus, extrahepatic spread, or distant metastasis; (3) they did not have any other associated malignancies; and (4) their initial treatment was liver resection, TACE or sorafenib at our hospital between January 2015 and December 2019. The exclusion criteria: (1) Patients were excluded if they had Child-Pugh class C liver function; (2) Patients had undergone palliative tumor resection; (3) Patients medical records were incomplete. The same inclusion and exclusion criteria were applied to the training and internal validation cohorts.
2.4ProceduresLiver resection was offered only to patients with Child-Pugh A function or to certain patients with Child-Pugh B liver function (score ≤7). Surgical procedures were conducted as described in this paper [12]. For TACE, a microcatheter was introduced and directed into the feeding artery. An emulsion of 5-15 mL of lipiodol and 5-fluorouracil (500 mg/m2) with or without Adriamycin (30 mg/m2) was infused into the feeding artery. Tumor response to TACE was assessed one month later using computed tomography (CT) and/or MRI. TACE was repeated once every 1-2 months for a total of 2-6 cycles. HCC patients in the sorafenib group were required to receive oral sorafenib 400 mg twice daily until unacceptable toxicity or loss of clinical benefit.
2.5Follow-upAfter treatment, patients were followed up every 3-4 months until death or loss to follow-up. On follow-up visits, patients were tested for alpha-fetoprotein (AFP) level, liver function, and blood tests, and they were subjected to abdominal ultrasonography, contrast-enhanced CT, and/or MRI.
2.6Statistical analysesContinuous data were reported as mean ± SD or as median (interquartile range), and differences were assessed for significance using the Mann-Whitney U test. Differences in categorical data were assessed for significance using the chi-squared or Fisher's exact (2-tailed) tests. The primary endpoint was OS, which was defined from the date of surgery to the date of death or last follow-up, and analyzed using the Kaplan-Meier method. Differences in OS were assessed using the log-rank test. All statistical analyses were performed using R studio v1.2.0 (https://rstudio.com/) and GraphPad v7.0 (https://www.graphpad.com/). Differences associated with P < 0.05 were considered statistically significant.
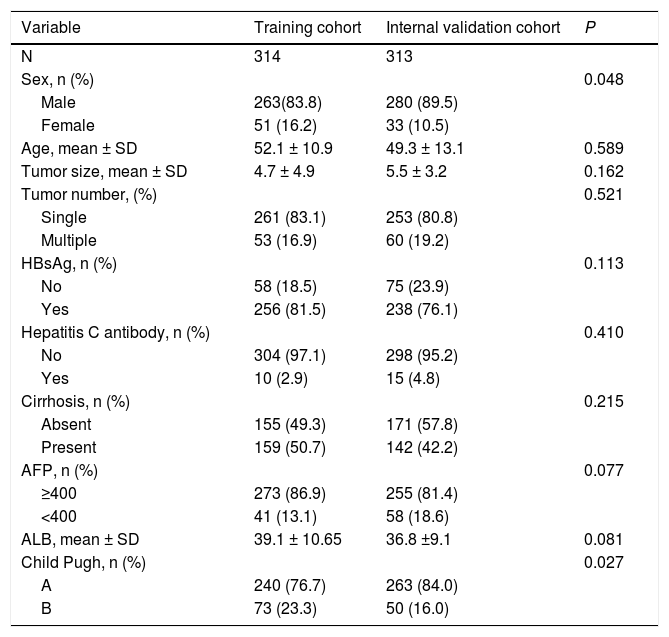
3Results3.1Characteristics of PatientsThe median age of all patients in both cohorts was 51 years, and more than 70% were male or were infected with hepatitis B virus. Approximately 3% were infected with hepatitis C virus (HCV). More than 50% patients had liver cirrhosis. All patients had compensated liver function. Most baseline clinicopathological data were similar between the training and validation cohorts. Patients in the training group showed a significantly higher age as well as better liver function (Child Pugh A) (Table 1).
