Background and aims. Patients with intrahepatic cholestasis of pregnancy (ICP) benefit from ursodeoxycholic acid (UDCA) treatment. Since there is still certain reluctance to use UDCA in pregnant women, mainly due to warnings in the official SPC information in respective drug leaflets, our objective was to assess the efficacy and safety of UDCA during pregnancy.
Material and methods. Our retrospective multicentric study was performed on 191 consecutive pregnant women with ICP treated with UDCA. Any maternal and/or fetal complications of the UDCA treatment were searched for; healthy pregnant women (n = 256) served as controls.
Results. The UDCA treatment improved liver disease status in the majority of the affected women (86.1%). This treatment was well tolerated, with only negligible skin reactions (0.5%) and mild diarrhea (4.7%). No complications attributable to UDCA treatment were detected during the fetal life, delivery, or the early neonatal period.
Conclusion. We confirmed the good efficacy and safety of UDCA treatment in pregnancy for both mothers and fetuses/neonates.
Deterioration of liver function tests is a common complication during pregnancy, with intrahepatic cholestasis of pregnancy (ICP) being the most common liver disorder in pregnant women.1 ICP manifests in the second half of a pregnancy, most often during the third trimester as pruritus, with deterioration in liver function tests, and typically with elevated serum total bile acids (BA) > 8 µmol/L. Signs of the disease resolve spontaneously after delivery, but the risk of recurrence in a subsequent pregnancy is 45-70%.2
ICP is usually considered a benign condition for pregnant woman despite the fact there is an association between ICP and later development of various liver and biliary diseases, including hepatitis C, nonalcoholic fatty liver disease, gallstones, cholecystitis, or pancreatitis.3 Far more significantly, ICP represents a serious threat to the fetus; in particular due to elevated maternal BA levels in the serum. In fact, BA exert important cardiac arrhythmogenic effects for the fetus, and increase the risk of pre-term delivery, meconium-stained amniotic fluid (with the subsequent risk of meconium aspiration syndrome), neonatal respiratory distress syndrome, or even sudden intrauterine fetal death (IUFD).2,4 The critical threshold of maternal serum BA, dramatically increasing the probability of fetal complications, was determined to be 40 µmol/ L.5 The treatment of ICP is based on oral administration of ursodeoxycholic acid (UDCA), and this treatment is regarded as “the first-line treatment for ICP, based on evidence obtained from randomized clinical trials” by recent EASL (European Association for the Study of the Liver) clinical practice guidelines on the management of cholestatic liver diseases.6 On the other hand, UDCA has still not been approved by the drug regulatory authorities as a pregnancy-safe drug; thus accounting for the certain reluctance of obstetricians to use UDCA in their pregnant patients,7 and also for much less definite guidelines of the official obstetrics authorities on UDCA use as a standard therapy for ICP.8
Thus, the aim of our study was to assess the efficacy and possible side effects of a UDCA therapy in patients with ICP recruited from four major centers for perinatal medicine in the Czech Republic.
Material and MethodsSubjectsOur retrospective, multicentric study was performed on 257 consecutive pregnant women, recruited between 2007-2013 from four large centers for perinatal medicine in the Czech Republic:
- •
General Faculty Hospital of the 1st Faculty of Medicine.
- •
Faculty Hospital in Motol of the 2nd Faculty of Medicine, both of the Charles University in Prague.
- •
Faculty Hospital of the Faculty of Health Sciences, Palacky University Olomouc.
- •
Regional Hospital Pardubice,
who developed signs of ICP, which, in turn, were treated with UDCA. All ICP patients included had elevation of liver function enzymes (either ALT > 47 IU/L; AST > 43 IU/L; or ALP > 132 IU/L), serum bile acids (> 8 µmol/L), and majority of pregnant women suffered from pruritus. Patients with other causes of liver dysfunction, including preeclampsia, HELLP syndrome, viral hepatitis, cholestasis due to bile duct stones, or suspect autoimmune hepatitis were excluded based on extensive routine laboratory and image diagnostics (viral serology, auto-antibodies, hematology work-up, liver ultrasonography) (n = 66). We scrutinized the medical records of all women with ICP (n = 191) to whom UDCA was administered during pregnancy, searching for the response to the UDCA treatment, any maternal and/or fetal complications of UDCA treatment, as well as neonatal status. The following parameters were specifically evaluated: maternal liver function tests, UDCA dosage, UDCA therapeutic effect, any side effects accompanying UDCA therapy, gestational age, quality of amniotic fluid, delivery course, and Apgar score of the neonates.