. Baseline characteristics of patients with HCC with portal vein tumor thrombus.
| Variable | Training cohort | Internal validation cohort | P |
|---|---|---|---|
| N | 314 | 313 | |
| Sex, n (%) | 0.048 | ||
| Male | 263(83.8) | 280 (89.5) | |
| Female | 51 (16.2) | 33 (10.5) | |
| Age, mean ± SD | 52.1 ± 10.9 | 49.3 ± 13.1 | 0.589 |
| Tumor size, mean ± SD | 4.7 ± 4.9 | 5.5 ± 3.2 | 0.162 |
| Tumor number, (%) | 0.521 | ||
| Single | 261 (83.1) | 253 (80.8) | |
| Multiple | 53 (16.9) | 60 (19.2) | |
| HBsAg, n (%) | 0.113 | ||
| No | 58 (18.5) | 75 (23.9) | |
| Yes | 256 (81.5) | 238 (76.1) | |
| Hepatitis C antibody, n (%) | 0.410 | ||
| No | 304 (97.1) | 298 (95.2) | |
| Yes | 10 (2.9) | 15 (4.8) | |
| Cirrhosis, n (%) | 0.215 | ||
| Absent | 155 (49.3) | 171 (57.8) | |
| Present | 159 (50.7) | 142 (42.2) | |
| AFP, n (%) | 0.077 | ||
| ≥400 | 273 (86.9) | 255 (81.4) | |
| <400 | 41 (13.1) | 58 (18.6) | |
| ALB, mean ± SD | 39.1 ± 10.65 | 36.8 ±9.1 | 0.081 |
| Child Pugh, n (%) | 0.027 | ||
| A | 240 (76.7) | 263 (84.0) | |
| B | 73 (23.3) | 50 (16.0) |
Values are n or n (%) or mean ± SD; ALB, albumin; ALT, alanine aminotransferase; PT, prothrombin time; AFP, alpha fetoprotein.
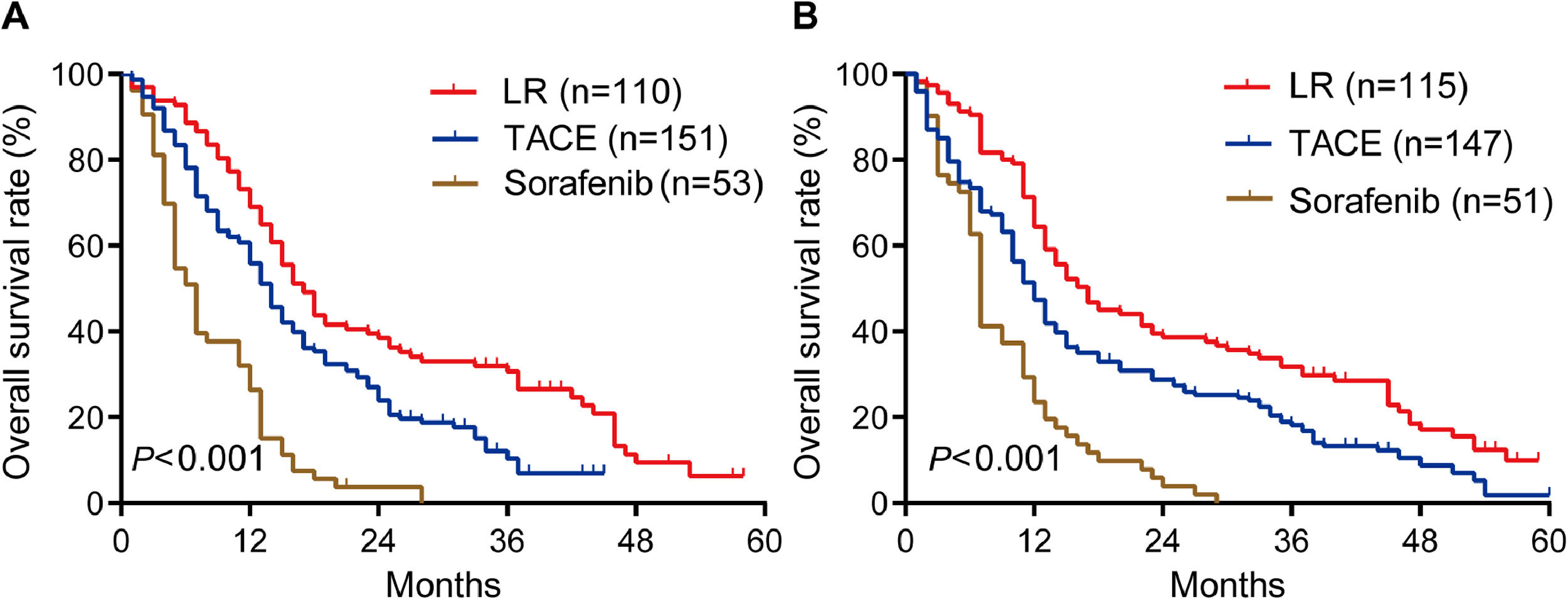
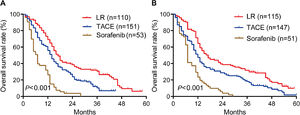
During follow-up for a median of 48 months (range, 23-71), OS rates in the training cohort were significantly longer in patients who underwent liver resection than in those who underwent TACE or sorafenib: the respective rates at 1 year were 69.0%, 56.0% and 26.4%; at 2 years, 38.4%, 23.9% and 3.8%; and at 3 years, 30.7%, 10.4% and 0% (P < 0.001; Fig. 2A). Median OS in this cohort was 17.0 months after liver resection, 14 months after TACE, and 7.0 months after sorafenib.
Similarly, OS rates in the internal validation cohort were significantly longer after liver resection than after TACE or sorafenib: the respective rates at 1 year were 64.3%, 47.4% and 23.5%; at 2 years, 38.6%, 27.3% and 3.9%; and at 3 years, 31.8%, 18.2% and 0% (P < 0.001; Fig. 2B). Median OS in this cohort was 16 months after liver resection, 12 months after TACE, and 7 months after sorafenib. These results suggest that, surgery is the best treatment for all PVTT types of HCC patients.
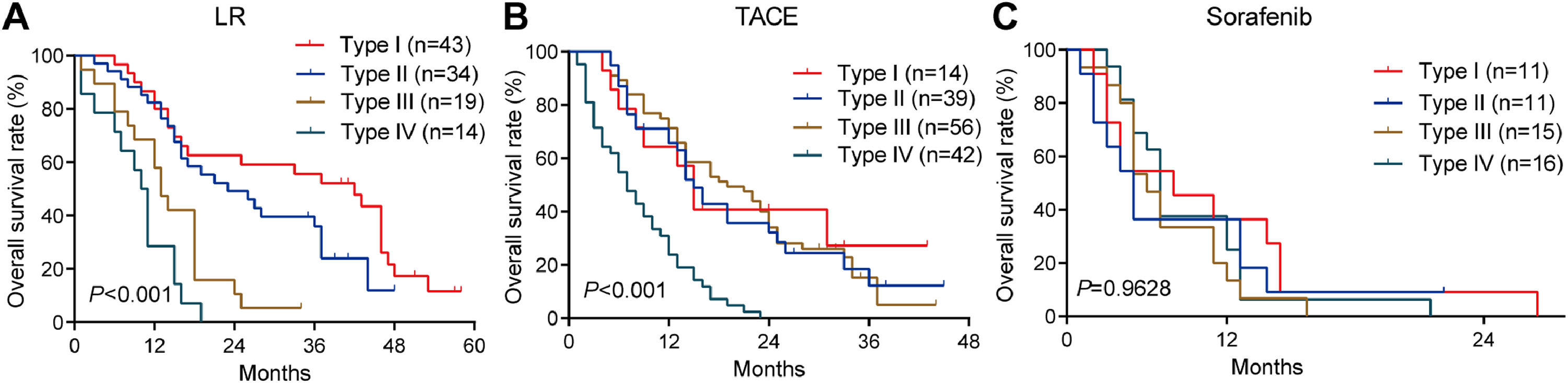
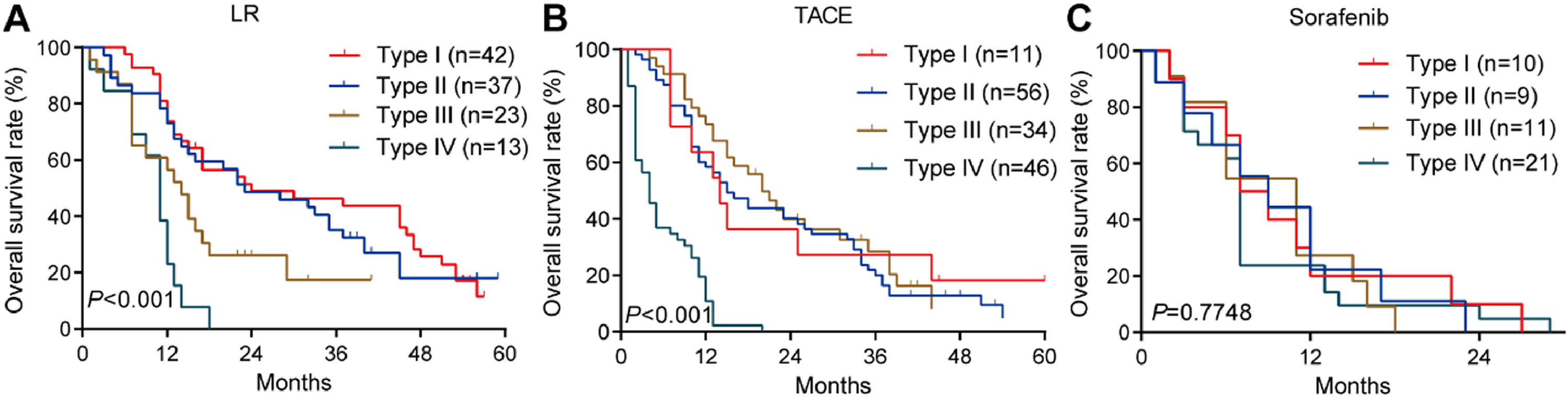
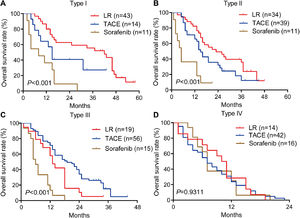
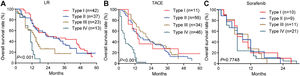
3.3OS for different PVTT types in the training cohortAmong patients who underwent liver resection in the training cohort, OS rates were significantly higher among those with type I or II PVTT than among those with type III or IV PVTT: the corresponding rates at 1 year were 