The control group consisted of 256 consecutive outpatient pregnant women followed in 2012 at the Department of Obstetrics and Gynecology, 1st Faculty of Medicine, Charles University in Prague for delivery of their babies. The exclusion criteria were presence of any liver disease or concomitant therapy.
The size of both cohorts was calculated to uncover at least 5% incidence of possible side effects of UDCA therapy (see Statistical analyses subsection).
Laboratory analysesRoutine serum biochemical markers were determined on an automatic analyzer (Modular analyzer, Roche Diagnostics GmbH, Germany) using standard laboratory assays. Serum BA levels were determined spectrophotometrically using a Bile Acids kit (Trinity Biotech, Jamestown, NY, USA).
Statistical analysesThe sample size needed to uncover at least 5% difference in the incidence of UDCA side effects compared to controls (at both alpha error of confidence and beta error levels set to 5%) was calculated using online DSS research statistical calculator (https://www.dssresearch.com/Knowledge-Center/toolkitcalculators/samplesizecalculators.aspx). Data are expressed either as the mean ± SD, or median and IQ range, depending on their normality. Comparison of the variables was performed using the t-test, Mann-Whitney test, or χ2 test. Wilcoxon Signed Rank Test was used to compare liver function tests prior to UDCA therapy and before delivery. We used the SigmaPlot 11.0 software package for all statistical analyses. All analyses were performed with alpha set to 0.05.
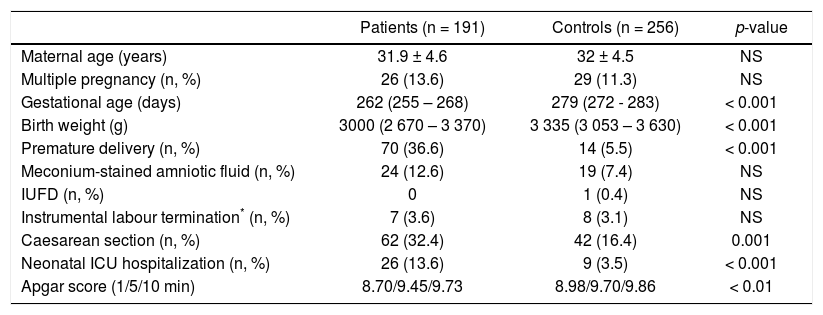
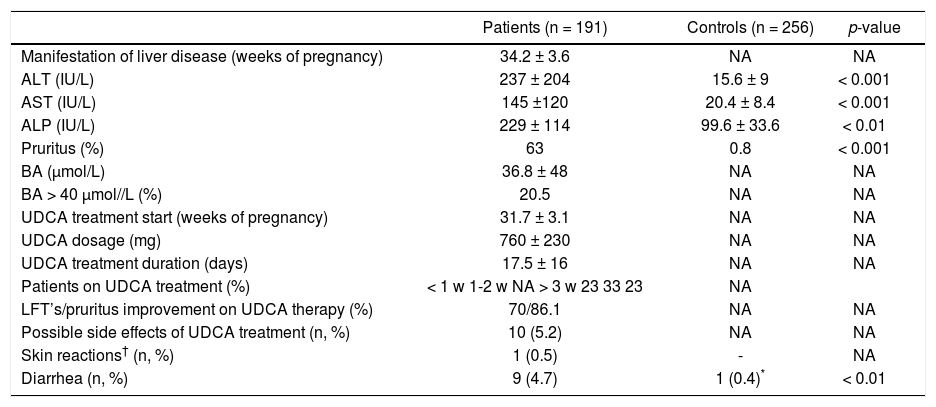
ResultsThe basic characteristics of all examined subjects are given in table 1. In all patients examined, the ICP manifested in the third trimester (34.4 ± 3.4 weeks of gestation) (Table 2); pruritus being present in a majority of the affected women (63%) (Table 2). The mean BA concentrations reached 37 μmol/L, and more than 20% had levels above the critical cut-off value of 40 μmol/L (Table 2). The highest serum BA level recorded was 290 μmol/L, indicating severe deterioration of biliary secretion functions.