86.7%, 82.3%, 57.9%, and 28.6%; at 2 years, 62.7%, 49.2%, 10.5%, and 0%; and at 3 years, 55.6%, 35.9%, 5.3%, and 0%. Median OS was 28, 23, 13 and 10.5 months for the four types of PVTT (Fig. 3A).
Among patients who underwent TACE, OS was also significantly worse among those with type IV PVTT than among those with types I, II or III: the corresponding rates at 1 year were 23.8%, 64.3%, 65.6%, and 65.8%; at 2 years, 0%, 40.8%, 32.1%, and 34.0%; and at 3 years, 0%, 27.2%, 12.2%, and 5.1%. Median OS was 16, 18, 17 and 7 months for the four types of PVTT (Fig. 3B).
Among patients who received sorafenib, OS did not differ significantly among the subgroups with different PVTT types, and median OS varied slightly from 5 to 8 months across the subgroups (Fig. 3C).
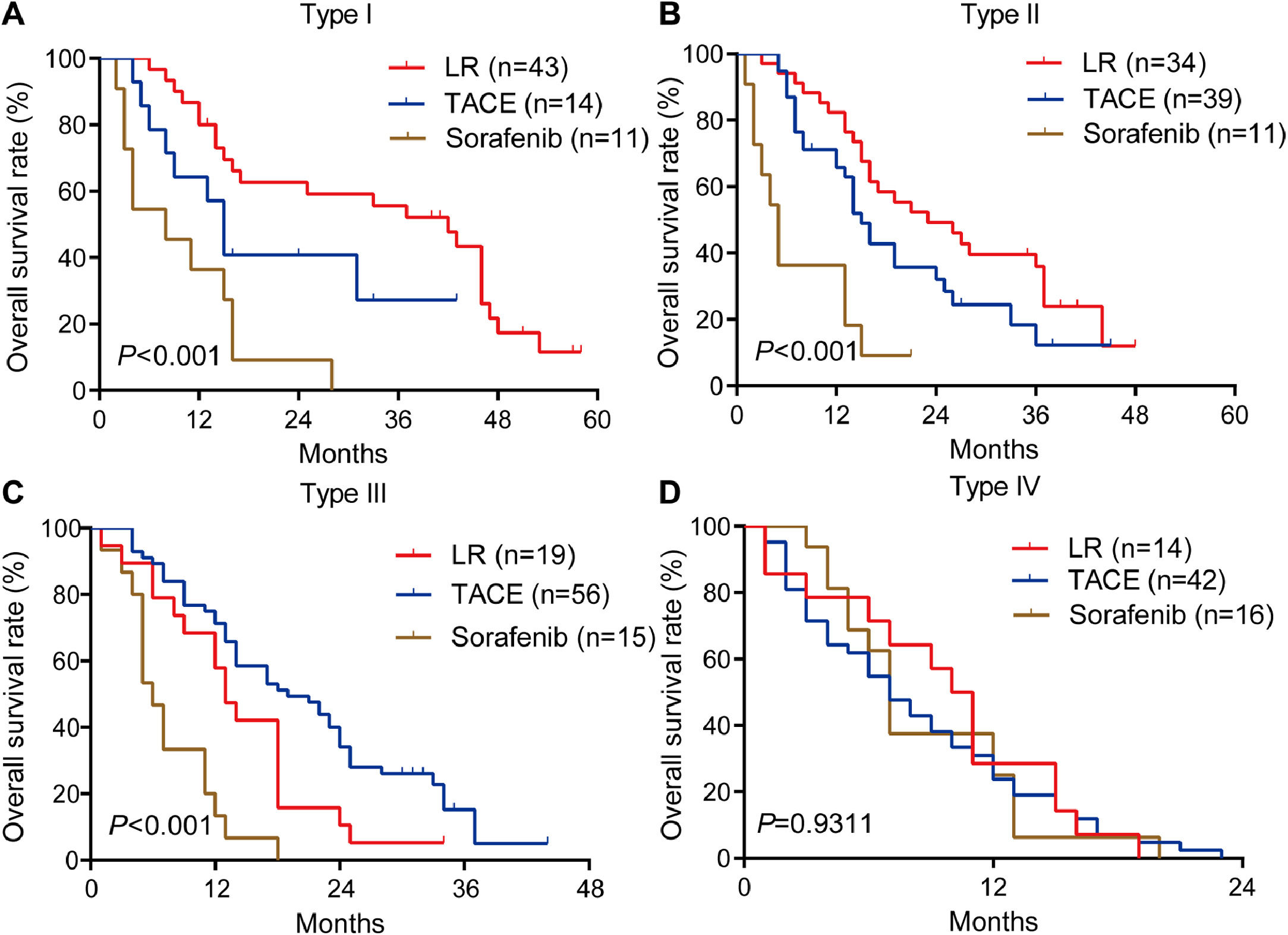
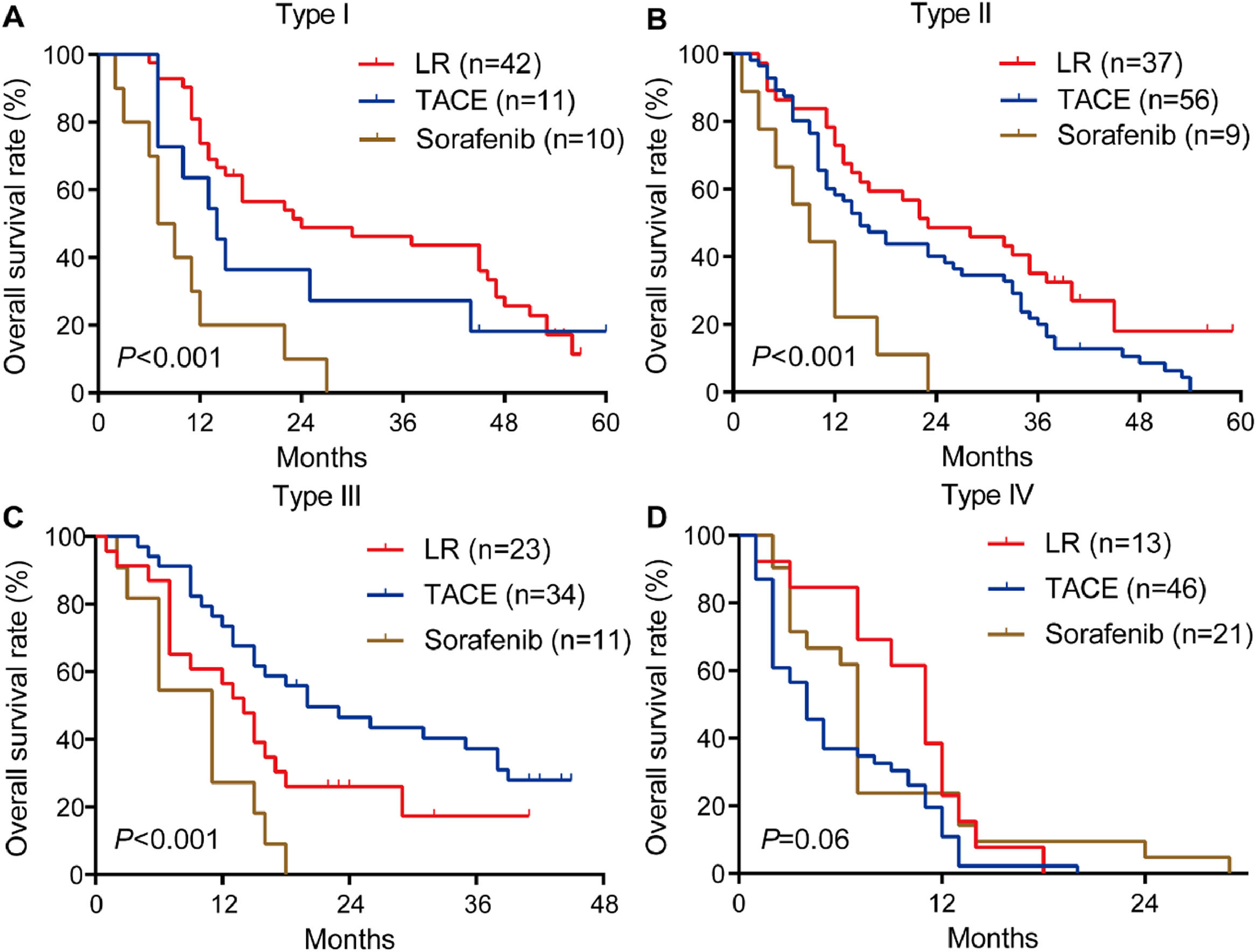
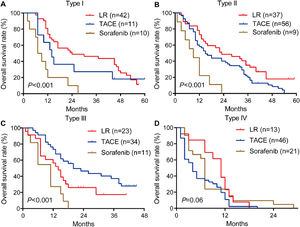
Among patients with type I or II PVTT, liver resection was associated with markedly better OS than TACE or sorafenib (Fig. 4A, 4B). In contrast, among patients with type III PVTT, TACE gave significantly better OS than liver resection or sorafenib (Fig. 4C). Among patients with type IV PVTT, the three treatments did not differ significantly (Fig. 4D).
3.4OS for different PVTT types in the internal validation cohortAmong patients who underwent liver resection in the internal validation cohort, OS was significantly higher among those with type I or II PVTT than among those with type III or IV PVTT: the corresponding rates at 1 year were 73.8%, 72.9%, 56.5%, and 23.1%; at 2 years, 48.9%, 45.9%, 26.0%, and 0%; and at 3 years, 43.7%, 32.4%, 17.4%, and 0%. Median survival was 24, 23, 14 and 11 months for patients with PVTT types I-IV (Fig. 5A).
Among patients who underwent TACE, OS was also significantly worse among those with type IV PVTT than among those with types I, II or III: the corresponding rates at 1 year were 10.8%, 54.5%, 58.3%, and 58.8%; at 2 years, 0%, 36.4%, 40.1%, and 39.9%; and at 3 years, 0%, 18.2%, 20.1%, and 20.4%. Median survival was 17, 16, 14 and 5 months for PVTT types I-IV (Fig. 5B).
Among patients who received sorafenib, OS did not differ significantly among the subgroups with different PVTT types, with median OS varying slightly between 6 and 9 months (Fig. 5C). These results are consistent with those from the training cohort.
Among patients with type I or II PVTT, liver resection was associated with significantly longer survival than TACE or sorafenib (Fig. 6A, 6B). Among patients with type III PVTT, TACE was associated with better OS than the other two treatments (Fig. 6C). Among patients with type IV PVTT, OS did not differ significantly among the three treatments (Fig. 6D). These results are consistent with those for the training cohort.