Basic characteristics of UDCA-treated ICP patients and control subjects.
| Patients (n = 191) | Controls (n = 256) | p-value | |
|---|---|---|---|
| Maternal age (years) | 31.9 ± 4.6 | 32 ± 4.5 | NS |
| Multiple pregnancy (n, %) | 26 (13.6) | 29 (11.3) | NS |
| Gestational age (days) | 262 (255 – 268) | 279 (272 - 283) | < 0.001 |
| Birth weight (g) | 3000 (2 670 – 3 370) | 3 335 (3 053 – 3 630) | < 0.001 |
| Premature delivery (n, %) | 70 (36.6) | 14 (5.5) | < 0.001 |
| Meconium-stained amniotic fluid (n, %) | 24 (12.6) | 19 (7.4) | NS |
| IUFD (n, %) | 0 | 1 (0.4) | NS |
| Instrumental labour termination* (n, %) | 7 (3.6) | 8 (3.1) | NS |
| Caesarean section (n, %) | 62 (32.4) | 42 (16.4) | 0.001 |
| Neonatal ICU hospitalization (n, %) | 26 (13.6) | 9 (3.5) | < 0.001 |
| Apgar score (1/5/10 min) | 8.70/9.45/9.73 | 8.98/9.70/9.86 | < 0.01 |
IUFD: intrauterine fetal death.
Detailed characteristics of UDCA-treated ICP patients and control subjects.
| Patients (n = 191) | Controls (n = 256) | p-value | |
|---|---|---|---|
| Manifestation of liver disease (weeks of pregnancy) | 34.2 ± 3.6 | NA | NA |
| ALT (IU/L) | 237 ± 204 | 15.6 ± 9 | < 0.001 |
| AST (IU/L) | 145 ±120 | 20.4 ± 8.4 | < 0.001 |
| ALP (IU/L) | 229 ± 114 | 99.6 ± 33.6 | < 0.01 |
| Pruritus (%) | 63 | 0.8 | < 0.001 |
| BA (µmol/L) | 36.8 ± 48 | NA | NA |
| BA > 40 μmol//L (%) | 20.5 | NA | NA |
| UDCA treatment start (weeks of pregnancy) | 31.7 ± 3.1 | NA | NA |
| UDCA dosage (mg) | 760 ± 230 | NA | NA |
| UDCA treatment duration (days) | 17.5 ± 16 | NA | NA |
| Patients on UDCA treatment (%) | < 1 w 1-2 w NA > 3 w 23 33 23 | NA | |
| LFT’s/pruritus improvement on UDCA therapy (%) | 70/86.1 | NA | NA |
| Possible side effects of UDCA treatment (n, %) | 10 (5.2) | NA | NA |
| Skin reactions† (n, %) | 1 (0.5) | - | NA |
| Diarrhea (n, %) | 9 (4.7) | 1 (0.4)* | < 0.01 |
Data expressed as mean ± SD. NA: not applicable.
The UDCA treatment was used in the range of 500-1,500 mg/d, 750 mg/d in most cases (corresponding to approximately 10 mg/kg/day). The mean duration of treatment was 17 days (Table 2). ALT/AST activities improved in 70% (ALT activities dropped from 237 ± 204 to 185 ± 270 IU/L, p < 0.001; AST from 145 ± 120 to 119 ± 156 IU/L, p = 0.006), pruritus was ameliorated in 86% of treated patients (Table 2).
The UDCA treatment was very well tolerated, with possible side effects having been recorded in only ten women (5.2%) (Table 2). Skin reactions occurred in one (0.5%), and mild diarrhea developed in nine women (4.7%). Because these side effects were only mild and minor in their frequency, in no patient UDCA treatment must have been stopped. Although gestational age, birth weight, the rates of preterm delivery and non-spontaneous labours, as well as the occurrence of neonatal complications were worse in the group of UDCA-treated women compared to control subjects (Table 1), this was most likely due to underlying liver disease and related neonatal prematurity. No complications attributable to the UDCA treatment were detected during fetal life, delivery, or the early neonatal period.
DiscussionLiver diseases unique for pregnancy are not uncommon and may have a serious impact on fetal and/or neonatal out-comes.1 One of the major areas of progress over the last decade in the hepatology field is the recognition and understanding of the pathogenesis of ICP, with defined accurate guidelines for its diagnosis and management.6 Although generally accepted by hepatologists, these guidelines are not commonly translated into routine care by obstetricians. The major problem is that serum BA determination is not recognized by all obstetricians as a major predictive marker of ICP;7 additionally, in many countries this laboratory test is even not available for routine laboratory use. Furthermore, obstetricians usually perceive a lack of robust clinical data on the safety of UDCA. This is also reflected by information on UDCA use in pregnancy in UDCA SPC’s (summary of product characteristics) drugs used in clinical practice. Thus, UDCA is not accepted as a treatment of choice in international obstetrics society guidelines for use in pregnant women;8 although in some countries, the opposite is true on the national level.9,10 Nevertheless, as implied from the recent detailed survey performed in Australia and New Zealand, the approach of obstetricians to patients with ICP is rather heterogeneous; one third using the RCOG guidelines,8 a third using their local hospital guidelines, and the last third did not use any.7 Interestingly, no respondent in this survey answered that they had used any hepatology society’s guidelines. Based on this data, the authors strongly urge for consensus guidelines.10
In our retrospective multicentric study, carried out on a large sample of pregnant women with deterioration of liver function tests, we were able to prove that UDCA administered to ICP patients was therapeutically efficient and not associated with serious side effects - for pregnant women, fetuses, or neonates. Our data are consistent with all previous clinical studies published thus far;11–21 but compared to the others, our study belongs to the largest in size (191 ICP patients treated with UDCA), and strongly supports the therapeutic potency as well as safety of this drug during in ICP patients. In fact, the first case report on pregnant woman with ICP, treated with UDCA, was published as early as 1991,16 with further studies following subsequently.11–15,17–21 Importantly, in none of these studies were any adverse reactions attributable to the UDCA administered to mothers recorded in their babies. Nevertheless, due to improper and insufficiently robust designs of the published studies, the data on safety (in particular for the babies) lead the appropriate drug administration authorities to reject the requests for the extension of the indication UDCA for the therapy of ICP in Australia (http://www.tga.gov.au/auspar/auspar-ursodeoxycholic-acid-accessed 30 Dec, 2015). However, it is necessary to stress that UDCA was found to be safe not only when searching for potential immediate adverse neonatal outcomes, but also in a long-term study by Zapata, et al. who followed infants whose mothers received UDCA during pregnancy for up to 12 years without any sign of adverse effects.22 In addition, a similar safety profile of UDCA is known from studies on preterm neonates on parenteral nutrition.23 These data fit with the observation of Mazzella, et al., who demonstrated negligible concentrations of UDCA in the cord blood of babies born to women treated with UDCA during pregnancy.24 Furthermore, several further recent papers might change the reluctance for UDCA use in pregnancy. First, the Cochrane review on treatment of ICP25 concluded that UDCA treatment significantly improves pruritus, with the potential to decrease fetal distress and asphyxia. Even more importantly, two recent meta-analyses strongly support the use of UDCA as the first line treatment of ICP, while confirming maternal symptom efficacy, decreased rates of premature deaths, fetal distress, as well as need for intensive care.26,27 Since these were done, additional clinical data have been published corroborating the efficacy and safety of UDCA in pregnancy.20,21 Together with the results from our large retrospective clinical observation, all the available data strongly suggests the therapeutic efficacy as well as safety of UDCA in ICP patients, which calls for a revision of the current obstetrics guidelines, changes of the current SPC information, which should lead to wider use of UDCA in pregnant patients.
The major limitations of our study, however, are the retrospective nature, lack of more detailed clinical data (such as scaling of pruritus severity, grading of UDCA treatment efficacy and non-availability of postnatal follow-up data.
In conclusion, in our large retrospective multicentric study we confirmed the therapeutic efficacy and safety of UDCA treatment in patients with ICP for both mothers and fetuses. These data support the need for a revision of current UDCA drug SPC information, which in turn, should translate in wider use of UDCA in patients with pregnancy-associated cholestatic liver diseases.
Grant SupportThis work was supported by grants given by the Czech Ministry of Health (grants IGA MZ CR NT 12211-5 and RVO-VFN64165/2015), and by Charles University in Prague (PRVOUK-P25/LF1/2).
Conflict of InterestThis study was also supported by PRO.MED.CS Praha a.s., a manufacturer of ursodeoxycholic acid. This supporter had no influence upon the collection, analysis, interpretation of the data, writing of the report, nor on the decision to submit the paper for publication.