Taken together, our results suggest that, liver resection may be more appropriate for type I or II PVTT; TACE, for type III; and sorafenib, for type IV. Evaluation of PVTT subtypes of HCC patients is of great significance in future clinical practice, and could provide potential treatment strategies for HCC patients with PVTT.
4DiscussionOur results suggest that liver resection may be associated with better OS than TACE or sorafenib for HCC patients with type I or II PVTT. In contrast, TACE may be more effective for HCC patients with type III PVTT, while sorafenib may be more appropriate for patients with type IV PVTT.
The optimal treatment for HCC patients with PVTT remains controversial. Some studies have highlighted the benefits of surgical treatment [14–17], since liver resection can unblock portal venous flow to decrease portal venous pressure and improve liver function, which can prolong survival and improve quality of life [7]. One study found that liver resection led to better outcomes than TACE for the treatment of HCC with PVTT [18], but the other study of a larger cohort found the opposite result [9]. Our study helps explain the controversy in the literature by demonstrating that the best treatment may depend on the PVTT type. For example, we found that HCC patients with type I or II PVTT showed better OS after liver resection than after the other two treatments, consistent with a Japanese study [7].
Our results support the efficacy of TACE for treating HCC patients with type III PVTT. TACE blocks blood vessels that carry nutrients to the tumor, allowing chemotherapy to be delivered at high concentrations to cancer cells, while minimizing damage to healthy liver cells. TACE also reduces portal venous pressure and prevents formation of intractable ascites and bleeding esophageal varices [19]. In addition, our results suggest that for HCC patients with type IV PVTT, sorafenib may be preferable to resection or TACE given that it is non-invasive and associated with fewer complications than invasive treatments [20]. However, the drug should be used with caution given that it has been associated with greater risk of in-hospital mortality, high blood pressure, skin toxicity, and adverse gastrointestinal reactions [6,21]. In addition, sorafenib is not well tolerated in patients with reduced liver function [6].
Sorafenib is multi-kinase inhibitor that is approved for first-line treatment of patients with advanced hepatocellular carcinoma. However, the efficacy of targeted agents is modest, and they confer limited survival benefits. Immunotherapies, including PD -1 and PD-L1 inhibitors, have shown clinical benefits in various cancers. However, several phase 3 studies did not show superiority of anti-PD-1 monotherapy compared with standard of care for the first-line or second-line treatment of hepatocellular carcinoma [22]. Considering differences in the treatment efficacy of PD-1 and PD-L1 inhibitors across various tumor types, combination treatment with an anti-PD-1 antibody and an anti-angiogenesis agent might be a potential first-line treatment for patients with hepatocellular carcinoma. Ren et al reported [23] that compared with similar studies of first-line therapies for patients with advanced hepatocellular carcinoma, Sintilimab–bevacizumab biosimilar reduced the risk of death and disease progression. Sintilizumab-bevacizumab might be the first-line therapy for patients with advanced hepatocellular carcinoma, however, for the population of HCC patients with PVTT, the potential benefit of Sintilizumab-bevacizumab therapy is not known, Whether HCC patients with PVTT could benefit from Sintilizumab-bevacizumab therapy warrants future large cohort studies.
While our retrospective analysis should be interpreted carefully given the risk of selection bias. In addition, the data could be affected by the differences in standards in surgical techniques and perioperative managements. And last, high prevalence of hepatitis B virus (HBV) may limit extrapolation of results to other patients.
5ConclusionIn conclusion, our study suggested that the optimal treatment for HCC patients with PVTT may depend on their PVTT type. Liver resection may be more appropriate for type I or II PVTT; TACE, for type III; and sorafenib, for type IV. Our results underscore the importance of evaluating the PVTT subtypes of HCC tumors in future clinical practice and provide a potential treatment strategy for HCC patients with PVTT.
CRediT authorship contribution statementYu Zhang: Funding acquisition, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Jun-Li Wu: Writing – original draft, Writing – review & editing. Le-Qun Li: Conceptualization, Data curation.
The authors acknowledge assistance from the staff in the Affiliated Tumor Hospital of Guangxi Medical University.